Writing Ionic Formulas Ionic Compounds o Things you



















- Slides: 29

Writing Ionic Formulas

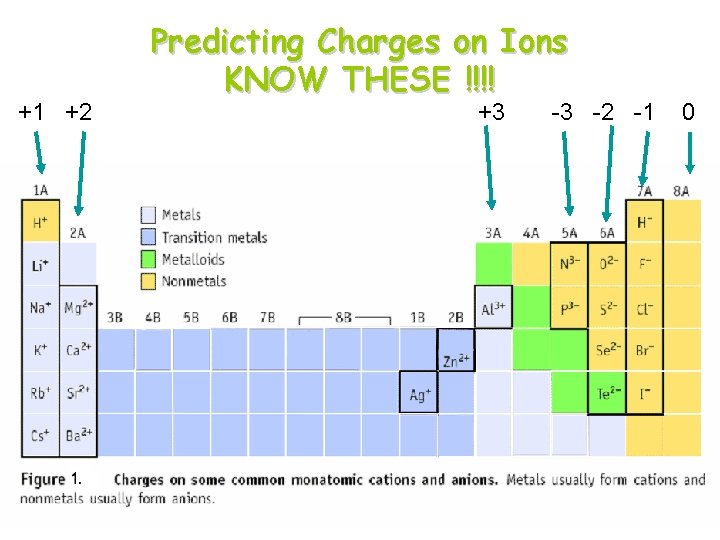
Ionic Compounds o Things you should know: n Ionic = metal-nonmetal combo of elements n Metal: loses e- to become stable; positive ions n Nonmetal: gains e- to become stable; negative ions n The charge value (1, 2, or 3) depends on how many e- were lost or gained. n The # lost or gained was the # needed to be “full”.

+1 +2 Predicting Charges on Ions KNOW THESE !!!! +3 -3 -2 -1 0

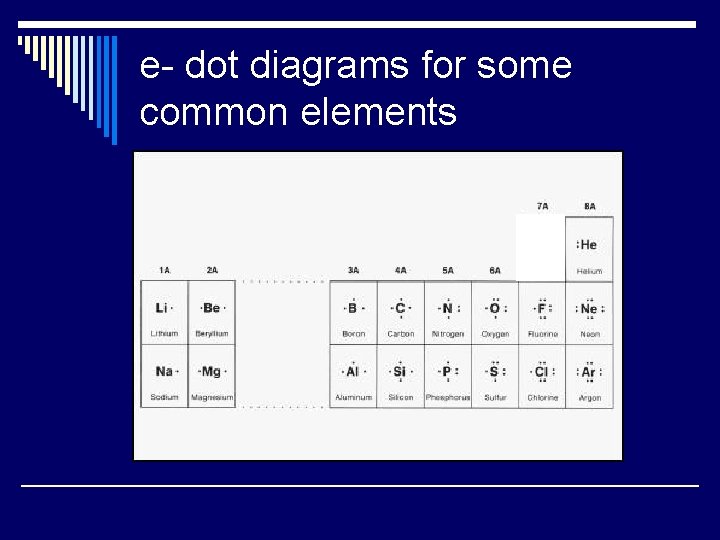
Method One: electron dot diagrams o. The # of dots shown = # of valence e-. o. Column # = # of valence e-. oe- are gained or lost in the # needed for each atom/ion to be stable.

e- dot diagrams for some common elements

Use e- dot diagrams to show the transfer of e- to make the metal and nonmetal atoms stable. e- dot diagram for Potassium (K) e- dot diagram for oxygen (O) Transfer of e- to form potassium oxide

Writing the Formula o If the ratio is 1: 1, no subscript is needed. n Ex: sodium chloride = Na. Cl o If the ratio is NOT 1: 1 use a subscript for each element which has more than 1 ion involved in the transfer. n Ex: potassium oxide = K 2 O

Your Turn! o What is the formula of barium iodide? n n n Show the e- dot diagram of each element. Show the transfer of e-. Write the formula based on the ratio of the ions.

Barium Iodide e- dot diagram for Barium (Ba) e- dot diagram for iodine (I) Transfer of e- to form barium iodide

Formula o. Ba. I 2

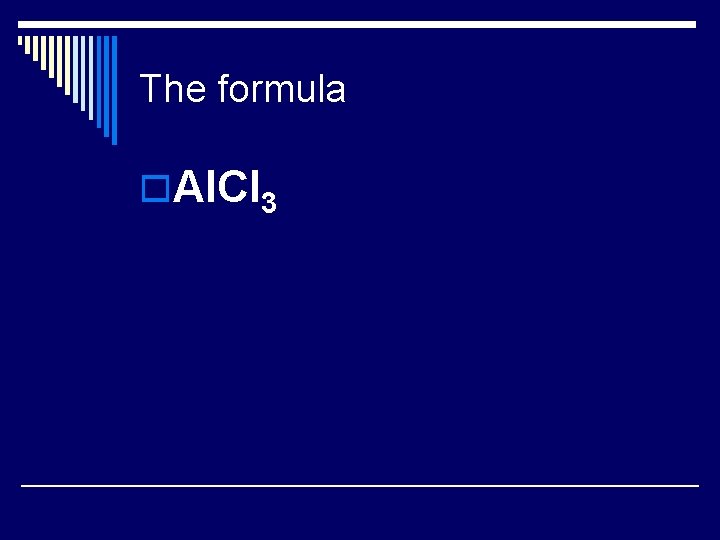
Method 2: using charges of the ions Ion charge for Aluminum (Al) Ion charge for chlorine (Cl) “Adding” the ions to get a neutral compound

The formula o. Al. CI 3

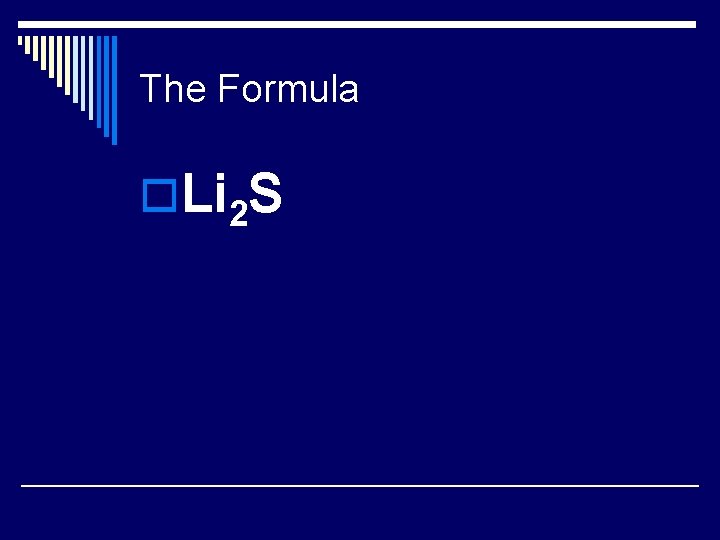
Your Turn! o. What is the formula of lithium sulfide? n n n Determine the ion charge of each element. Determine the # of each ion needed to form a neutral compound. Write the formula based on the ratio of the ions.

The Formula o. Li 2 S

Transition Metals o Transition metals = B column elements o Charge can vary. It may be: +1, +2, +3, or +4. o A number in parentheses following the name of the metal gives the ion charge. n Ex: Iron (II) = Fe+2 Iron (III) = Fe+3

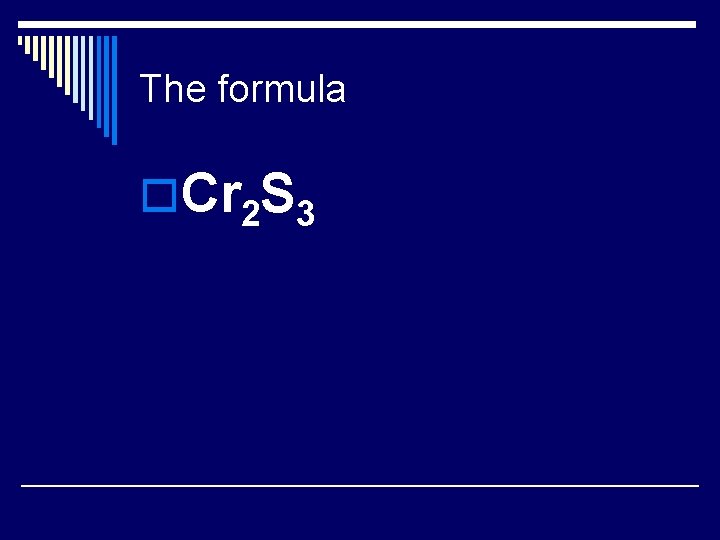
Teacher Example o What is the formula of chromium (III) sulfide? Ion charge for Chromium (Cr) Ion charge for sulfide “Adding” the ions to get a neutral compound

The formula o. Cr 2 S 3

Your Turn! o What is the formula for silver (I) chloride?

The formula o. Ag. CI

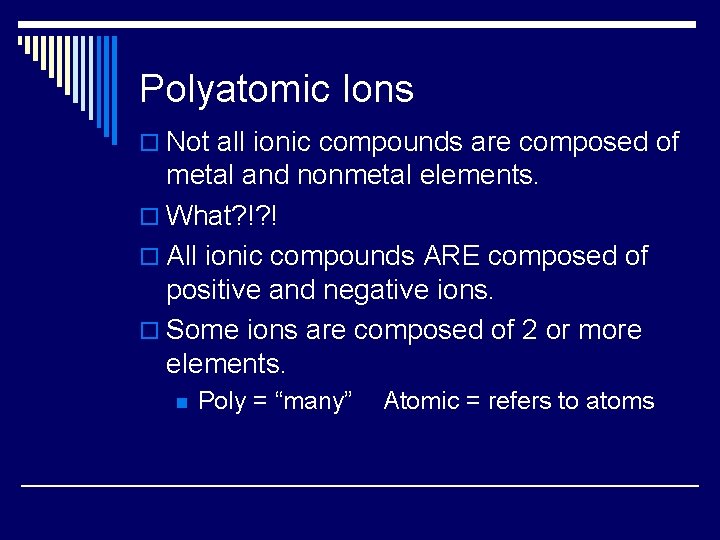
Polyatomic Ions o Not all ionic compounds are composed of metal and nonmetal elements. o What? !? ! o All ionic compounds ARE composed of positive and negative ions. o Some ions are composed of 2 or more elements. n Poly = “many” Atomic = refers to atoms

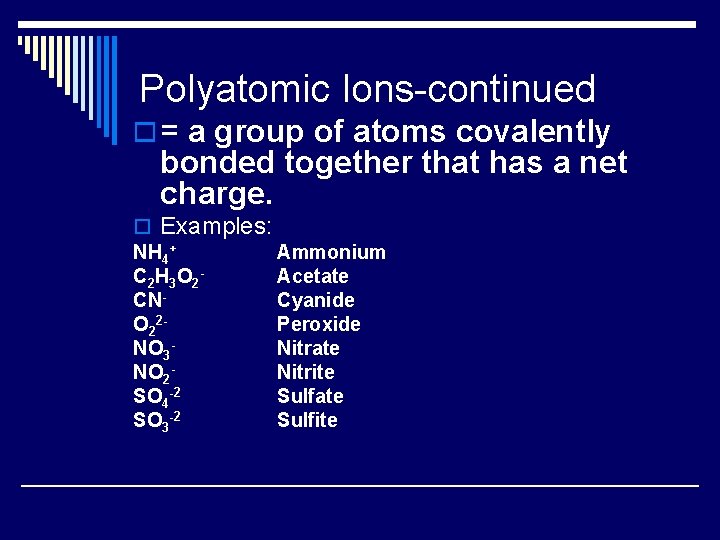
Polyatomic Ions-continued o = a group of atoms covalently bonded together that has a net charge. o Examples: NH 4+ Ammonium C 2 H 3 O 2 Acetate CNCyanide O 22 Peroxide NO 3 Nitrate NO 2 Nitrite SO 4 -2 Sulfate SO 3 -2 Sulfite

