4 3 CHEMICAL EQUATIONS Pages 202 211 CHEMICAL




- Slides: 12

4. 3 CHEMICAL EQUATIONS Pages 202 -211

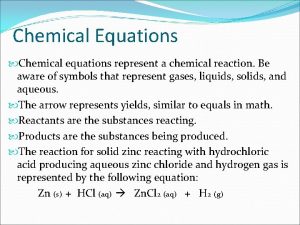
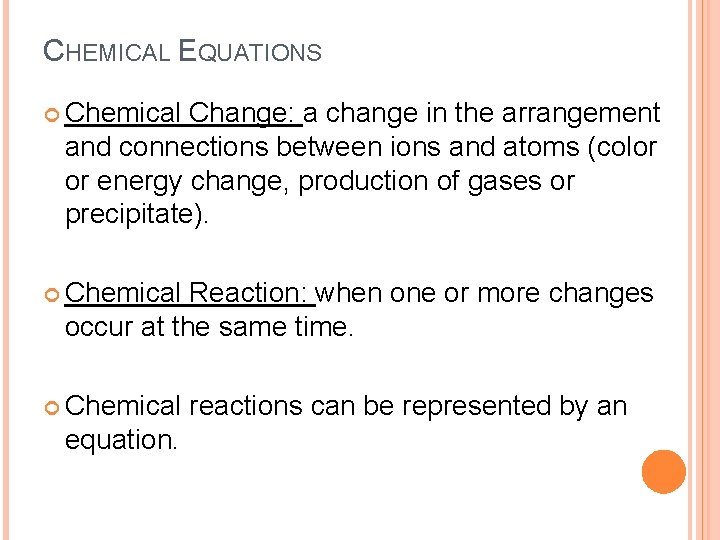
CHEMICAL EQUATIONS Chemical Change: a change in the arrangement and connections between ions and atoms (color or energy change, production of gases or precipitate). Chemical Reaction: when one or more changes occur at the same time. Chemical equation. reactions can be represented by an

CHEMICAL EQUATIONS Reactants: pure substances that react in a chemical change (written to the left). Products: pure substances formed in a chemical change that have different properties than those of the reactants (written to the right). Reactants Products Word Equation: nitrogen monoxide + oxygen nitrogen dioxide Symbolic Equation: 2 NO + O 2 2 NO 2

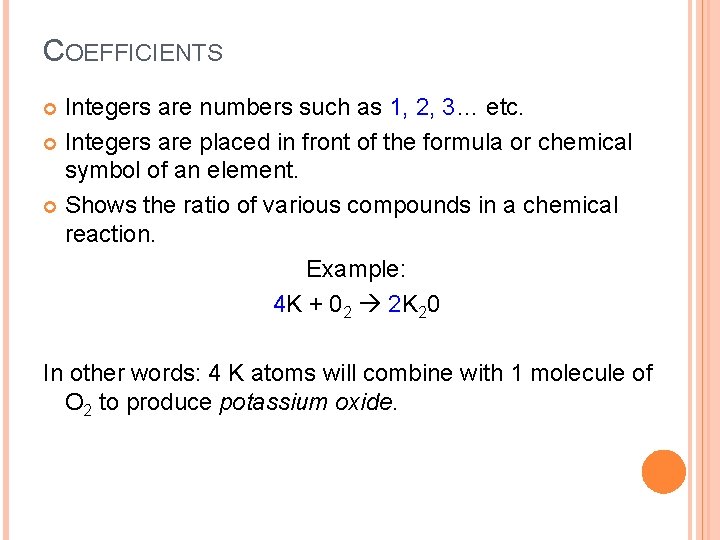
COEFFICIENTS Integers are numbers such as 1, 2, 3… etc. Integers are placed in front of the formula or chemical symbol of an element. Shows the ratio of various compounds in a chemical reaction. Example: 4 K + 02 2 K 20 In other words: 4 K atoms will combine with 1 molecule of O 2 to produce potassium oxide.

LAW OF CONSERVATION OF MASS “Mass is conserved in a chemical reaction; the total mass of products is always equal to the total mass of the reactants in any chemical equation. ” Atoms are conserved; never made or destroyed. WHO SAID THIS? ? French chemist, Antoine Lavoisier and his wife Marie. Anne (1789), measured the masses of reactants and their products of many chemical reactions. Discovered that the total mass never changed during a chemical reaction. This law is true for all chemical equations.


