Chemical Equations and Chemical Reactions Chemical Equations Representing















- Slides: 24

Chemical Equations and Chemical Reactions

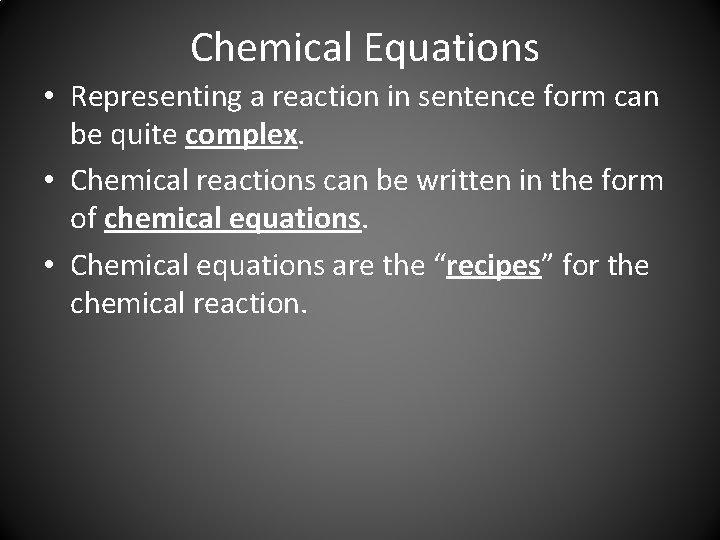
Chemical Equations • Representing a reaction in sentence form can be quite complex. • Chemical reactions can be written in the form of chemical equations. • Chemical equations are the “recipes” for the chemical reaction.

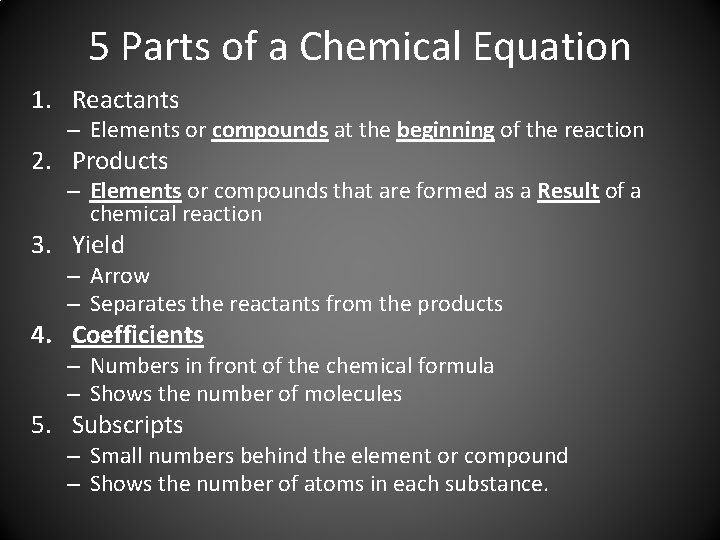
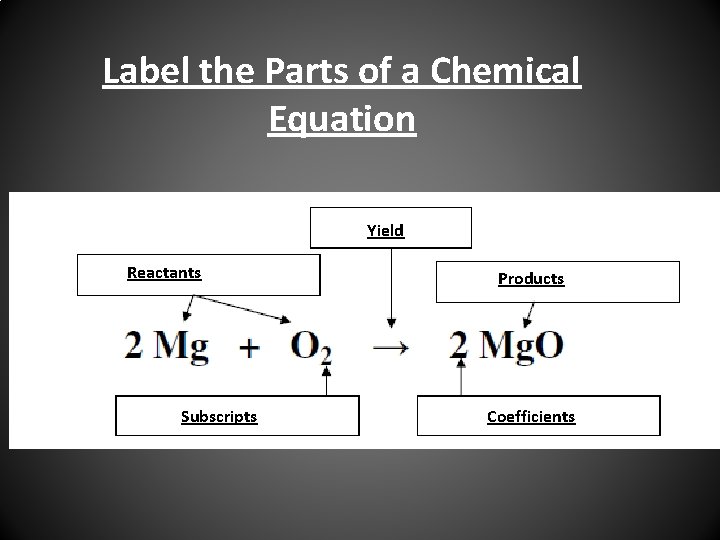
5 Parts of a Chemical Equation 1. Reactants – Elements or compounds at the beginning of the reaction 2. Products – Elements or compounds that are formed as a Result of a chemical reaction 3. Yield – Arrow – Separates the reactants from the products 4. Coefficients – Numbers in front of the chemical formula – Shows the number of molecules 5. Subscripts – Small numbers behind the element or compound – Shows the number of atoms in each substance.