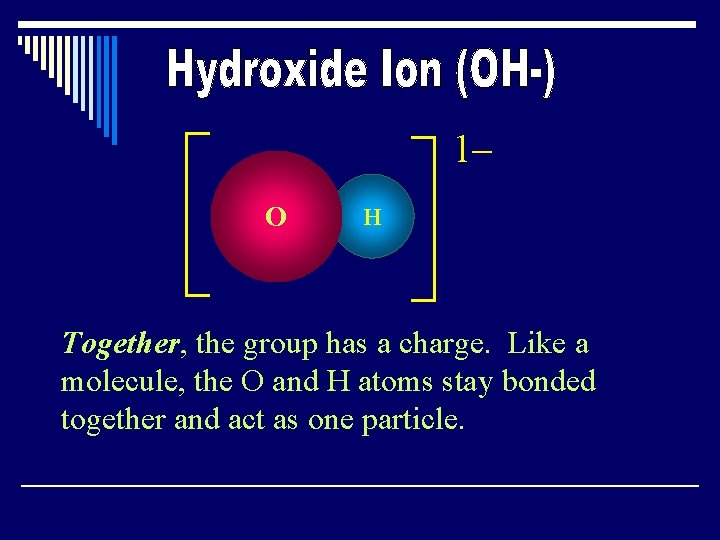
1– O H Together, the group has a charge. Like a molecule, the O and H atoms stay bonded together and act as one particle.

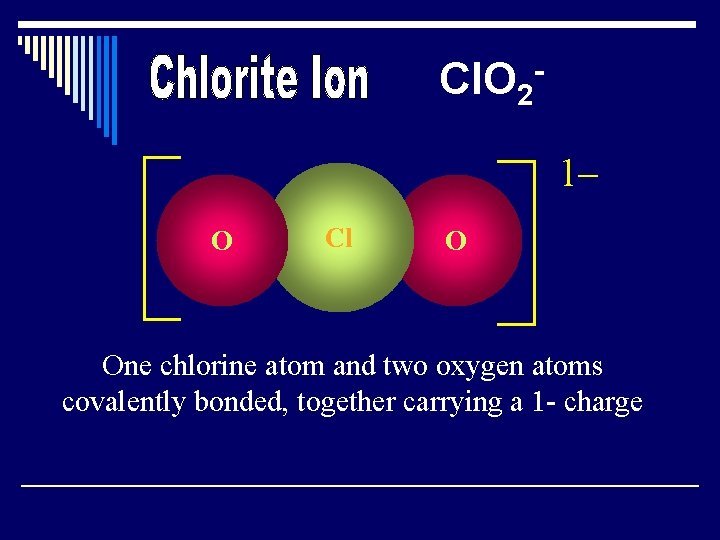
Cl. O 21– O Cl O One chlorine atom and two oxygen atoms covalently bonded, together carrying a 1 - charge

Writing a formula that contains a polyatomic ion o Use the charge method. “Adding” the charges of the ions, the compound must be neutral. o Compound: Magnesium hydroxide

Magnesium hydroxide Ion charge for Magnesium (Mg) Ion charge for hydroxide (OH-) “Adding” the ions to get a neutral compound

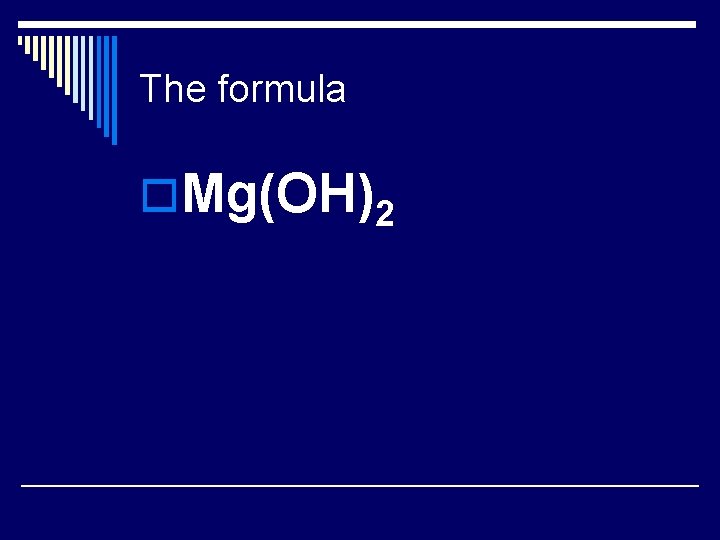
The formula o. Mg(OH)2

Your Turn! o. Write the formula for lithium phosphate.

The formula o. Li. PO 4

Let’s Put It All Together Transitional metal and Polyatomic Ion o What is the formula of chromium (III) sulfate? Ion charge for Chromium (Cr) Ion charge for sulfate “Adding” the ions to get a neutral compound