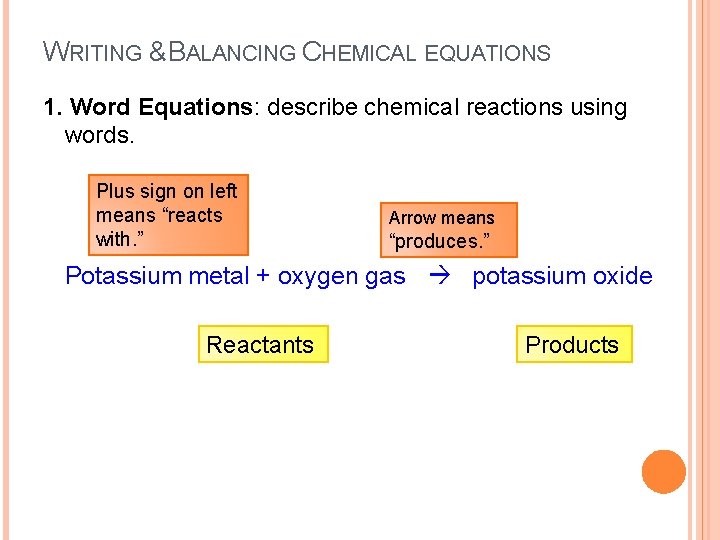
WRITING & BALANCING CHEMICAL EQUATIONS 1. Word Equations: describe chemical reactions using words. Plus sign on left means “reacts with. ” Arrow means “produces. ” Potassium metal + oxygen gas potassium oxide Reactants Products

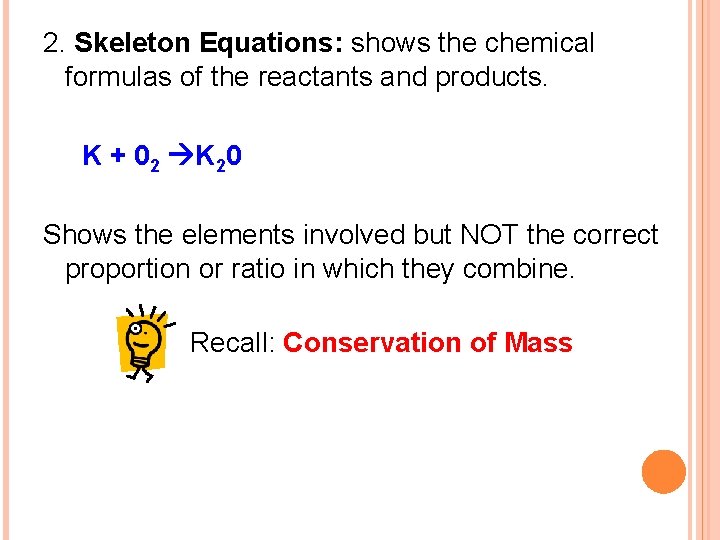
2. Skeleton Equations: shows the chemical formulas of the reactants and products. K + 02 K 20 Shows the elements involved but NOT the correct proportion or ratio in which they combine. Recall: Conservation of Mass

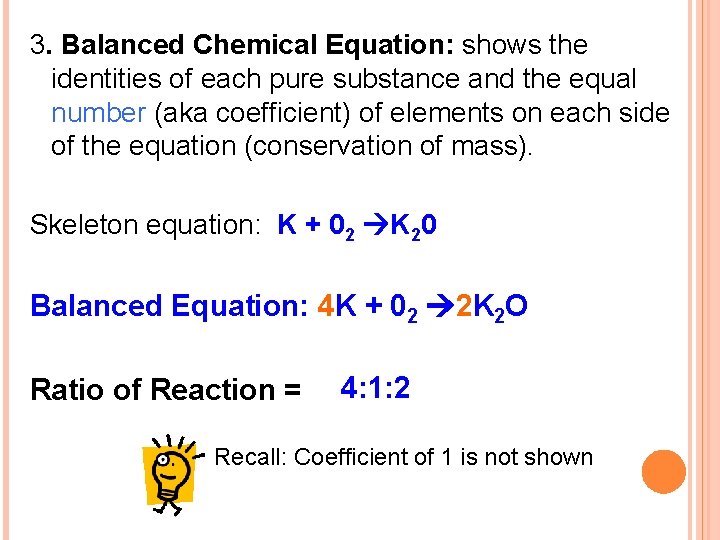
3. Balanced Chemical Equation: shows the identities of each pure substance and the equal number (aka coefficient) of elements on each side of the equation (conservation of mass). Skeleton equation: K + 02 K 20 Balanced Equation: 4 K + 02 2 K 2 O Ratio of Reaction = 4: 1: 2 Recall: Coefficient of 1 is not shown

