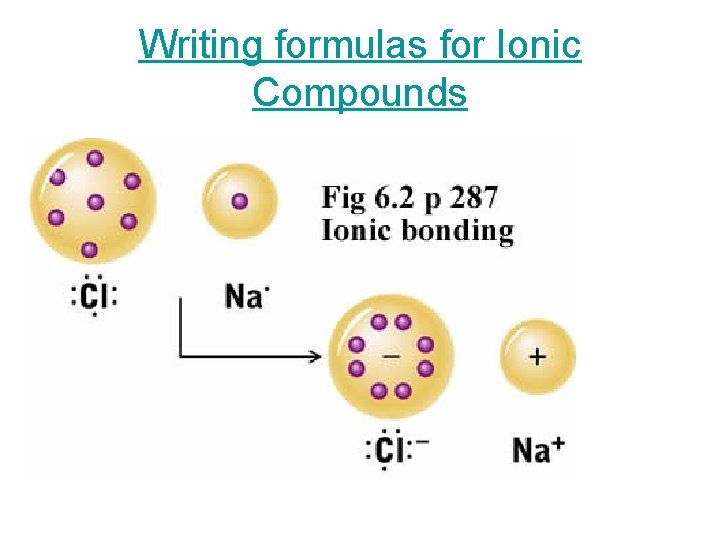
Writing formulas for Ionic Compounds Review Ionic compounds








- Slides: 15

Writing formulas for Ionic Compounds

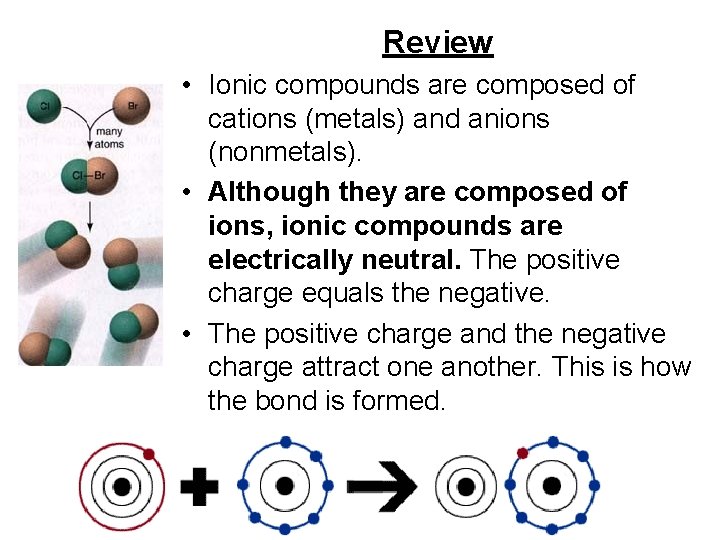
Review • Ionic compounds are composed of cations (metals) and anions (nonmetals). • Although they are composed of ions, ionic compounds are electrically neutral. The positive charge equals the negative. • The positive charge and the negative charge attract one another. This is how the bond is formed.

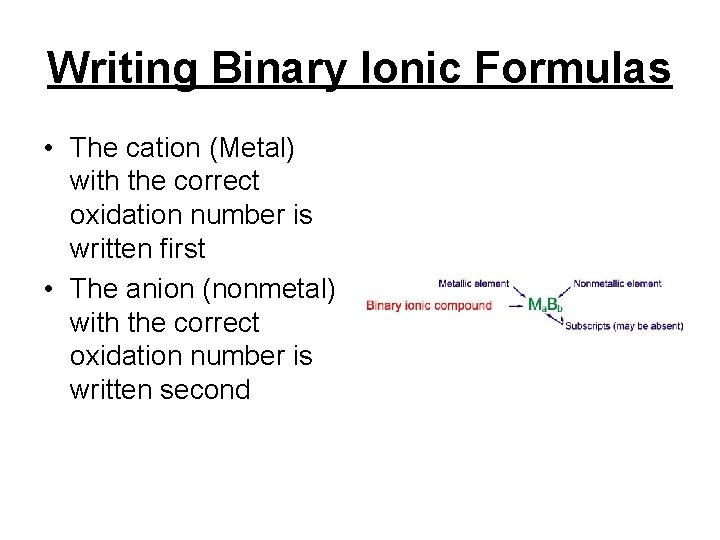
Writing Binary Ionic Formulas • The cation (Metal) with the correct oxidation number is written first • The anion (nonmetal) with the correct oxidation number is written second

Binary Ionic Formulas • Criss– cross the charges from superscripts to subscripts using only their absolute value