Label the Parts of a Chemical Equation Yield Reactants Subscripts Products Coefficients

R C P 6 C 6 O 18 = 6 + 12 H 12 12 + 6 = 18 12 How Many Oxygen Atoms are on the Product Side? How Many Elements are in this Chemical Formula? How Many Water Molecules are on the Reactant Side?

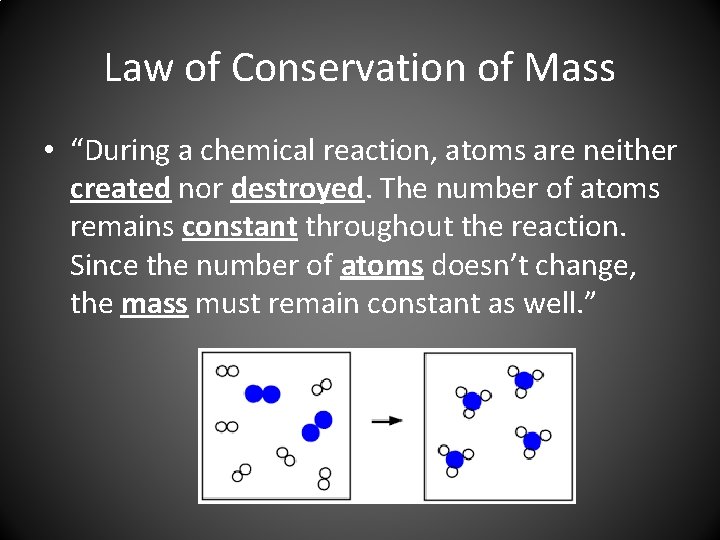
Law of Conservation of Mass • “During a chemical reaction, atoms are neither created nor destroyed. The number of atoms remains constant throughout the reaction. Since the number of atoms doesn’t change, the mass must remain constant as well. ”

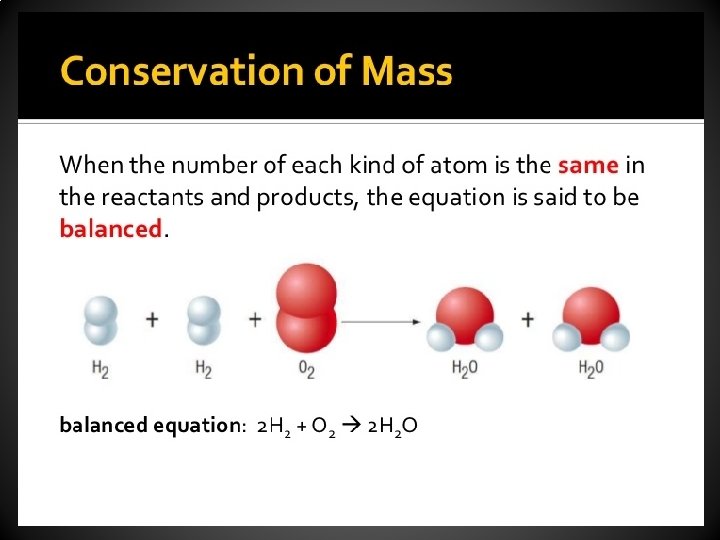
Conservation of Mass • No atoms are destroyed and no new atoms are produced during a chemical reaction. • Instead, the atoms in the reactants are simply rearranged to form the products. • Chemical bonds between atoms are broken and new ones are formed and the atom simply reconnect in new ways.



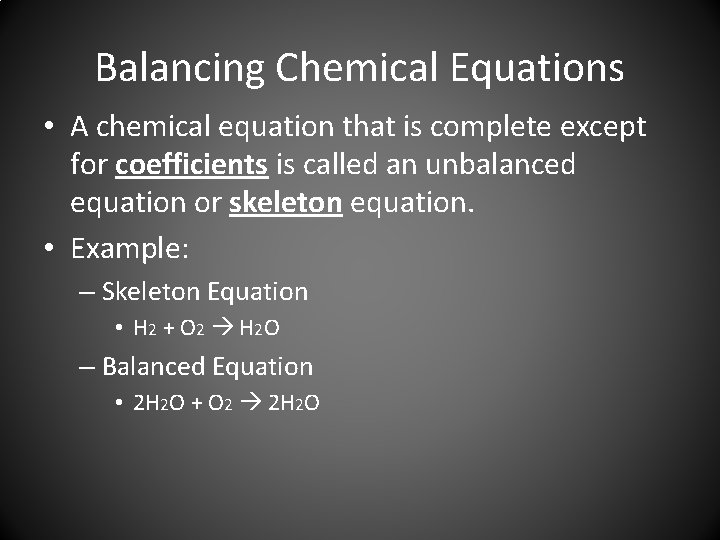
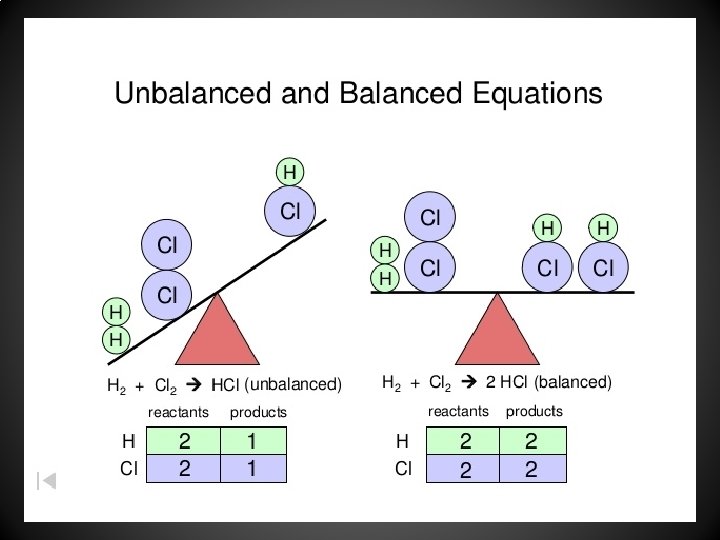
Balancing Chemical Equations • A chemical equation that is complete except for coefficients is called an unbalanced equation or skeleton equation. • Example: – Skeleton Equation • H 2 + O 2 H 2 O – Balanced Equation • 2 H 2 O + O 2 2 H 2 O

How to Balance Equation • Begin by counting the number of atoms of each element. • Balance by placing COEFFICIENTS in front of the chemical formulas until the number of atoms in the reactants equals the products. • USE ONLY WHOLE NUMBERS • NEVER change a subscript.



Ask yourself “Has something new been created/destroyed? ” If so a chemical change/reactions has occurred.

Chemical Change • When a formation of new substances takes place with different chemical properties it is called chemical changes. • A chemical reaction is always accompanied by a chemical change. • Reaction is the term used for depicting a change or transformation.

Physical Change • A physical change in a substance doesn't change what the substance is. – whipping egg whites (air is forced into the fluid, but no new substance is produced) – H 2 O transforming from Solid to Liquid State. The Ice cube is melting but the chemical substance is still H 2 O – boiling water (water molecules are forced away from each other when the liquid changes to vapor, but the molecules are still H 2 O. )

Chemical Change • In a chemical change where there is a chemical reaction, a new substance is formed and energy is either given off or absorbed. – iron rusting (iron oxide forms) – gasoline burning (water vapor and carbon dioxide form) – eggs cooking (fluid protein molecules uncoil and crosslink to form a network) – bread rising (yeast converts carbohydrates into carbon dioxide gas)

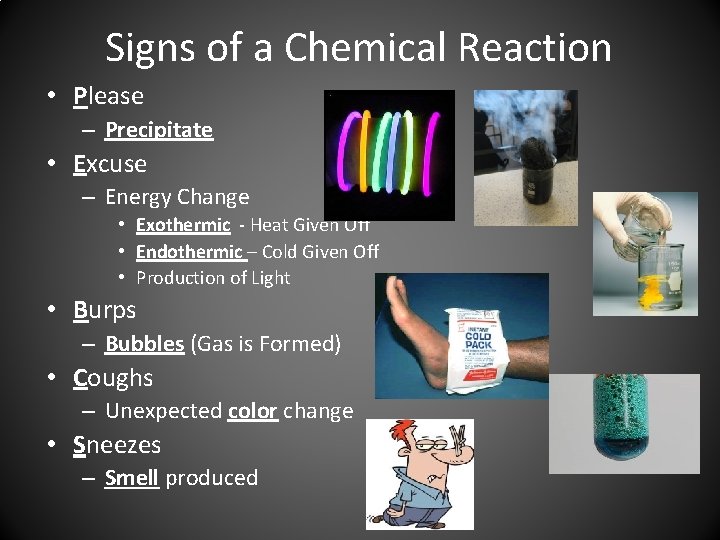
Signs of a Chemical Reaction • Please – Precipitate • Excuse – Energy Change • Exothermic - Heat Given Off • Endothermic – Cold Given Off • Production of Light • Burps – Bubbles (Gas is Formed) • Coughs – Unexpected color change • Sneezes – Smell produced

Please • P in Please stands for PRECIPITATE • 2 liquid make a Solid • Precipitate is an insoluble solid that emerges from a liquid solution.

Excuse • E in Excuse stands for Energy Change. • Light is produced • Two types of energy change: – Endothermic (COLD) • Energy is absorbed, causing temperatures to DECREASE – Exothermic (HOT) • Energy is released, causing temperatures to INCREASE

Energy Change • Light Production

Burps • B in Burps stands for Bubbles • If you see bubbles, a gas has been produced

Coughs • C in Coughs stands for Color change • This change in color must be UNEXPECTED

Sneezes • S in Sneezes stands for production of smell
 Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Are kc and kp equal
Are kc and kp equal Types of reactions
Types of reactions Chapter 8 review chemical equations and reactions
Chapter 8 review chemical equations and reactions Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Chemical equations and reactions chapter 8 review
Chemical equations and reactions chapter 8 review I intro
I intro Unit 5 chemical equations and reactions
Unit 5 chemical equations and reactions Unit 4: toxins lesson 73 worksheet answers
Unit 4: toxins lesson 73 worksheet answers Synthesis reaction
Synthesis reaction Toxic reactions chemical equations
Toxic reactions chemical equations 10 examples of redox reaction
10 examples of redox reaction Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Section 1 chemical changes
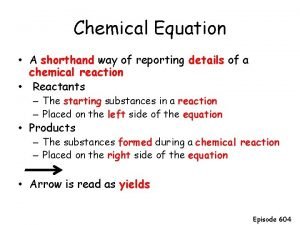
Section 1 chemical changes Chemist shorthand way of representing chemical reaction
Chemist shorthand way of representing chemical reaction Chemist shorthand way of representing chemical reaction
Chemist shorthand way of representing chemical reaction Chemist shorthand way of representing chemical reaction.
Chemist shorthand way of representing chemical reaction. Reactants and products
Reactants and products Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Chemical reactions of copper and percent yield
Chemical reactions of copper and percent yield What is released or absorbed whenever chemical
What is released or absorbed whenever chemical Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes