Terpenes Terpenoids Terpenes class of 20 000 compounds
































- Slides: 94

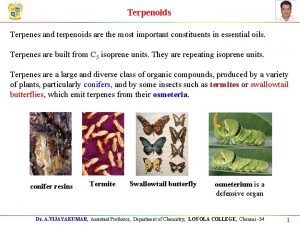
Terpenes & Terpenoids

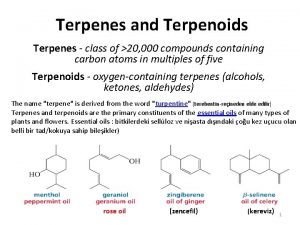
Terpenes - class of >20, 000 compounds containing carbon atoms in multiples of five Terpenoids - oxygen-containing terpenes (alcohols, ketones, aldehydes

The name "terpene" is derived from the word "turpentine" Terpenes and terpenoids are the primary constituents of the essential oils of many types of plants and flowers. rose oil (zenc 3 kereviz) (


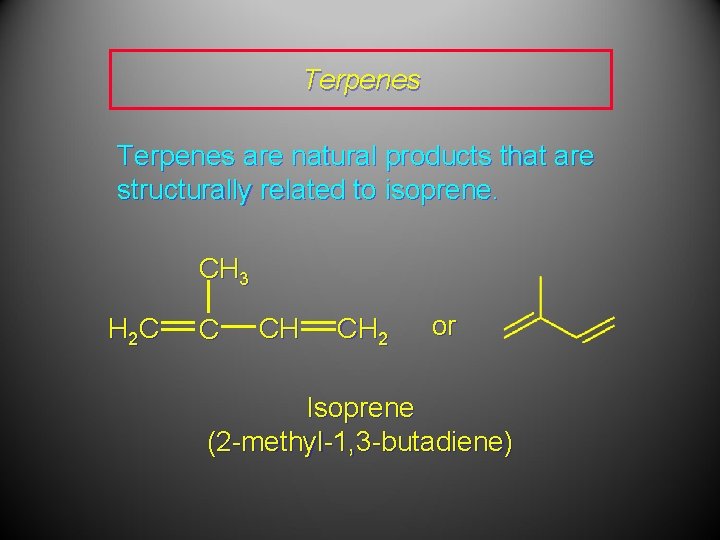
Terpenes are natural products that are structurally related to isoprene. CH 3 H 2 C C CH CH 2 or Isoprene (2 -methyl-1, 3 -butadiene)

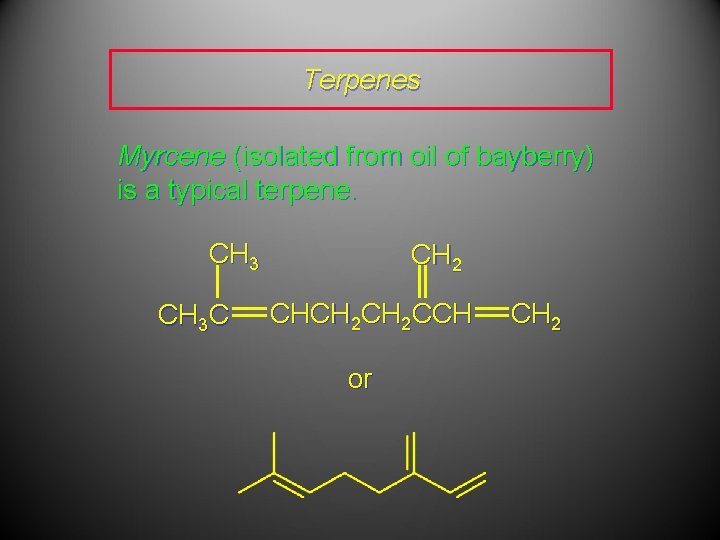
Terpenes Myrcene (isolated from oil of bayberry) is a typical terpene. CH 3 C CH 2 CHCH 2 CCH or CH 2

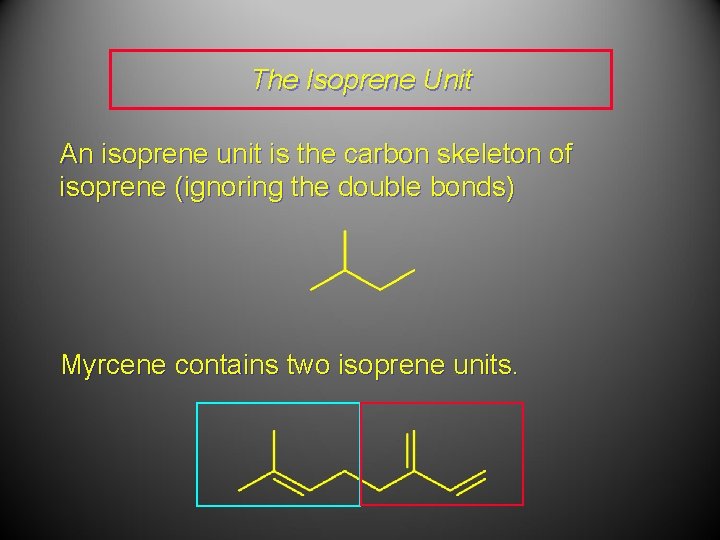
The Isoprene Unit An isoprene unit is the carbon skeleton of isoprene (ignoring the double bonds) Myrcene contains two isoprene units.

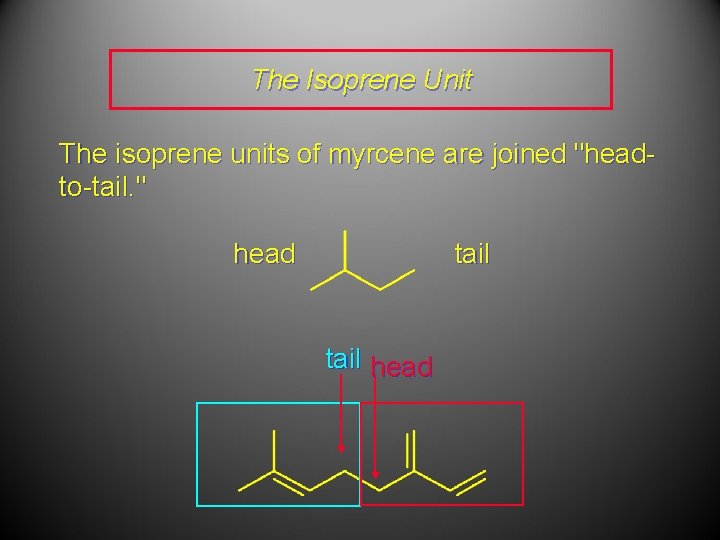
The Isoprene Unit The isoprene units of myrcene are joined "headto-tail. " head tail head

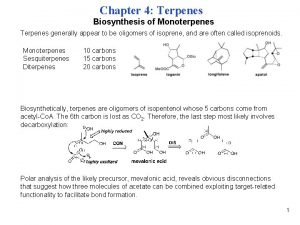
Classification of Terpenes Class Number of carbon atoms Monoterpene 10 Sesquiterpene 15 Diterpene 20 Sesterpene 25 Triterpene 30 Tetraterpene 40

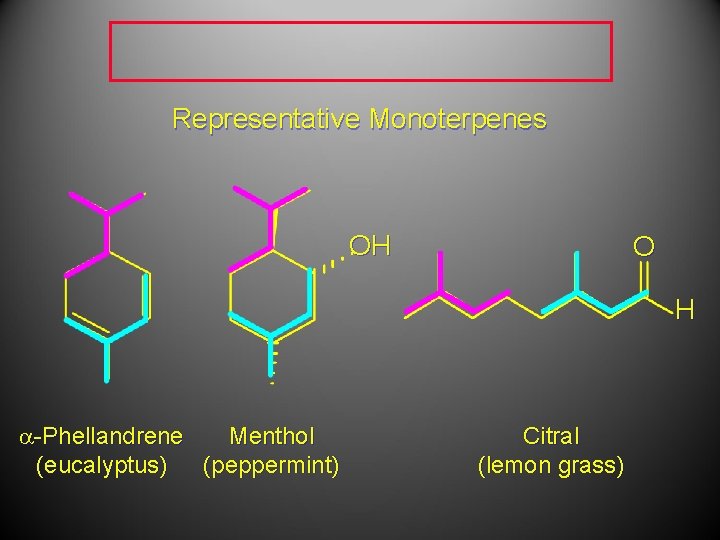
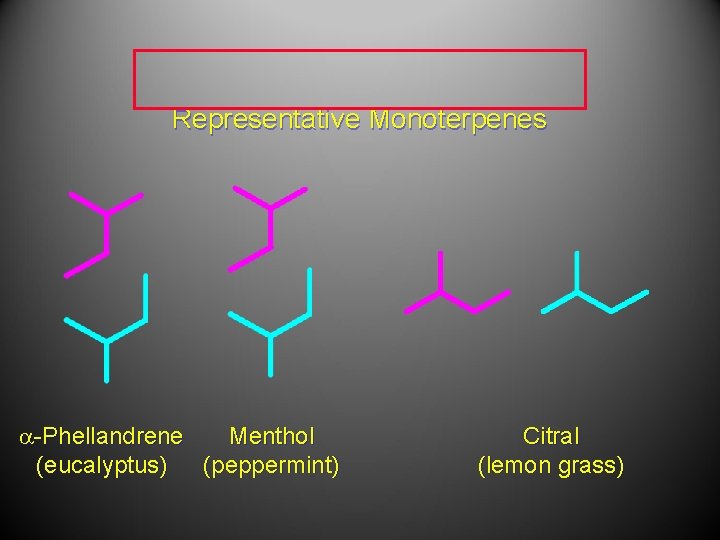
Representative Monoterpenes OH O H a-Phellandrene Menthol (eucalyptus) (peppermint) Citral (lemon grass)

Representative Monoterpenes OH O H a-Phellandrene Menthol (eucalyptus) (peppermint) Citral (lemon grass)

Representative Monoterpenes a-Phellandrene Menthol (eucalyptus) (peppermint) Citral (lemon grass)

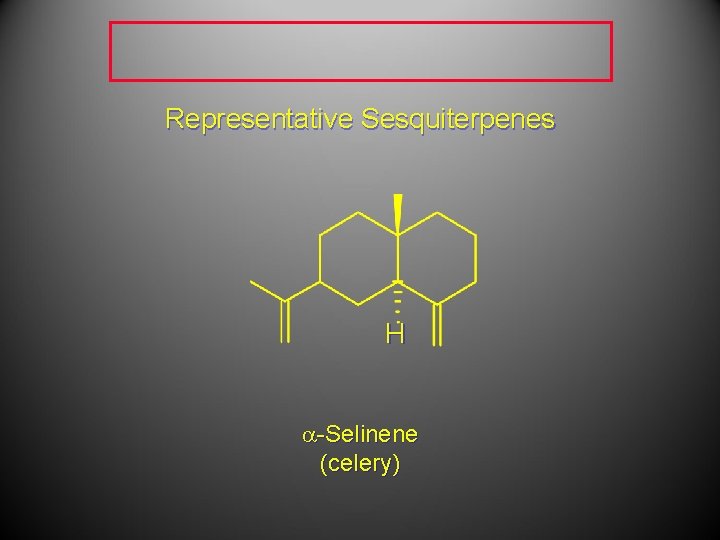
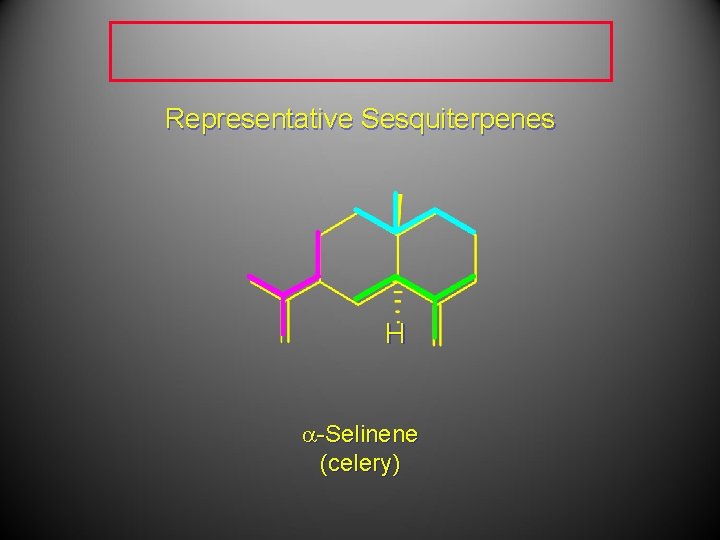
Representative Sesquiterpenes H a-Selinene (celery)

Representative Sesquiterpenes H a-Selinene (celery)

Representative Sesquiterpenes a-Selinene (celery)

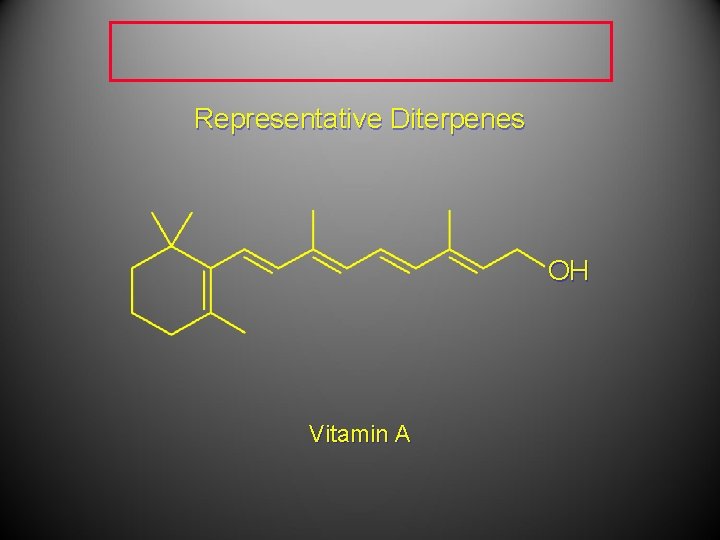
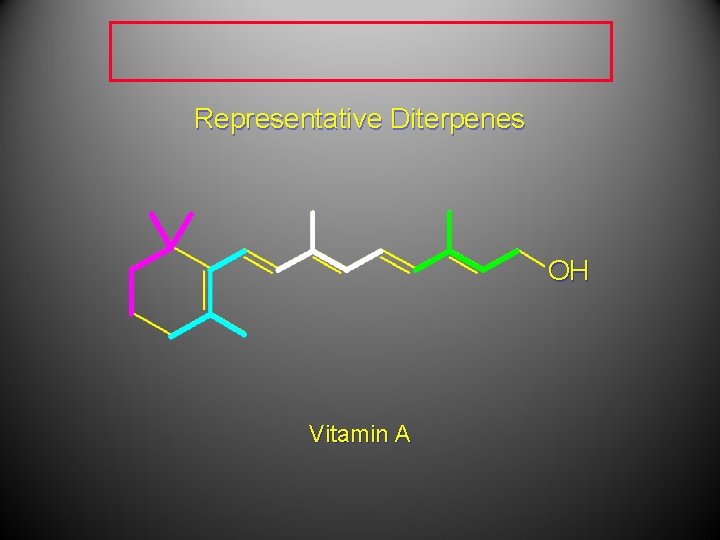
Representative Diterpenes OH Vitamin A

Representative Diterpenes OH Vitamin A

Representative Diterpenes Vitamin A

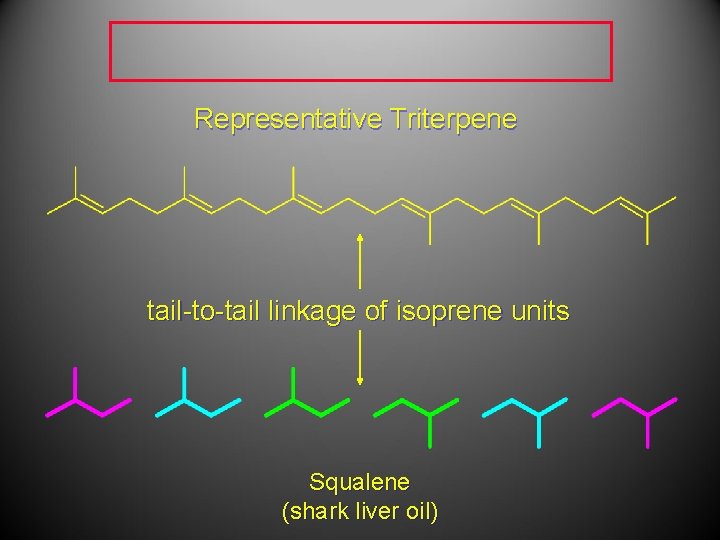
Representative Triterpene tail-to-tail linkage of isoprene units Squalene (shark liver oil)

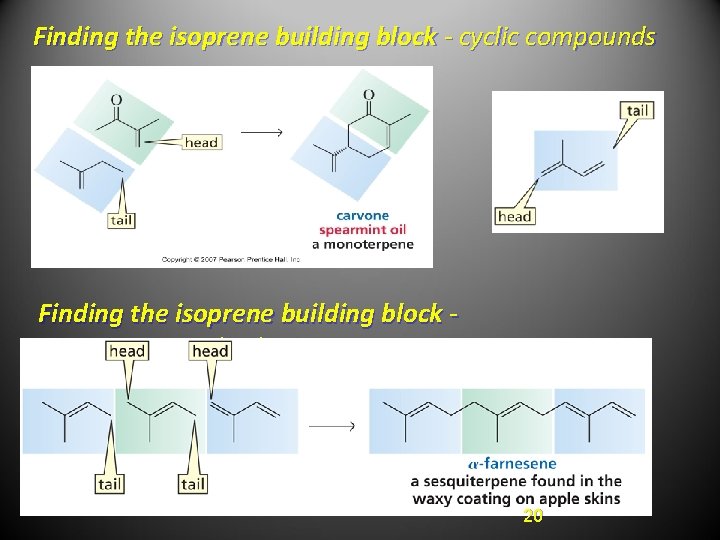
Finding the isoprene building block - cyclic compounds Finding the isoprene building block sesquiterpenes (C 15) 20

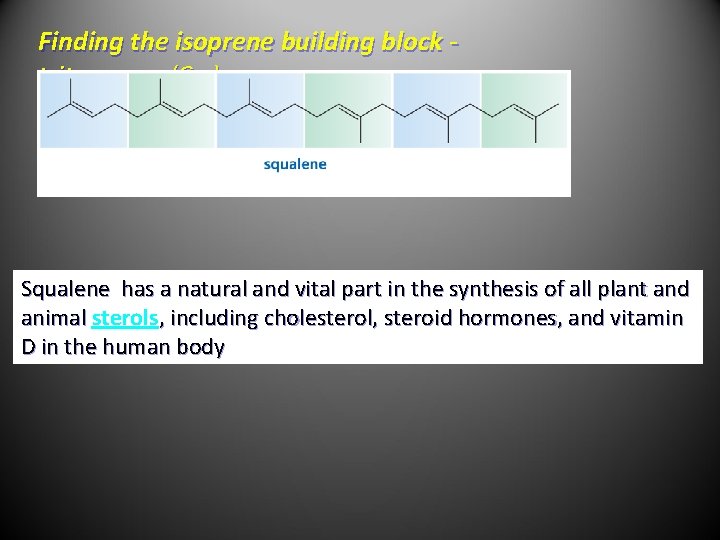
Finding the isoprene building block triterpenes (C 30) - Squalene has a natural and vital part in the synthesis of all plant and animal sterols, including cholesterol, steroid hormones, and vitamin D in the human body

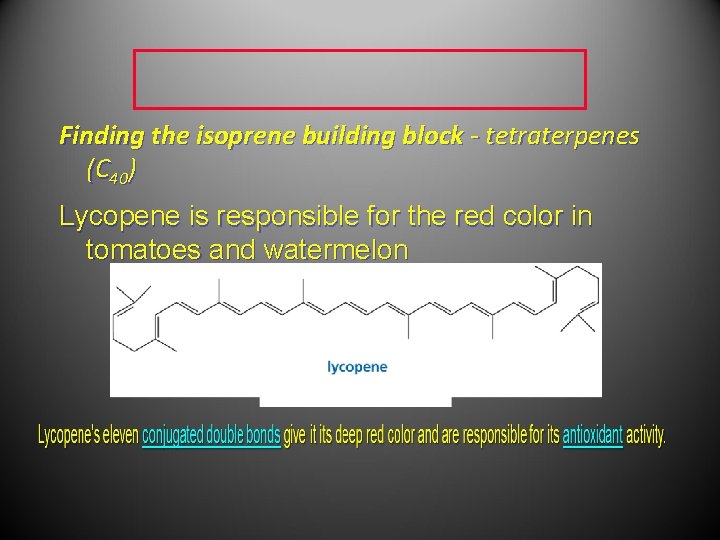
Finding the isoprene building block - tetraterpenes (C 40) Lycopene is responsible for the red color in tomatoes and watermelon

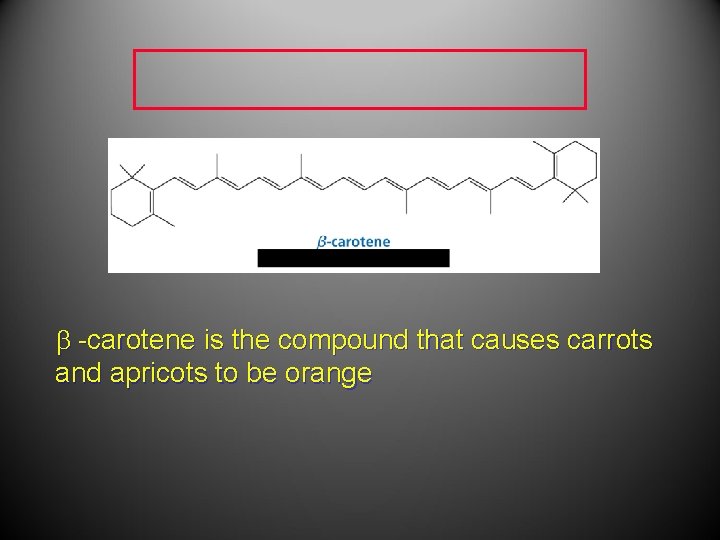
-carotene is the compound that causes carrots and apricots to be orange

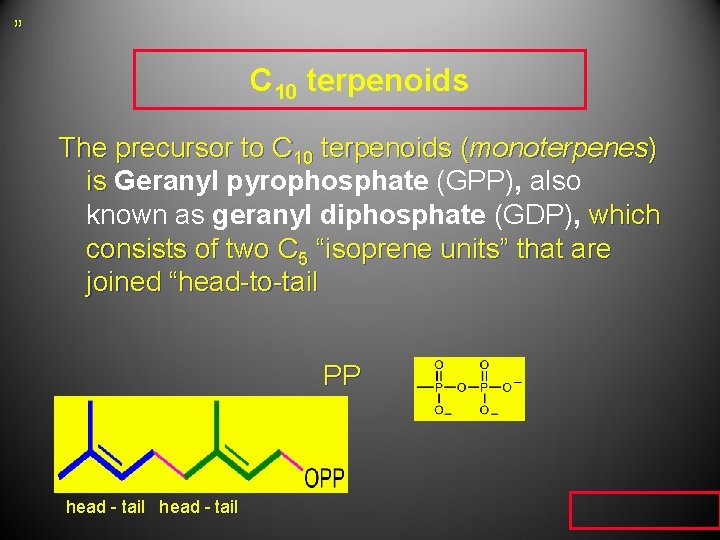
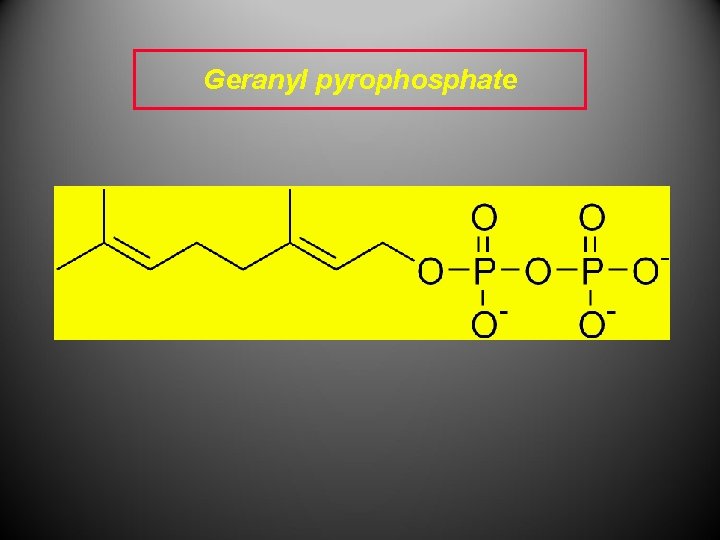
” C 10 terpenoids The precursor to C 10 terpenoids (monoterpenes) is Geranyl pyrophosphate (GPP), also known as geranyl diphosphate (GDP), which consists of two C 5 “isoprene units” that are joined “head-to-tail PP head - tail

Geranyl pyrophosphate

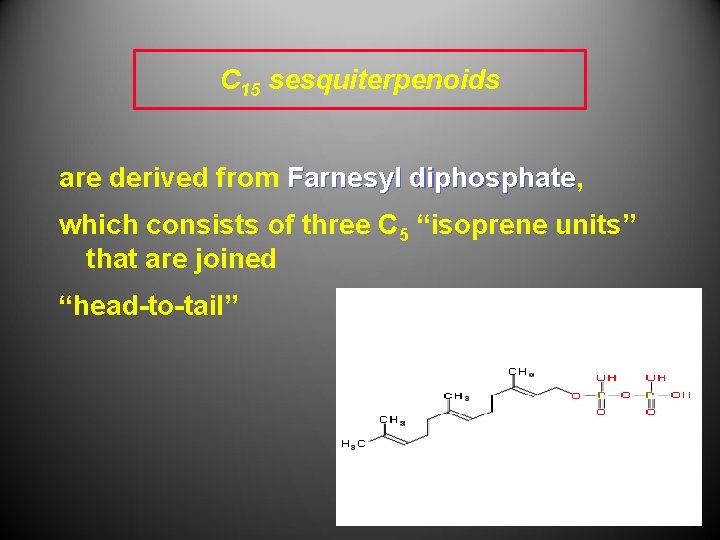
C 15 sesquiterpenoids are derived from Farnesyl diphosphate, diphosphate which consists of three C 5 “isoprene units” that are joined “head-to-tail”


C 20 diterpenoids are derived from Geranylgeranyl diphosphate, which consists of four C 5 “isoprene units” that are joined “head-to-tail”

Terpenoid nomenclature • Groups and subgroups • Based on pathways • Classification • IUPAC • CAS • Trivial name(derived from the structural family Or relate to natural source)

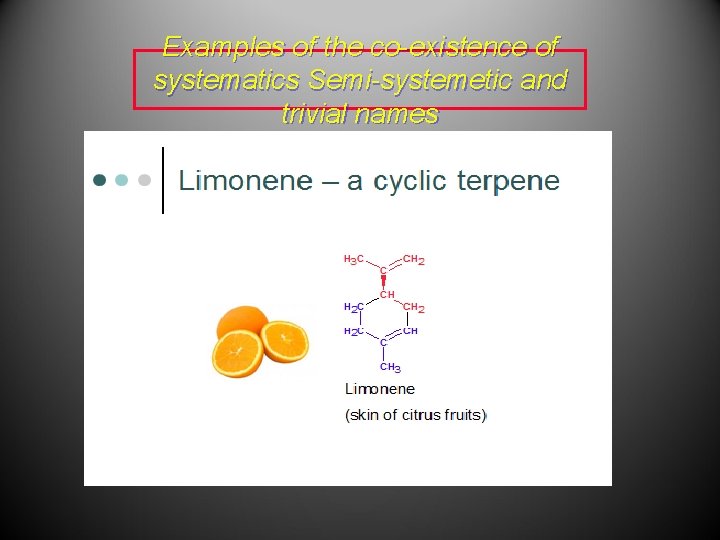
Examples of the co-existence of systematics Semi-systemetic and trivial names

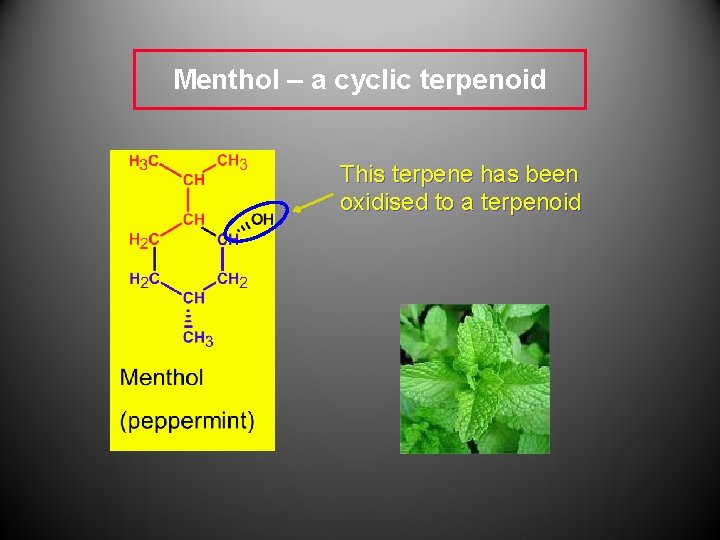
Menthol – a cyclic terpenoid This terpene has been oxidised to a terpenoid

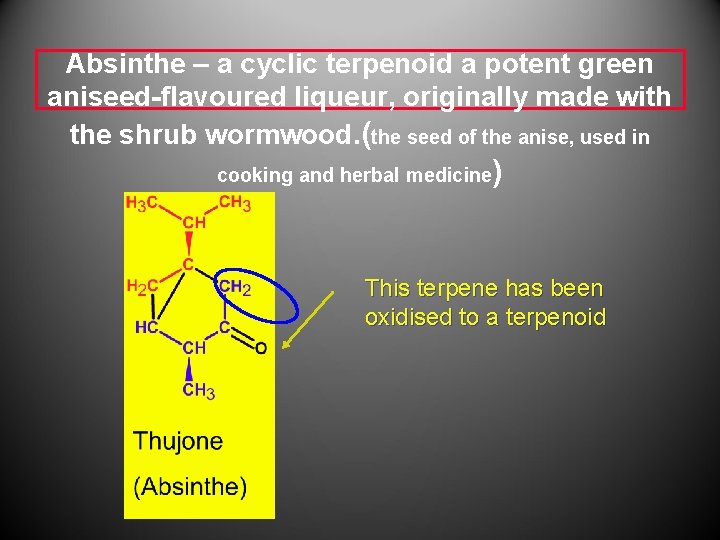
Absinthe – a cyclic terpenoid a potent green aniseed-flavoured liqueur, originally made with the shrub wormwood. (the seed of the anise, used in cooking and herbal medicine ) This terpene has been oxidised to a terpenoid

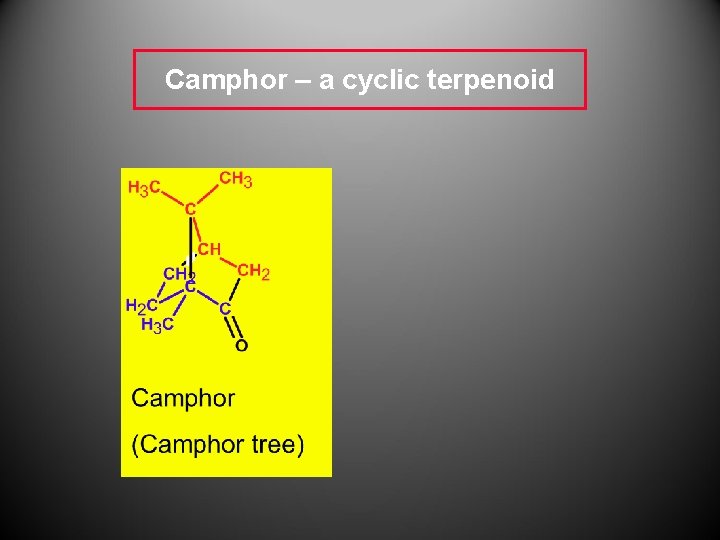
Camphor – a cyclic terpenoid

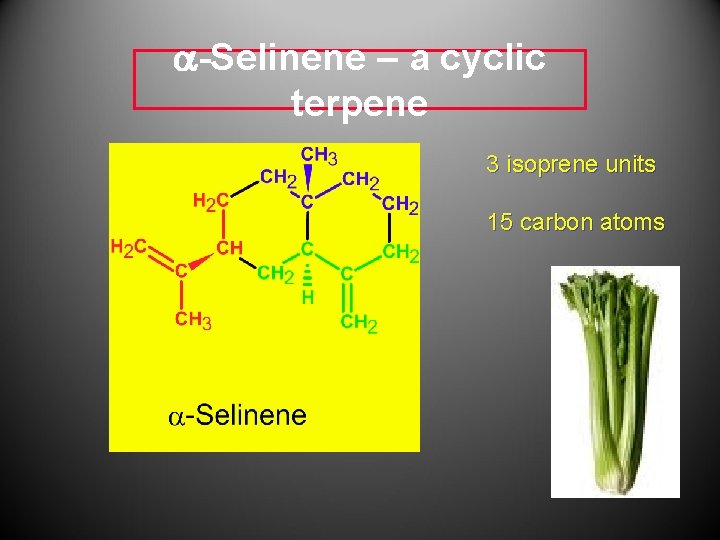
a-Selinene – a cyclic terpene 3 isoprene units 15 carbon atoms

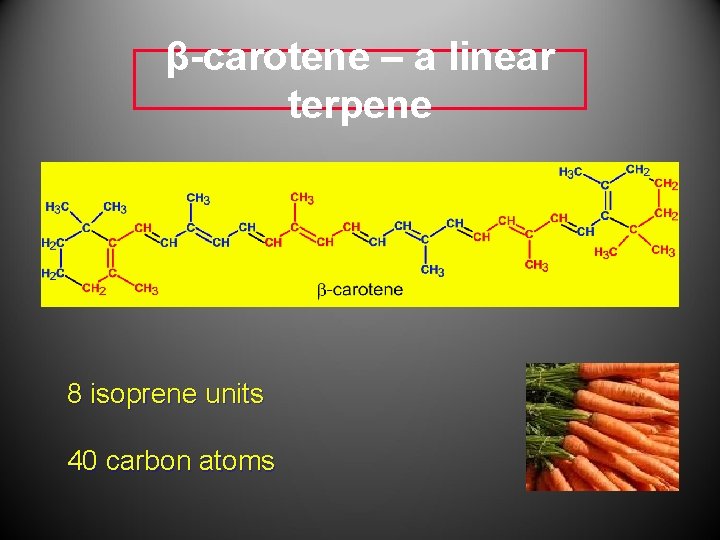
β-carotene – a linear terpene 8 isoprene units 40 carbon atoms

Questions Which unit makes up every terpene? Isoprene Unit How many carbons are there in an isoprene unit? Five What is the systematic name for isoprene? 2 -methylbuta-1, 3 -diene What is an oxidised terpene known as? Terpenoid

Carvone • Occurs in Enantiomeric forms • Also known as Meridian fennel, Persian carrot • Uses • Carvone –Latin name for Caraway, carum carvi • Basic carbon skeleton 1 -isopropyl-4 -methylcyclohexane common in nature the genus mentha includes various types of mint

saturated ketone of the p- menthane family of monoteroenoids Greek letters Use to distinguish between isomeric terpenoids Depends on • In order in which the isomers were discovered • Their abundance

α- Pinene by weight ¾ component of turpentine oil β- Pinene next most significant component

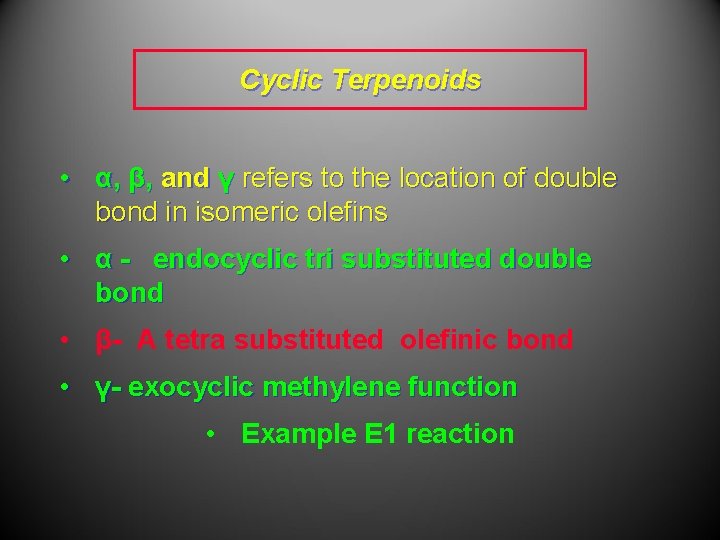
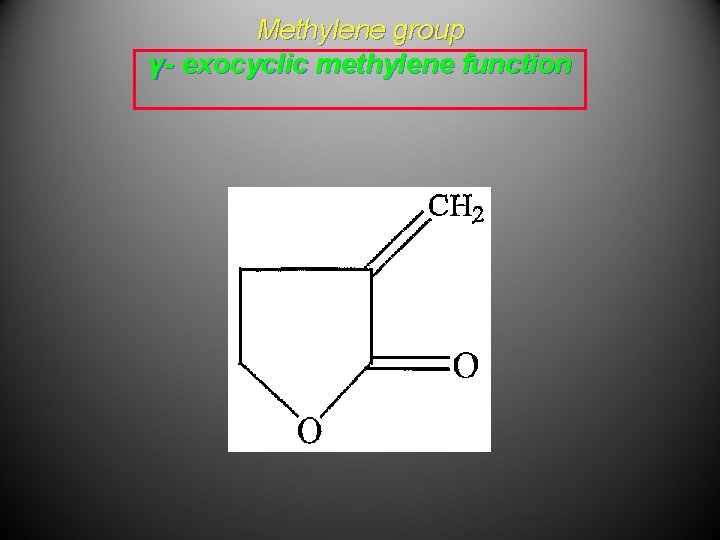
Cyclic Terpenoids • α, β, and γ refers to the location of double bond in isomeric olefins • α - endocyclic tri substituted double bond • β- A tetra substituted olefinic bond • γ- exocyclic methylene function • Example E 1 reaction


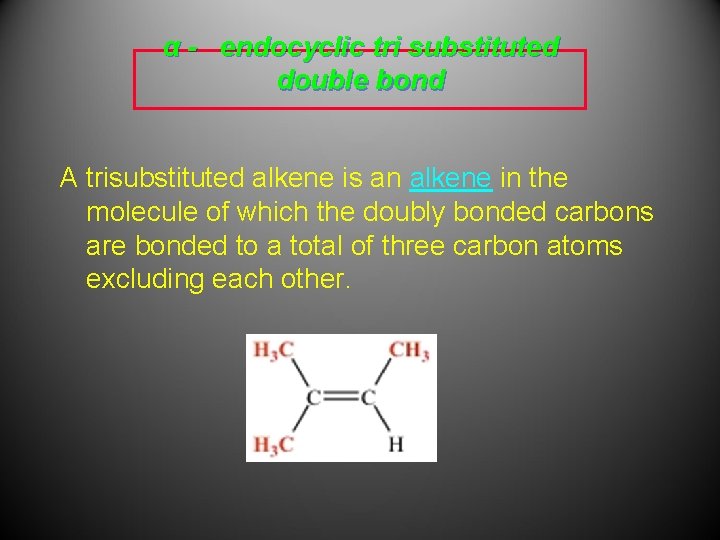
α - endocyclic tri substituted double bond A trisubstituted alkene is an alkene in the molecule of which the doubly bonded carbons are bonded to a total of three carbon atoms excluding each other.

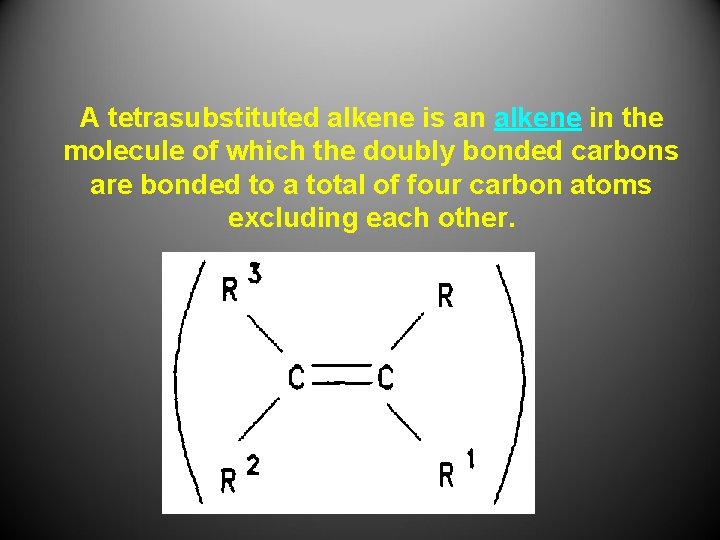
A tetrasubstituted alkene is an alkene in the molecule of which the doubly bonded carbons are bonded to a total of four carbon atoms excluding each other.

Methylene group γ- exocyclic methylene function

THE ROLE OF TERPENOIDS IN NATURE • Terpenoids are produced by a wide variety of plants, animals and microorganisms. • As for all metabolites, the synthesis of terpenoids places a metabolic load on the organism which produces them and so, almost invariably, there is a role which the material plays and for which it is synthesized.

• The roles which the terpenoids play in living organisms can be grouped into three classes: • Functional • Defense and (producing resins and gums Acacia gummiferae) • Communication.

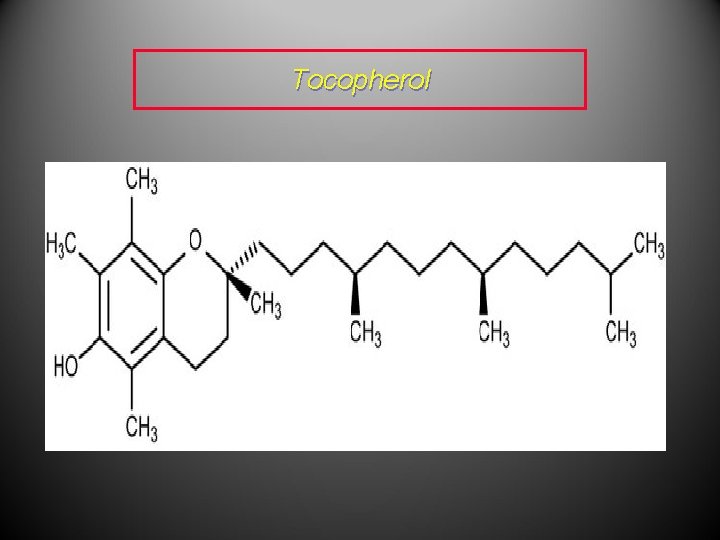
Examples • Vitamin A, or retinol, is the precursor for the pigment in eyes which detects light and is therefore responsible for the sense of sight. • Vitamin E, or tocopherol, is an important antioxidant which prevents oxidative damage to cells.

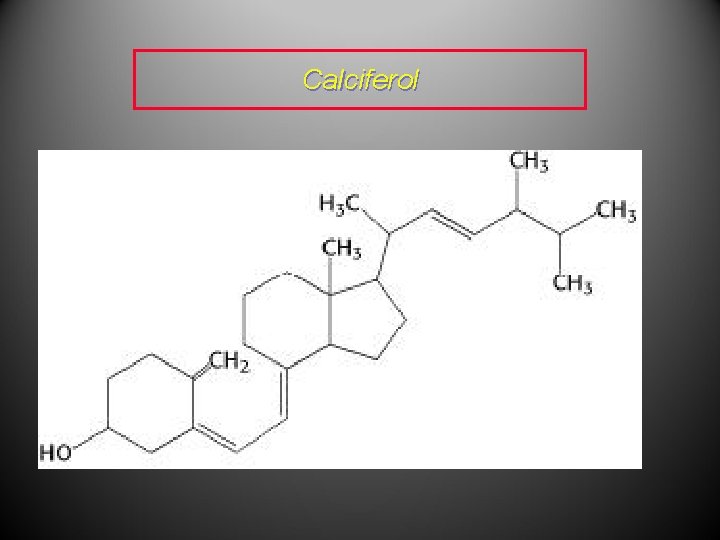
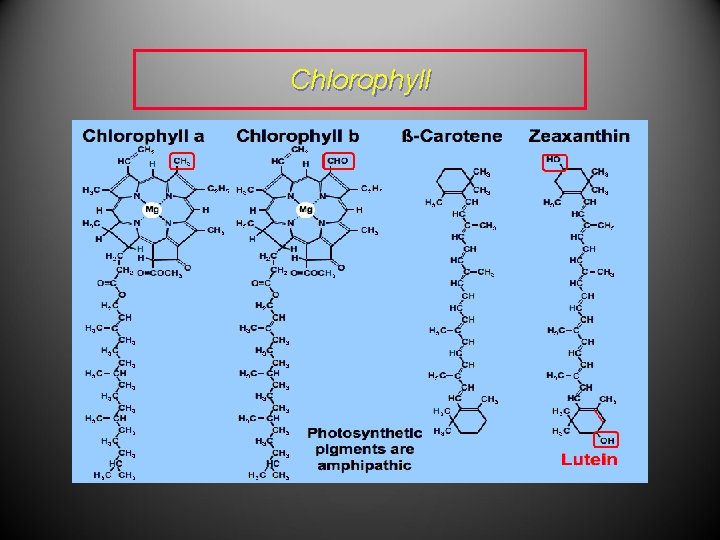
• Vitamin D 2, also known as calciferol, regulates calcium metabolism in the body and is therefore vital for the building and maintenance of bone. • Chlorophyll-a is a green pigment found, for example, in plant leaves and is a key factor of photosynthesis through which atmospheric carbon dioxide is converted to glucose.


Calciferol

Tocopherol

Chlorophyll

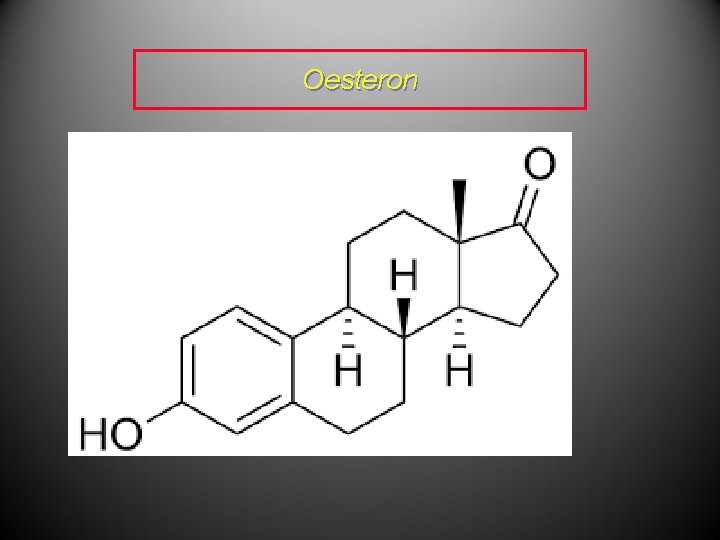
Communication • Terpenoids are also used as chemical messengers. • If the communication is between different parts of the same organism, the messenger is referred to as a hormone. • Giberellic acid is a hormone used by plants to control their rate of growth. • Testosterone and oestrone are mammalian sex hormones.



Oesteron

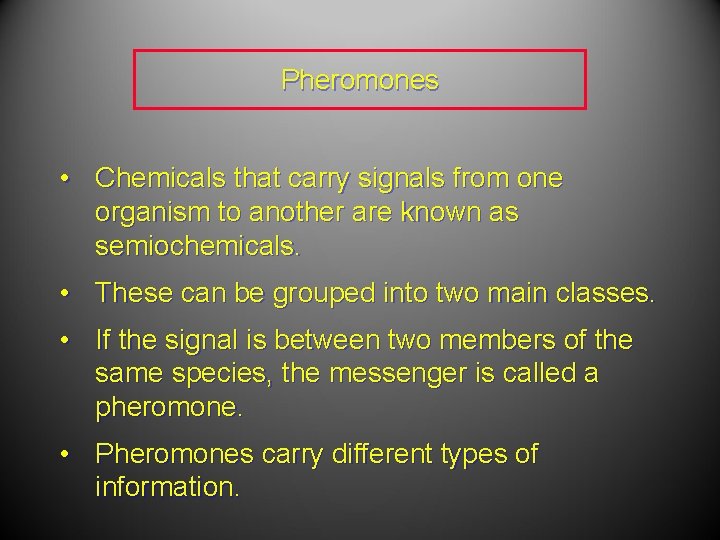
Pheromones • Chemicals that carry signals from one organism to another are known as semiochemicals. • These can be grouped into two main classes. • If the signal is between two members of the same species, the messenger is called a pheromone. • Pheromones carry different types of information.

• Not all species use pheromones. • In those which do, some may use only one or two pheromones while others, in particular the social insects such as bees, ants and termites, use an array (arrangement)of chemical signals to organise most aspects of their lives.

• Ants and termites use trail pheromones to mark a path between the nest and a food source. • This explains why ants are often seen walking in single file over long distances. • One such trail pheromone is Neocembrene-A which is produced and used by termites of the Australian species Nasutitermes exitiosus. .

• The social insects also use alarm, aggregation, dispersal and social pheromones to warn of danger and to control group behaviour.

Allelochemicals • Chemicals which carry messages between members of different species are known as Allelochemicals. Within this group, • Allomones benefit the sender of the signal, • Kairomones its receiver and with • Synomones both the sender and receiver benefit. Examples are shown in

Examples • Camphor and d-limonene are Allomones in that the trees which produce them are protected from insect attack by their presence

• Similarly, antifeedants could be considered to be allomones since the signal generator, the plant, receives the benefit of not being eaten. • Myrcene is a kairomone, in that it is produced by the ponderosa pine and its presence attracts the females of the bark beetle, Dendroctonous brevicomis.

• Geraniol is found in the scent of many flowers such as the rose. • Its presence attracts insects to the flower and it can be classified as a Synomones since the attracted insect finds nectar and the plant obtains a pollinator.

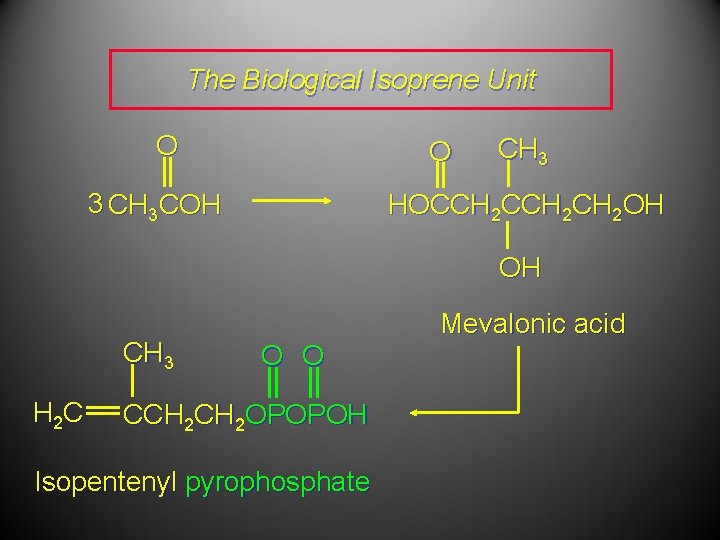
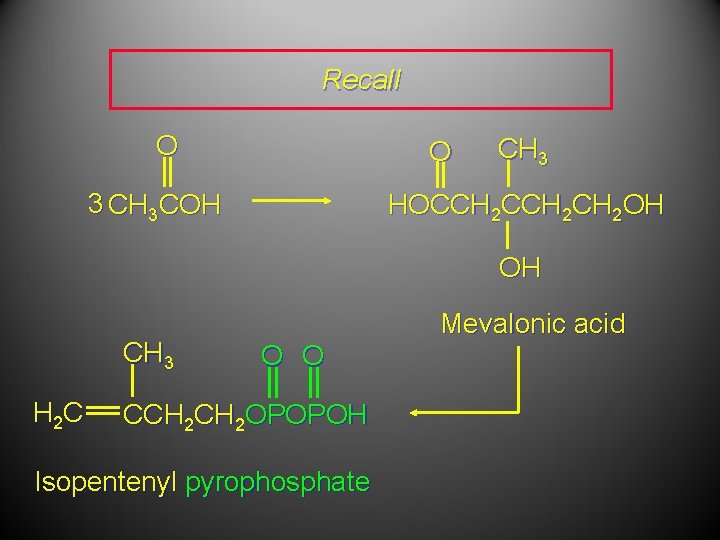
The Biological Isoprene Unit The isoprene units in terpenes do not come from isoprene. They come from isopentenyl pyrophosphate. Isopentenyl pyrophosphate (5 carbons) comes from acetate (2 carbons) via mevalonate (6 carbons).

The Biological Isoprene Unit O O 3 CH 3 COH CH 3 HOCCH 2 CH 2 OH OH CH 3 H 2 C Mevalonic acid O O CCH 2 OPOPOH Isopentenyl pyrophosphate

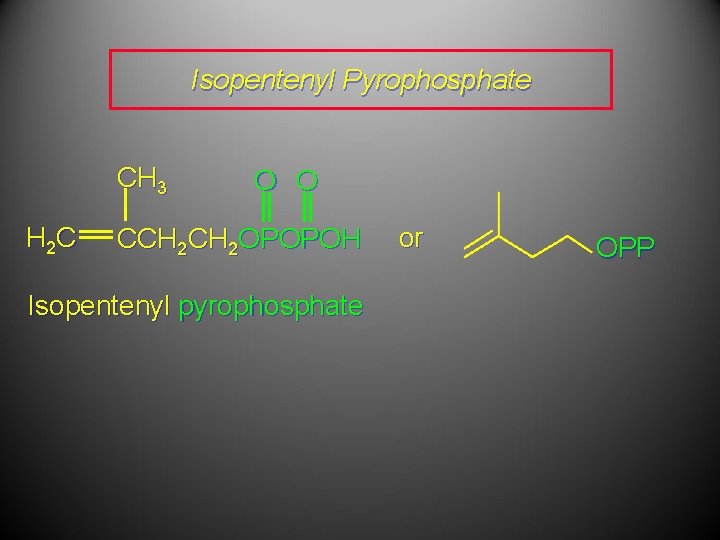
Isopentenyl Pyrophosphate CH 3 H 2 C O O CCH 2 OPOPOH Isopentenyl pyrophosphate or OPP

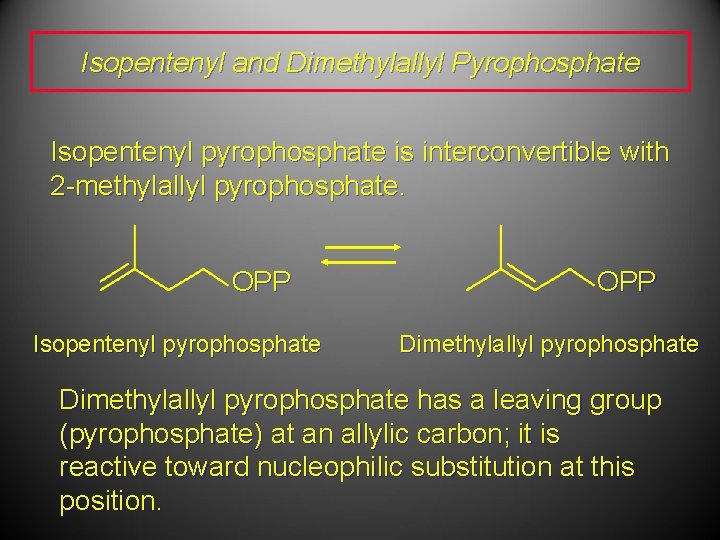
Isopentenyl and Dimethylallyl Pyrophosphate Isopentenyl pyrophosphate is interconvertible with 2 -methylallyl pyrophosphate. OPP Isopentenyl pyrophosphate OPP Dimethylallyl pyrophosphate has a leaving group (pyrophosphate) at an allylic carbon; it is reactive toward nucleophilic substitution at this position.

26. 9 Carbon-Carbon Bond Formation in Terpene Biosynthesis

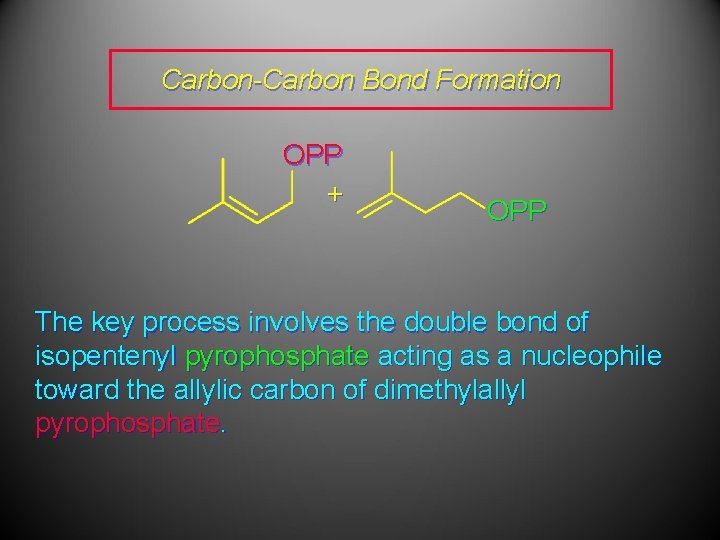
Carbon-Carbon Bond Formation OPP + OPP The key process involves the double bond of isopentenyl pyrophosphate acting as a nucleophile toward the allylic carbon of dimethylallyl pyrophosphate.

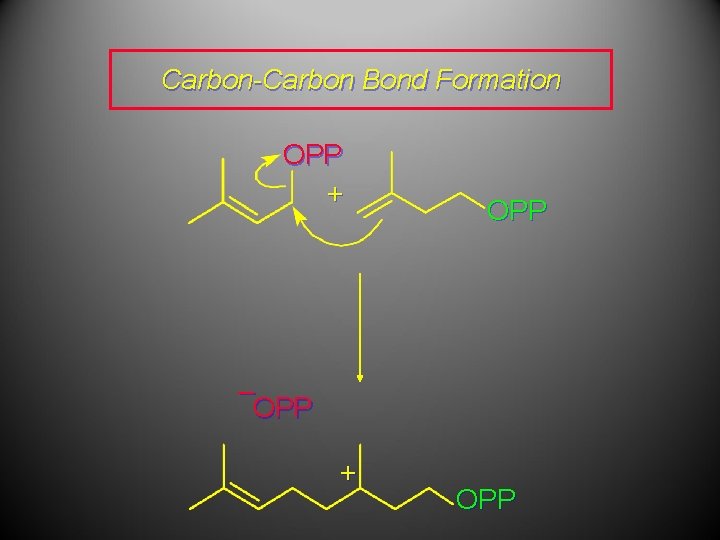
Carbon-Carbon Bond Formation OPP + OPP – OPP + OPP

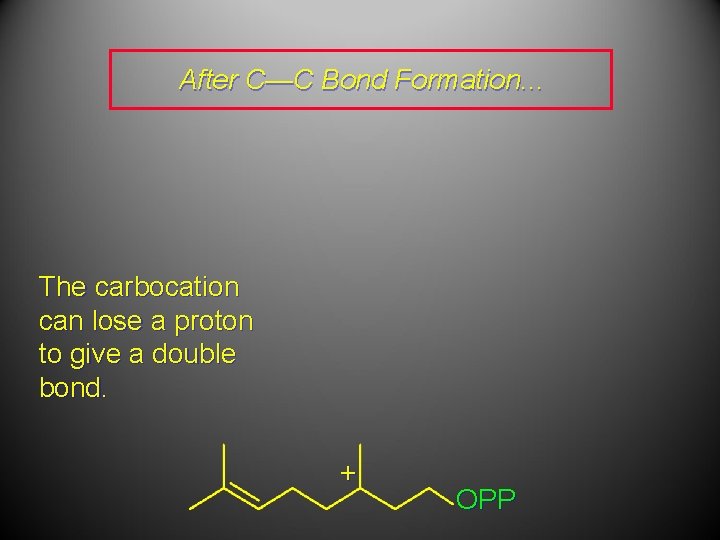
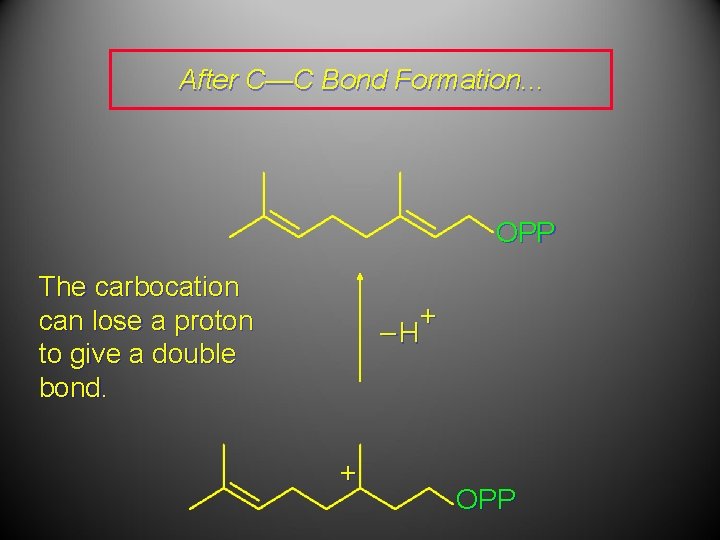
After C—C Bond Formation. . . The carbocation can lose a proton to give a double bond. + OPP

After C—C Bond Formation. . . OPP The carbocation can lose a proton to give a double bond. + –H + OPP

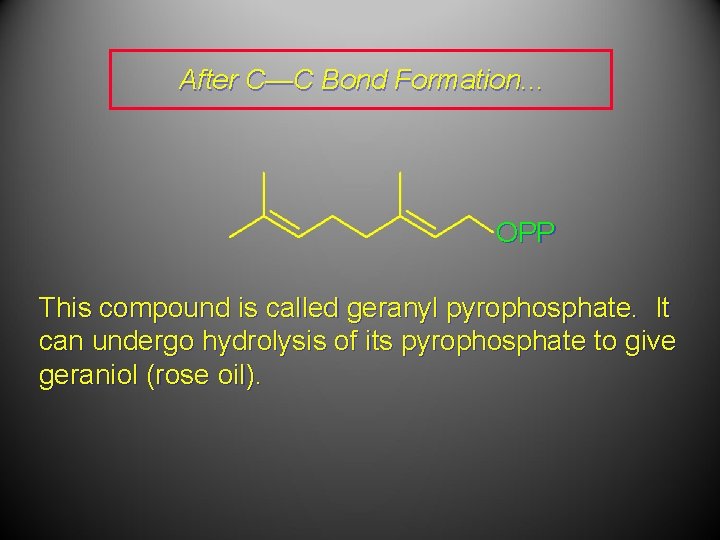
After C—C Bond Formation. . . OPP This compound is called geranyl pyrophosphate. It can undergo hydrolysis of its pyrophosphate to give geraniol (rose oil).

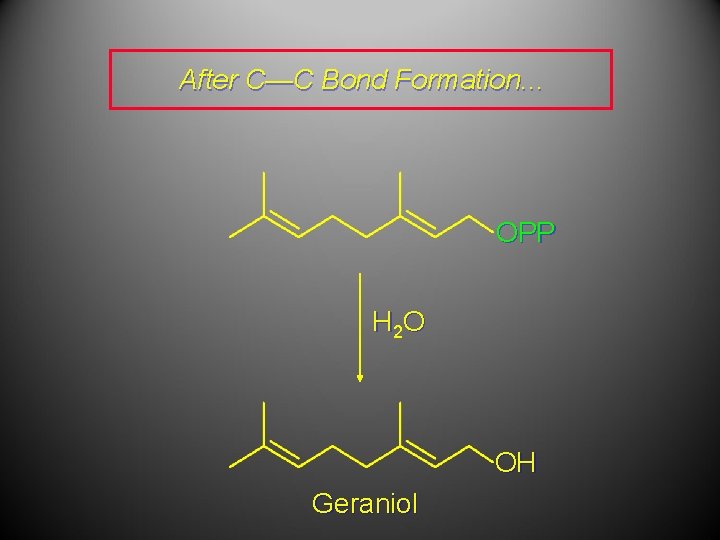
After C—C Bond Formation. . . OPP H 2 O OH Geraniol

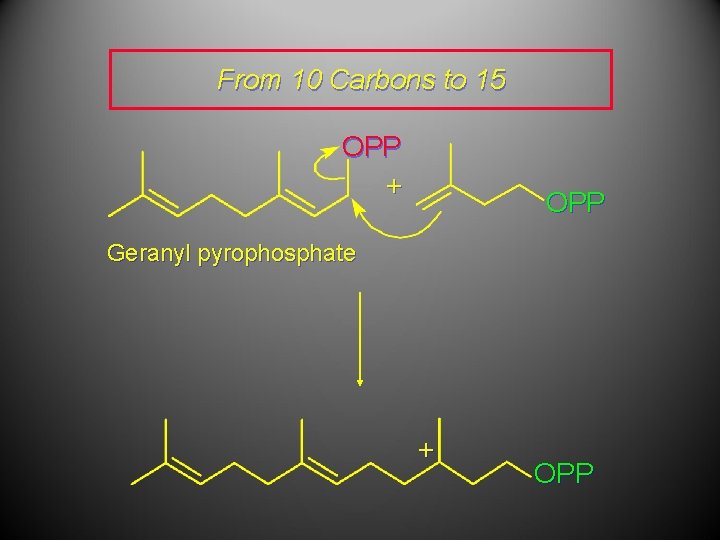
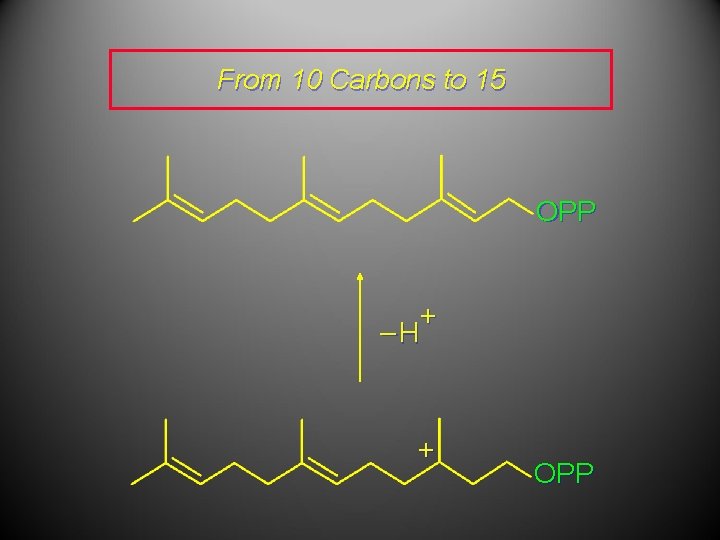
From 10 Carbons to 15 OPP + OPP Geranyl pyrophosphate + OPP

From 10 Carbons to 15 OPP + –H + OPP

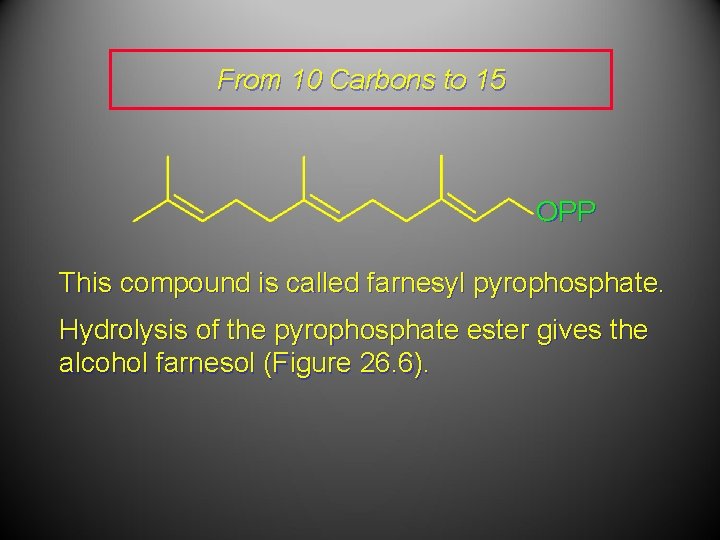
From 10 Carbons to 15 OPP This compound is called farnesyl pyrophosphate. Hydrolysis of the pyrophosphate ester gives the alcohol farnesol (Figure 26. 6).

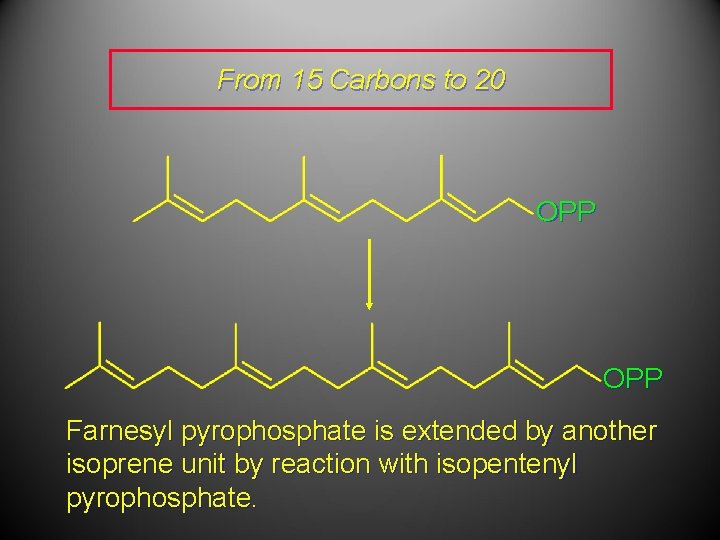
From 15 Carbons to 20 OPP Farnesyl pyrophosphate is extended by another isoprene unit by reaction with isopentenyl pyrophosphate.

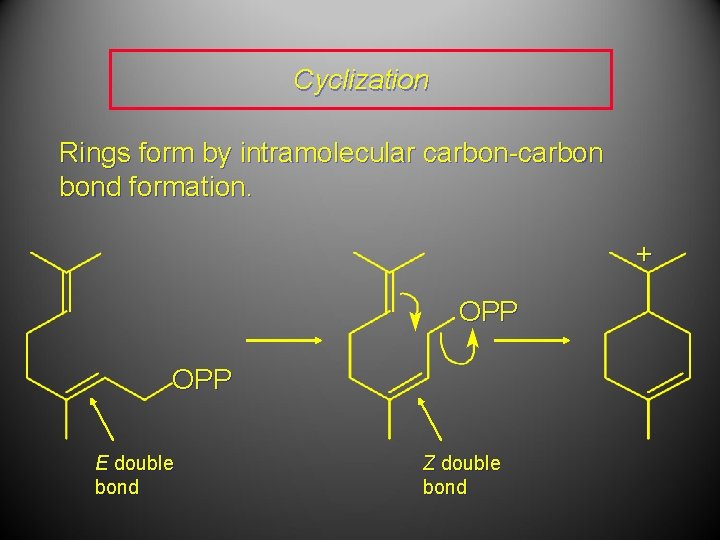
Cyclization Rings form by intramolecular carbon-carbon bond formation. + OPP E double bond Z double bond

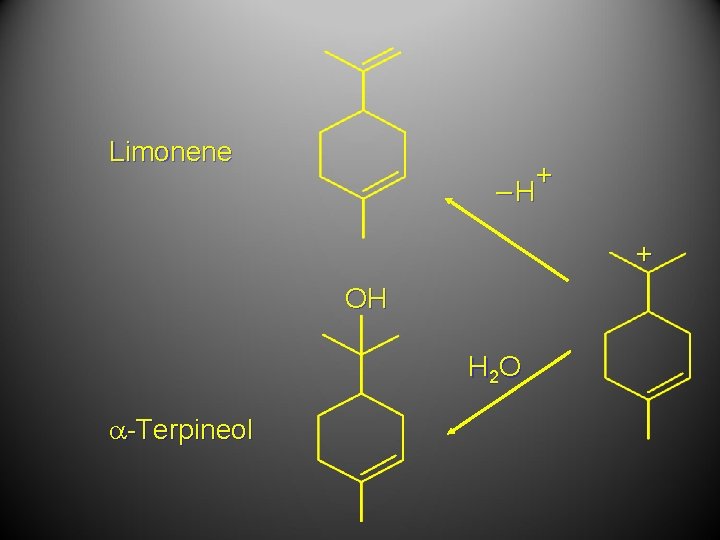
Limonene + –H + OH H 2 O a-Terpineol

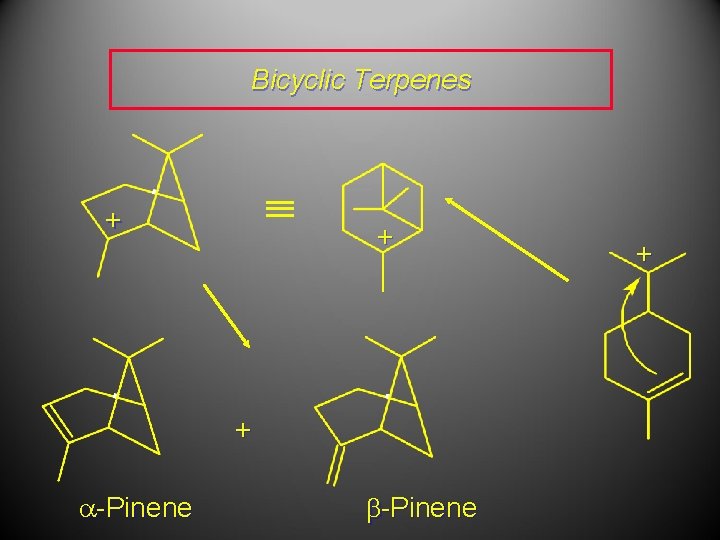
Bicyclic Terpenes + + + a-Pinene +

26. 10 The Pathway from Acetate to Isopentenyl Pyrophosphate

Recall O O 3 CH 3 COH CH 3 HOCCH 2 CH 2 OH OH CH 3 H 2 C Mevalonic acid O O CCH 2 OPOPOH Isopentenyl pyrophosphate

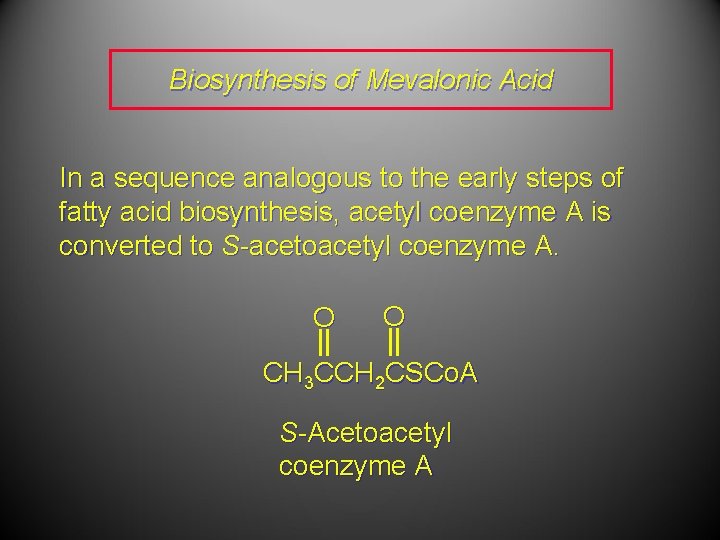
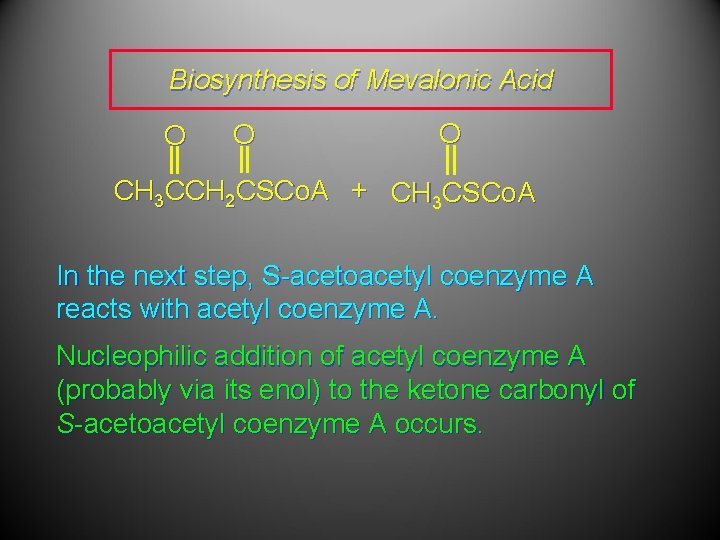
Biosynthesis of Mevalonic Acid In a sequence analogous to the early steps of fatty acid biosynthesis, acetyl coenzyme A is converted to S-acetoacetyl coenzyme A. O O CH 3 CCH 2 CSCo. A S-Acetoacetyl coenzyme A

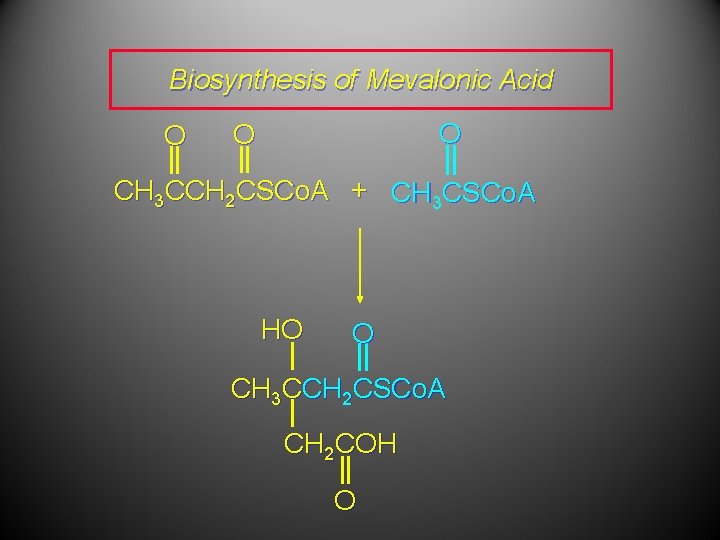
Biosynthesis of Mevalonic Acid O O O CH 3 CCH 2 CSCo. A + CH 3 CSCo. A In the next step, S-acetoacetyl coenzyme A reacts with acetyl coenzyme A. Nucleophilic addition of acetyl coenzyme A (probably via its enol) to the ketone carbonyl of S-acetoacetyl coenzyme A occurs.

Biosynthesis of Mevalonic Acid O O O CH 3 CCH 2 CSCo. A + CH 3 CSCo. A HO O CH 3 CCH 2 CSCo. A CH 2 COH O

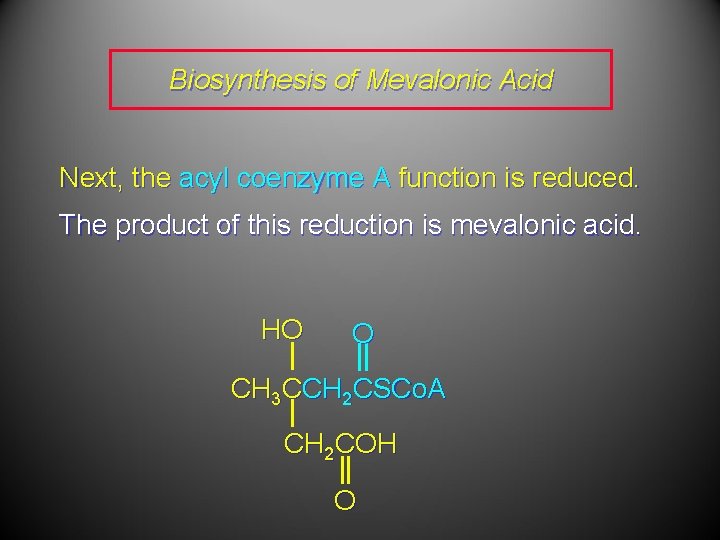
Biosynthesis of Mevalonic Acid Next, the acyl coenzyme A function is reduced. The product of this reduction is mevalonic acid. HO O CH 3 CCH 2 CSCo. A CH 2 COH O

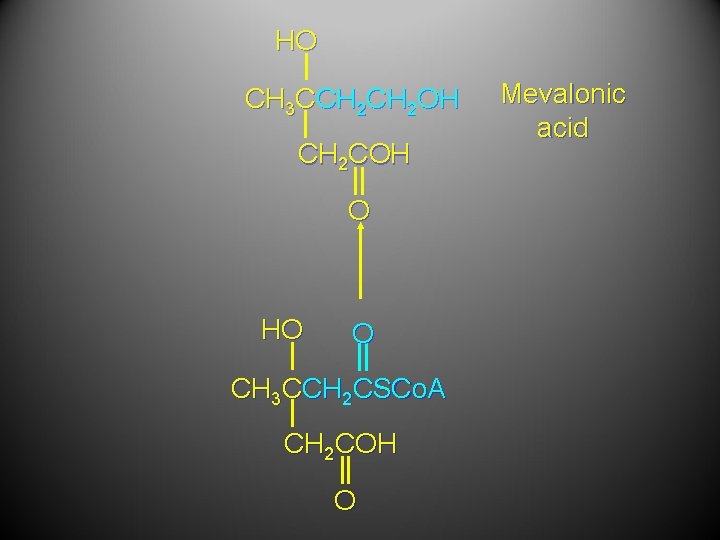
HO CH 3 CCH 2 OH CH 2 COH O HO O CH 3 CCH 2 CSCo. A CH 2 COH O Mevalonic acid

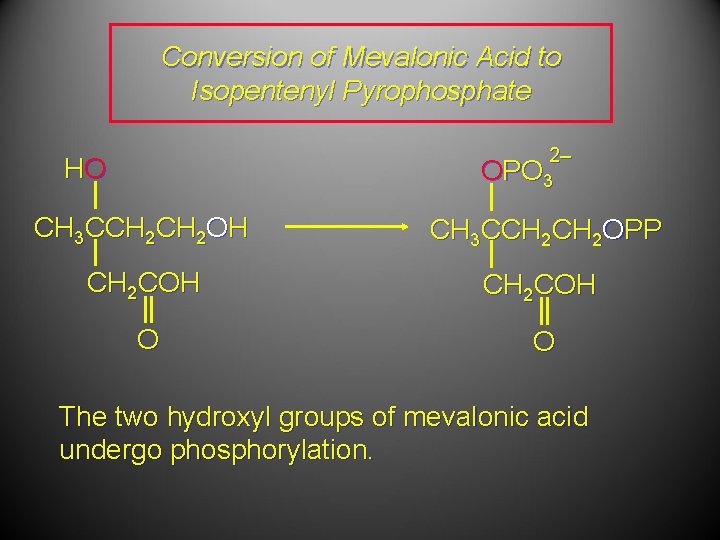
Conversion of Mevalonic Acid to Isopentenyl Pyrophosphate 2– HO OPO 3 CH 3 CCH 2 OH CH 3 CCH 2 OPP CH 2 COH O O The two hydroxyl groups of mevalonic acid undergo phosphorylation.