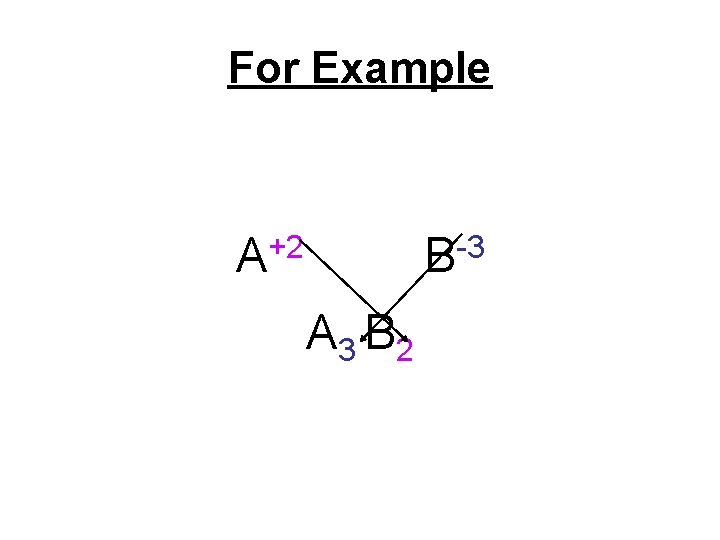
For Example +2 A -3 B A 3 B 2

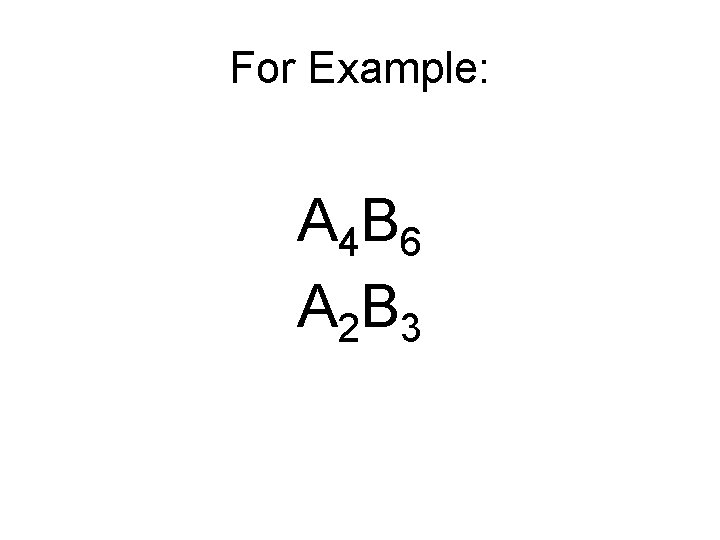
• Check to make sure the subscripts are in the lowest possible ratio to one another

For Example: A 4 B 6 A 2 B 3

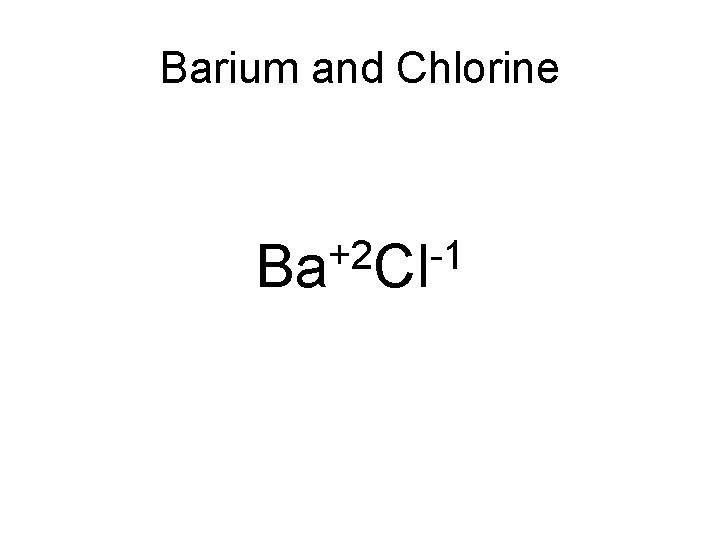
Example Write the correct ionic formula for Barium and Chlorine. Step 1: Write the cation (with correct oxidation number) first And the anion (with the correct oxidation number second)

Barium and Chlorine +2 -1 Ba Cl

Step 2: Criss-cross the superscripts to subscripts using only the absolute value Ba+2 Cl-1 Ba. Cl 2 Why is there no subscript for Ba?

Step 3: Make sure all subscripts are in the lowest possible ratio. Ba. Cl 2 If there are in the lowest possible ratio you are done!!

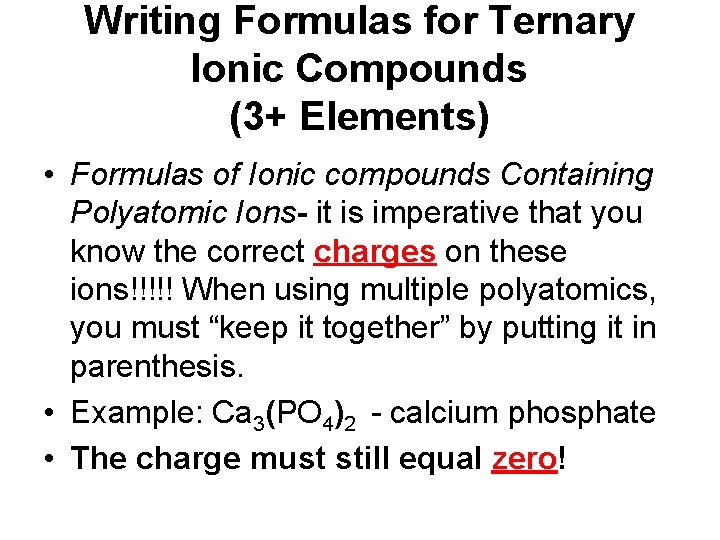
Writing Formulas for Ternary Ionic Compounds (3+ Elements) • Formulas of Ionic compounds Containing Polyatomic Ions- it is imperative that you know the correct charges on these ions!!!!! When using multiple polyatomics, you must “keep it together” by putting it in parenthesis. • Example: Ca 3(PO 4)2 - calcium phosphate • The charge must still equal zero!


Write the correct formula for the following binary ionic compounds: • • Potassium and Iodine Calcium and Fluorine Aluminum and Sulfur Iron(III) and Oxygen (watch out for the transition metal)

Writing Formulas for Ternary Ionic Compounds (3+ Elements) • Write the formula for: sodium phosphate: ammonium sulfide: