REPRESENTING CHEMICAL REACTIONS Representing Chemical Reactions with Equations



- Slides: 15

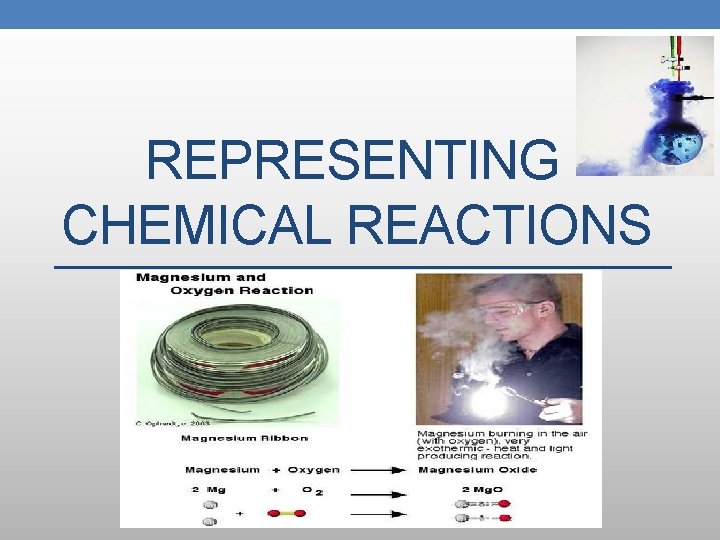
REPRESENTING CHEMICAL REACTIONS

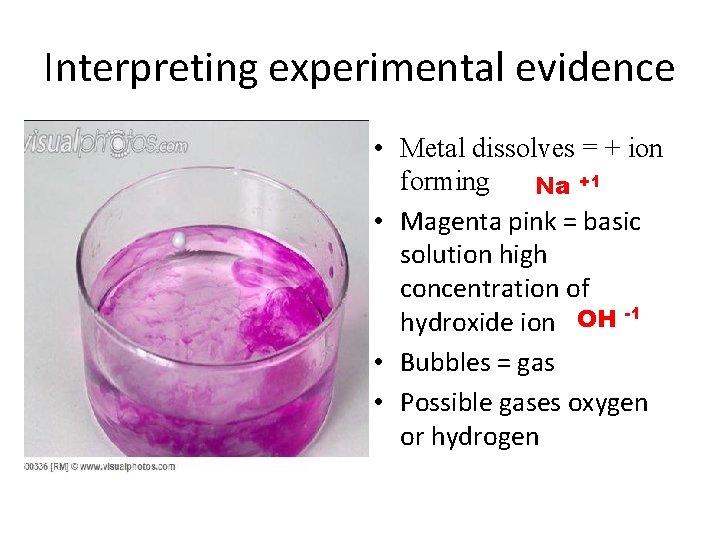
Representing Chemical Reactions with Equations • Reaction of sodium with water ((Phenolphthalein added) Acid, neutral in phenolphthalein = colorless Base in phenolphthalein = pink

Interpreting experimental evidence • Metal dissolves = + ion forming Na +1 • Magenta pink = basic solution high concentration of hydroxide ion OH -1 • Bubbles = gas • Possible gases oxygen or hydrogen

Actual Product is highly Flammable • Rxn is very exothermic (releases heat); • Hydrogen can ignite • Is Hydrogen (monoatomic or diatomic? ) • H 2