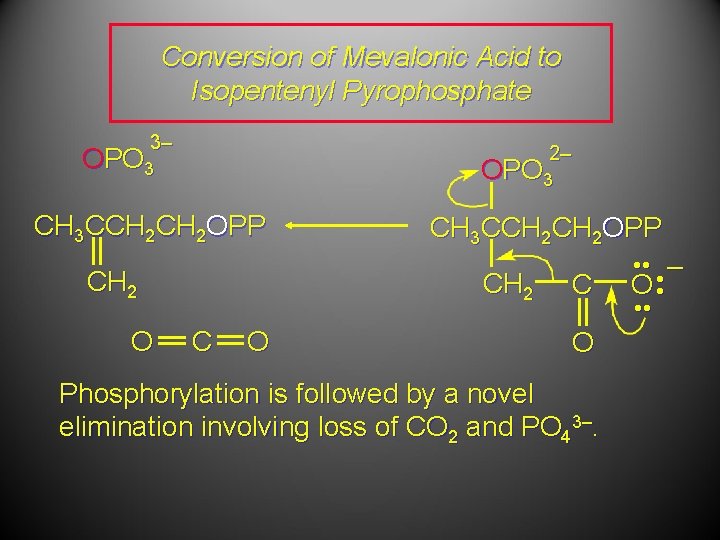
Conversion of Mevalonic Acid to Isopentenyl Pyrophosphate 3– 2– OPO 3 CH 3 CCH 2 OPP CH 2 O CH 3 CCH 2 OPP CH 2 C O Phosphorylation is followed by a novel elimination involving loss of CO 2 and PO 43–. • • – O • •

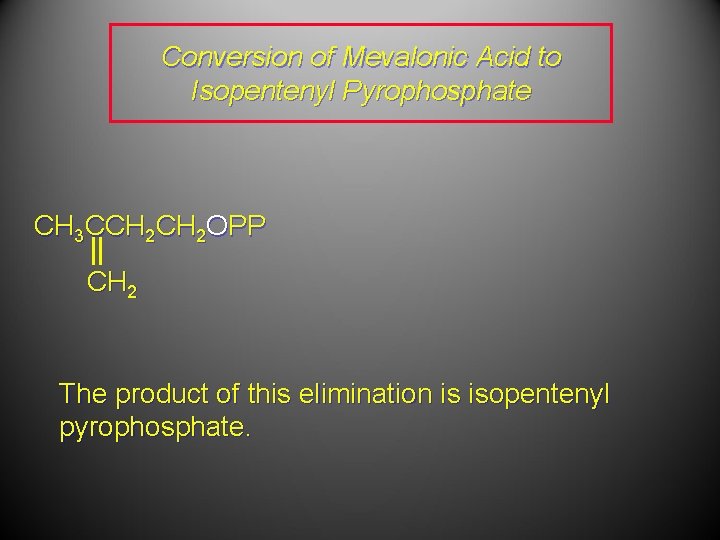
Conversion of Mevalonic Acid to Isopentenyl Pyrophosphate CH 3 CCH 2 OPP CH 2 The product of this elimination is isopentenyl pyrophosphate.

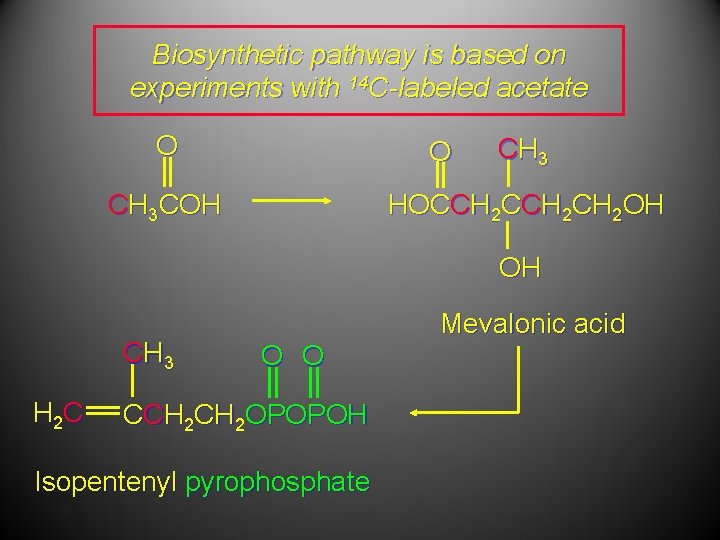
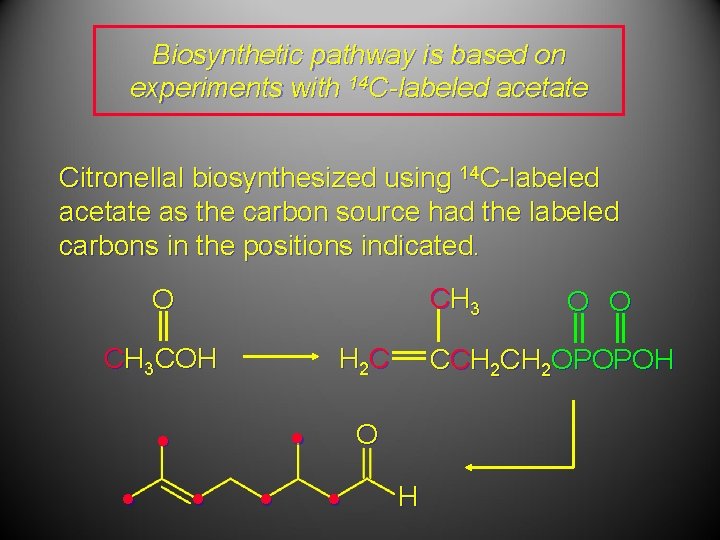
Biosynthetic pathway is based on experiments with 14 C-labeled acetate O O CH 3 COH CH 3 HOCCH 2 CH 2 OH OH CH 3 H 2 C Mevalonic acid O O CCH 2 OPOPOH Isopentenyl pyrophosphate

Biosynthetic pathway is based on experiments with 14 C-labeled acetate Citronellal biosynthesized using 14 C-labeled acetate as the carbon source had the labeled carbons in the positions indicated. CH 3 O CH 3 COH H 2 C • • • CCH 2 OPOPOH O • O O H
 97 000 000 in scientific notation
97 000 000 in scientific notation 602 200 000 000 000 000 000 000 in scientific notation
602 200 000 000 000 000 000 000 in scientific notation 7 500 000 000 000 000 000 in scientific notation
7 500 000 000 000 000 000 in scientific notation 090-0000-0000
090-0000-0000 What is terpenoids
What is terpenoids Isoprene structure
Isoprene structure Enfleurage method ppt
Enfleurage method ppt Terpenes to steroids
Terpenes to steroids Terpenes steroids prostaglandins
Terpenes steroids prostaglandins Venn diagram ionic and covalent bonds
Venn diagram ionic and covalent bonds Express 4,980,000, 000 in scientific notation
Express 4,980,000, 000 in scientific notation How to do scientific notation
How to do scientific notation 2,340,000,000
2,340,000,000 20000/12/2
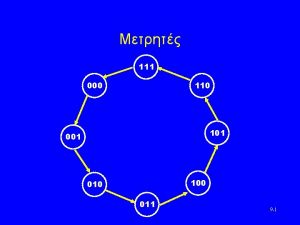
20000/12/2 110-000-110 & 111-000-111
110-000-110 & 111-000-111 1 100 1000 10000
1 100 1000 10000 What are some of the advantages of scientific notation?
What are some of the advantages of scientific notation? 4 500 000
4 500 000 1-000-000-0000
1-000-000-0000 Milli micro nano
Milli micro nano 450 000 000 in scientific notation
450 000 000 in scientific notation 123 000 000 in scientific notation
123 000 000 in scientific notation 1 600 000
1 600 000 1,000 x 3,000
1,000 x 3,000 Cancion lemuriana
Cancion lemuriana 4 500 000 000
4 500 000 000 4 500 000 000
4 500 000 000 240 000
240 000 210 000 in scientific notation
210 000 in scientific notation 1 200 000 000
1 200 000 000 Frans cooijmans
Frans cooijmans 4 000 000
4 000 000 How was today's class
How was today's class Package mypackage class first class body
Package mypackage class first class body Introduction to ooad
Introduction to ooad Lower boundary of the modal class
Lower boundary of the modal class Class i vs class ii mhc
Class i vs class ii mhc Difference between abstract class and concrete class
Difference between abstract class and concrete class How to get the class mark
How to get the class mark Stimulus
Stimulus Discriminative stimulus
Discriminative stimulus 7 rights of medication administration in order
7 rights of medication administration in order Class maths student student1 class student string name
Class maths student student1 class student string name What is the class width for the given class (28-33)
What is the class width for the given class (28-33) In greenfoot, you can cast an actor class to a world class?
In greenfoot, you can cast an actor class to a world class? Static vs dynamic class loading in java
Static vs dynamic class loading in java Esd class levels
Esd class levels Analysis class diagram example
Analysis class diagram example Class 2 class 3
Class 2 class 3 Public class test subject extends test class
Public class test subject extends test class Package mypackage class first class body
Package mypackage class first class body Class third class
Class third class Uml private public protected
Uml private public protected Component class has composite class as collaborator
Component class has composite class as collaborator Criss cross method steps
Criss cross method steps Ternary ionic compounds
Ternary ionic compounds Is maple syrup a homogeneous mixture
Is maple syrup a homogeneous mixture Physical state of covalent compounds
Physical state of covalent compounds Giant molecular structure vs simple molecular structure
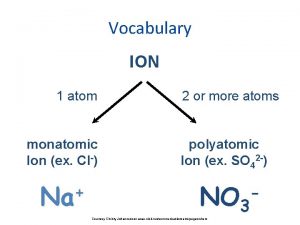
Giant molecular structure vs simple molecular structure Monatomic ion
Monatomic ion Vitamins are tasteless organic compounds
Vitamins are tasteless organic compounds Vitamin classification chart
Vitamin classification chart M(aa)3 isomers
M(aa)3 isomers Naming metallic compounds
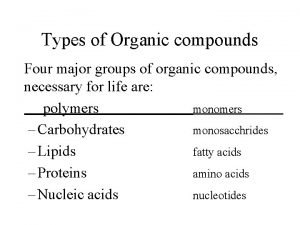
Naming metallic compounds The four types of organic compounds
The four types of organic compounds Ionic compound brittle
Ionic compound brittle Are compounds pure substances
Are compounds pure substances Solubility formula class 9
Solubility formula class 9 Decomposition of organic matter equation
Decomposition of organic matter equation Ammonia bond angle and shape
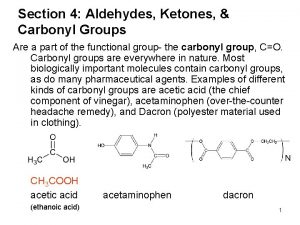
Ammonia bond angle and shape Carbonyl group
Carbonyl group Adjectivizer
Adjectivizer Polyatomic compounds
Polyatomic compounds Is sugar a pure substance
Is sugar a pure substance Mixture
Mixture Which are pure substances
Which are pure substances Mixture matter graphic organizer
Mixture matter graphic organizer Organic and inorganic compounds experiment
Organic and inorganic compounds experiment Intensive properties examples
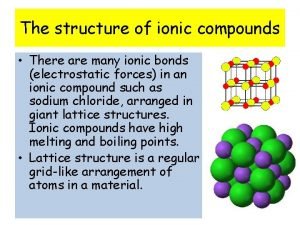
Intensive properties examples Ionic compound
Ionic compound Preparation of alicyclic compounds
Preparation of alicyclic compounds Polyatomic ion
Polyatomic ion Plant secondary compounds
Plant secondary compounds Homologous series definition
Homologous series definition Combustion reaction
Combustion reaction These are organic compounds made by living things
These are organic compounds made by living things All organic compounds must contain the element
All organic compounds must contain the element Organic compounds must contain:
Organic compounds must contain: Oxo functional group
Oxo functional group Organic vs inorganic compounds
Organic vs inorganic compounds Coordination compound nomenclature
Coordination compound nomenclature P2o5 compound name
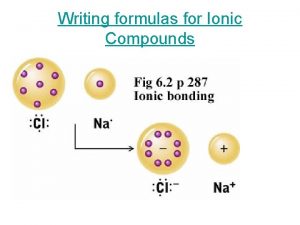
P2o5 compound name How to name ionic compounds
How to name ionic compounds Covalent bond naming
Covalent bond naming Covalent compound formula phosphorus pentafluoride
Covalent compound formula phosphorus pentafluoride