TIPS FOR WRITING EQUATIONS Common compounds: methane (CH 4), ammonia (NH 3) and water (H 20) (need to memorize). Diatomic elements: elements that exist on their own in nature, exist as 2 atom molecules: H 2, N 2, 02, F 2, Cl 2, Br 2, and I 2 Written this way when on their own and NOT part of a compound.

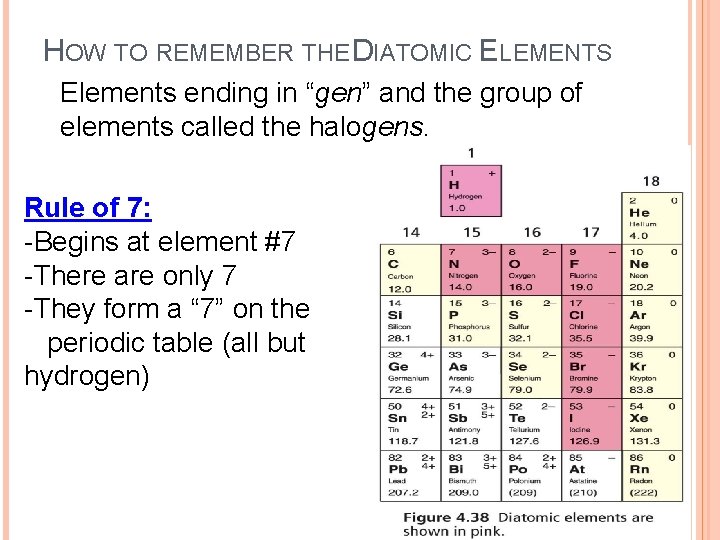
HOW TO REMEMBER THE DIATOMIC ELEMENTS Elements ending in “gen” and the group of elements called the halogens. Rule of 7: -Begins at element #7 -There are only 7 -They form a “ 7” on the periodic table (all but hydrogen)

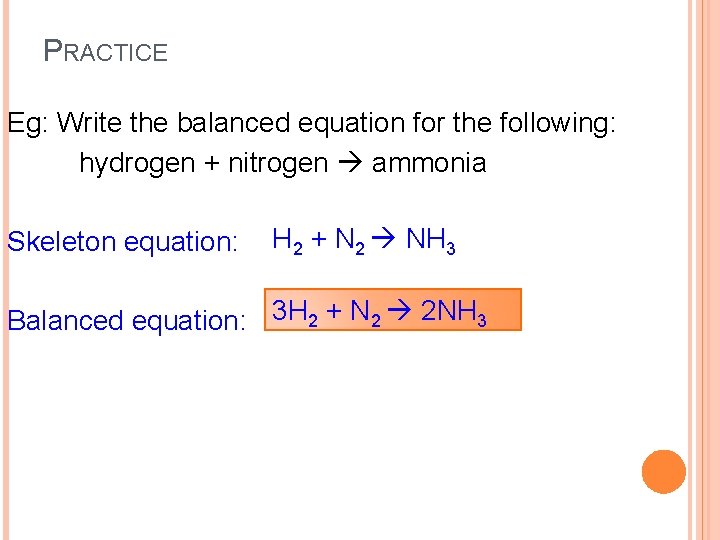
PRACTICE Eg: Write the balanced equation for the following: hydrogen + nitrogen ammonia Skeleton equation: H 2 + N 2 NH 3 Balanced equation: 3 H 2 + N 2 2 NH 3
 Printed pages vs web pages
Printed pages vs web pages Translate word equations to chemical equations
Translate word equations to chemical equations Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Opwekking 211
Opwekking 211 Miller indices 211
Miller indices 211 Coordinated entry snohomish county
Coordinated entry snohomish county Poli 211
Poli 211 Physics 211 exam 1
Physics 211 exam 1 Legea 211 din 2011 privind regimul deseurilor
Legea 211 din 2011 privind regimul deseurilor Mgt 211
Mgt 211 Mgt 211
Mgt 211 Is 211 nationwide
Is 211 nationwide Csce 211
Csce 211