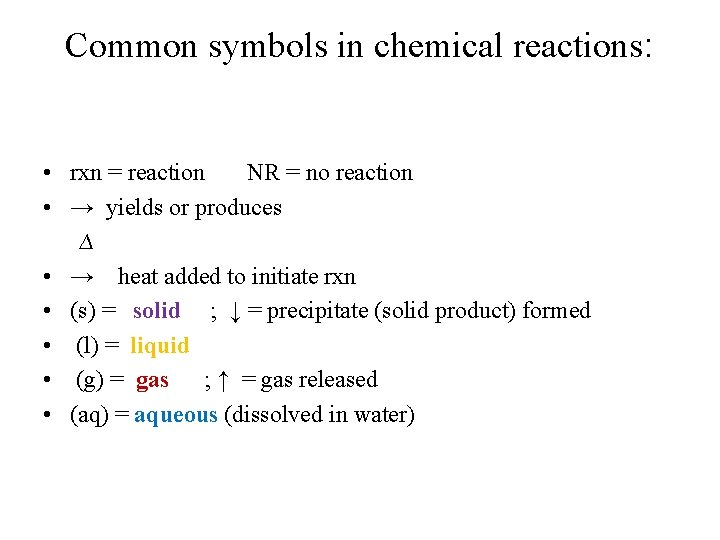
Common symbols in chemical reactions: • rxn = reaction NR = no reaction • → yields or produces ∆ • → heat added to initiate rxn • (s) = solid ; ↓ = precipitate (solid product) formed • (l) = liquid • (g) = gas ; ↑ = gas released • (aq) = aqueous (dissolved in water)

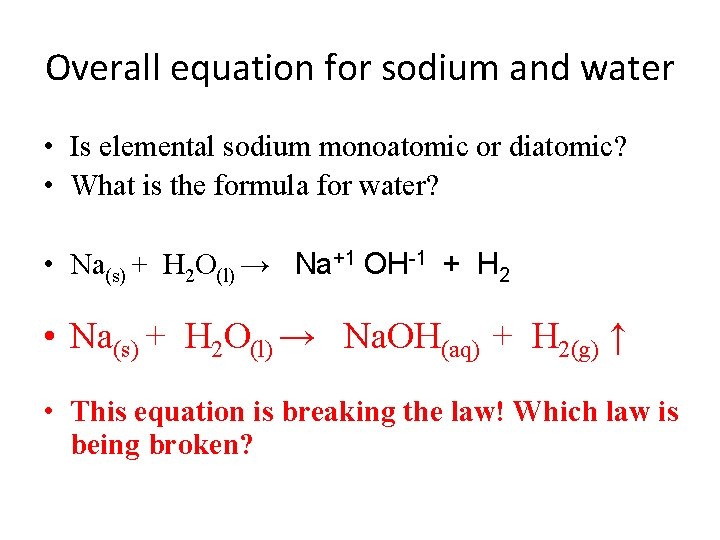
Overall equation for sodium and water • Is elemental sodium monoatomic or diatomic? • What is the formula for water? • Na(s) + H 2 O(l) → Na+1 OH-1 + H 2 • Na(s) + H 2 O(l) → Na. OH(aq) + H 2(g) ↑ • This equation is breaking the law! Which law is being broken?

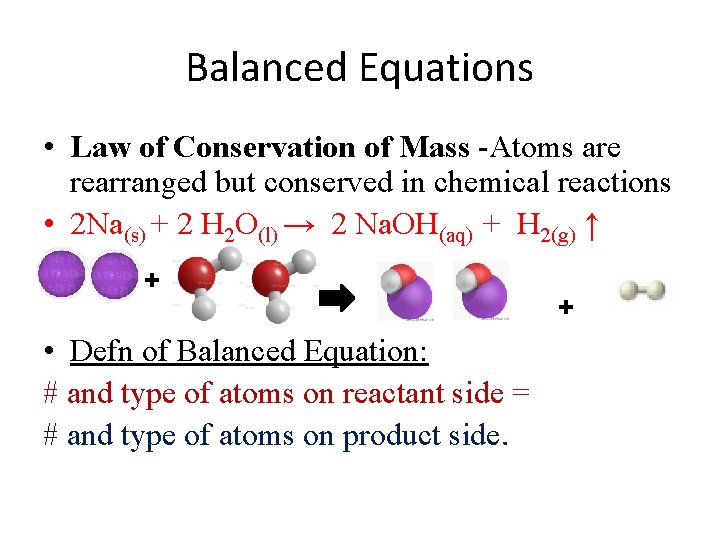
Balanced Equations • Law of Conservation of Mass -Atoms are rearranged but conserved in chemical reactions • 2 Na(s) + 2 H 2 O(l) → 2 Na. OH(aq) + H 2(g) ↑ + • Defn of Balanced Equation: # and type of atoms on reactant side = # and type of atoms on product side. +

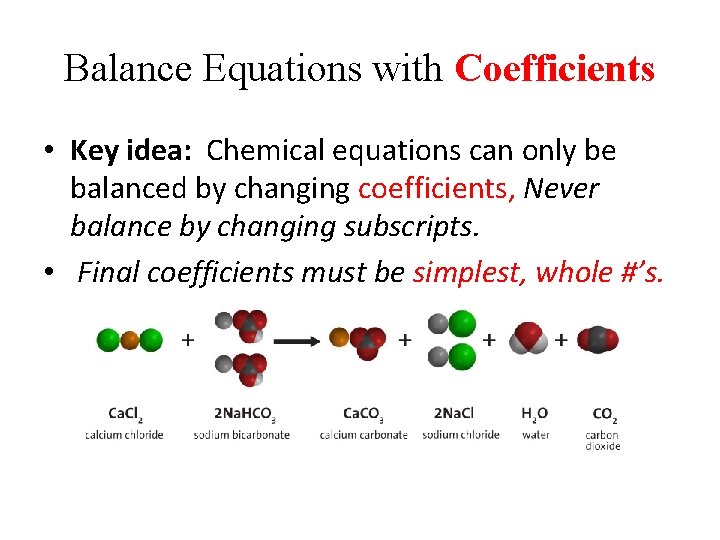
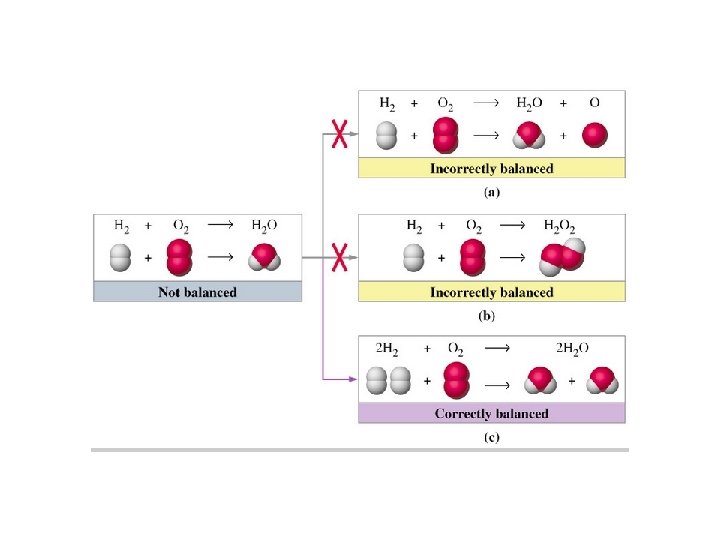
Balance Equations with Coefficients • Key idea: Chemical equations can only be balanced by changing coefficients, Never balance by changing subscripts. • Final coefficients must be simplest, whole #’s.

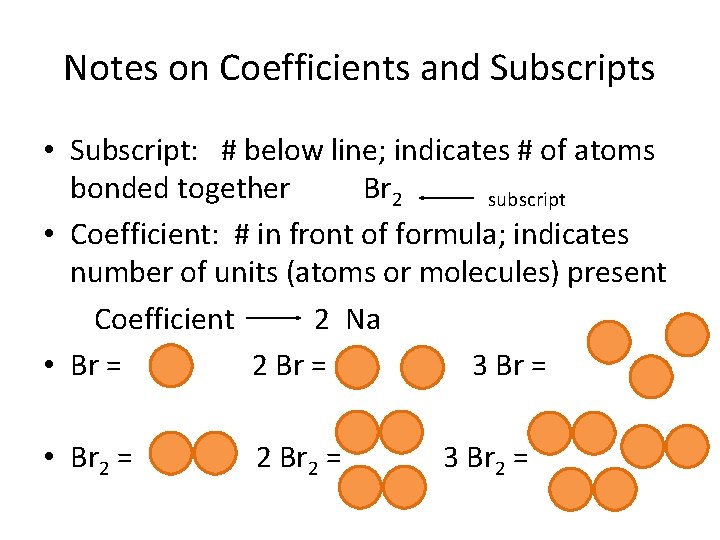
Notes on Coefficients and Subscripts • Subscript: # below line; indicates # of atoms bonded together Br 2 subscript • Coefficient: # in front of formula; indicates number of units (atoms or molecules) present Coefficient 2 Na • Br = 2 Br = 3 Br = • Br 2 = 2 Br 2 = 3 Br 2 =

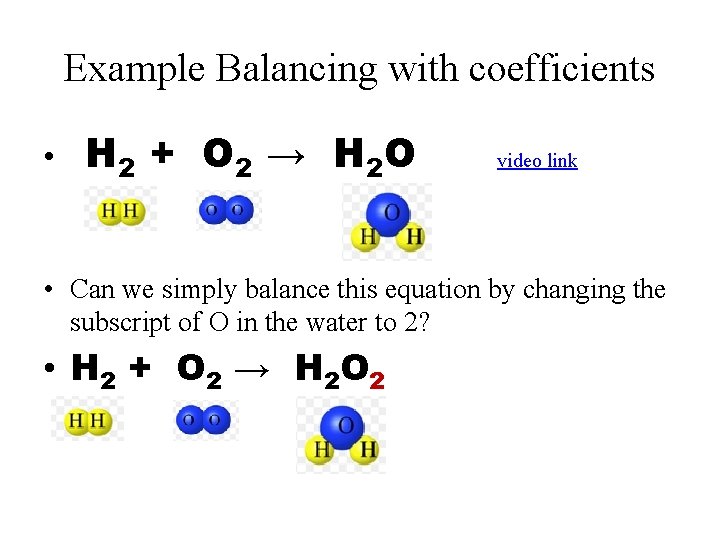
Example Balancing with coefficients • H 2 + O 2 → H 2 O video link • Can we simply balance this equation by changing the subscript of O in the water to 2? • H 2 + O 2 → H 2 O 2

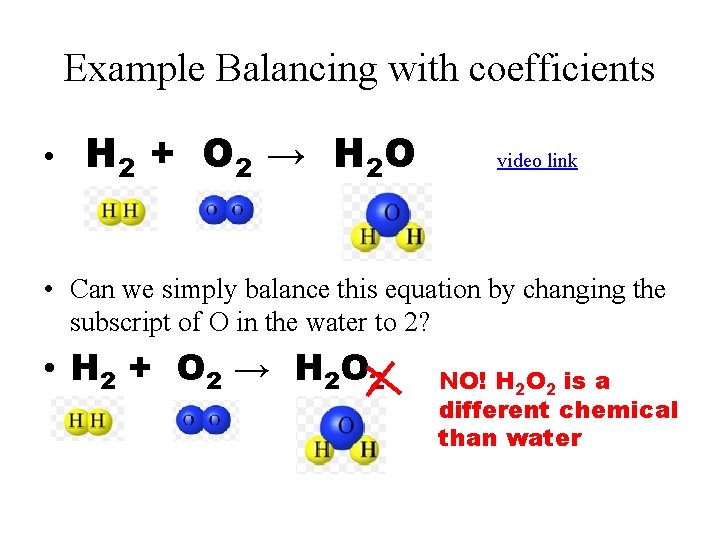
Example Balancing with coefficients • H 2 + O 2 → H 2 O video link • Can we simply balance this equation by changing the subscript of O in the water to 2? • H 2 + O 2 → H 2 O 2 NO! H 2 O 2 is a different chemical than water

HYDROGEN PEROXIDE REACTS WITHMn. O 2 WHILE WATER DOES NOT!!VIDEO LINK Water + Mn. O 2 = NR Mn. O 2 water Hydrogen peroxide + Mn. O 2 =


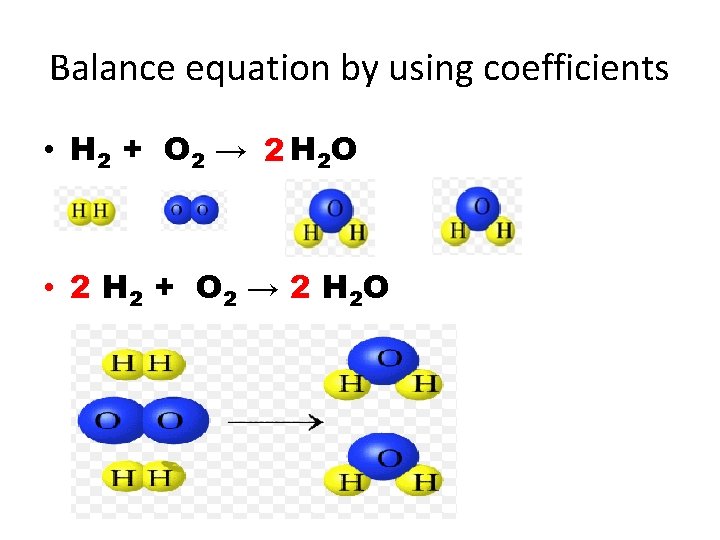
Balance equation by using coefficients • H 2 + O 2 → 2 H 2 O • 2 H 2 + O 2 → 2 H 2 O

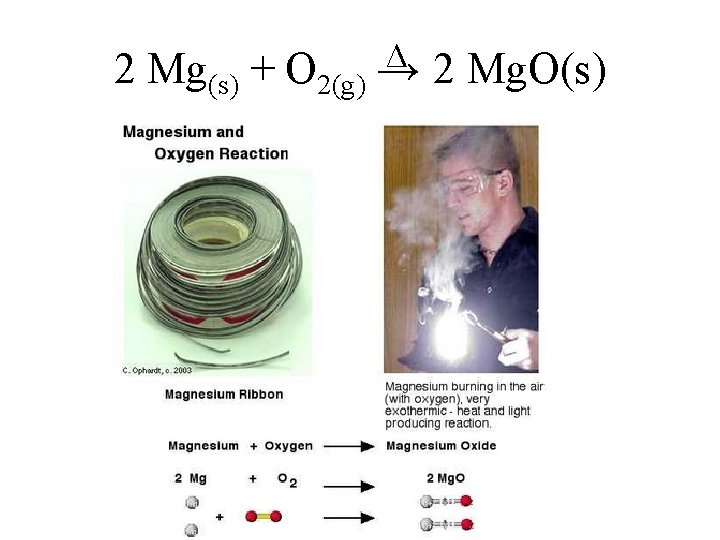
2 Mg(s) + O 2(g) ∆ → 2 Mg. O(s)
 Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Types of reactions
Types of reactions Toxic reactions chemical equations
Toxic reactions chemical equations Chemistry chapter 8 review chemical equations and reactions
Chemistry chapter 8 review chemical equations and reactions Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Balancing equations chapter 8
Balancing equations chapter 8 Balancing chemical equations definition
Balancing chemical equations definition Unit 5 chemical equations and reactions
Unit 5 chemical equations and reactions Chapter 19 chemical reactions simple word equations
Chapter 19 chemical reactions simple word equations Toxic reactions chemical equations
Toxic reactions chemical equations Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Translating chemical equations
Translating chemical equations A chemists shorthand way of representing chemical reaction
A chemists shorthand way of representing chemical reaction A chemist shorthand way
A chemist shorthand way