More Ionic Compounds Rules for Writing Ionic Formulas













- Slides: 14

More Ionic Compounds

Rules for Writing Ionic Formulas n n The cation comes first in the chemical formula for ionic compounds. The anion is written second in the chemical formula for ionic compounds. Write the symbol and the charge of the cation next to the symbol and charge of the anion. Drop the charges down and swap them with the other symbol.

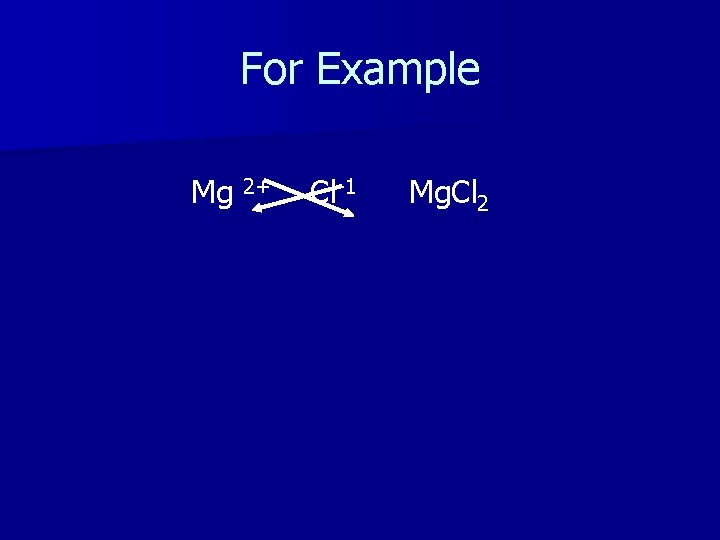
For Example Mg 2+ Cl-1 Mg. Cl 2

Let’s Practice Together: 1. Ca 2+ 2. Al 3+ Cl-1 NO 3 -1 Ca. Cl 2 Al(NO 3)3

Let’s Practice Together n 3. Fe 3+ Cl – = Fe. Cl 3 n 4. Na + OH – = Na. OH n 5. Na + S 2 - = Na 2 S

Rules for Naming Ionic Compounds Name the cation first. It is the same as the element name of the metal. n Name the anion second, but change the ending of the anion to –ide. For polyatomic ions, you write the name of the polyatomic ion. n

Practice – – – KBr = Potassium Bromide Mg. Cl 2 = Magnesium Chloride Li 3 N = Lithium Nitride Al 2 O 3 = Aluminum Oxide Na. OH = Sodium Hydroxide Na 2 CO 3 = Sodium Carbonate

Names Including Transition Elements: First write the name of the cation element. n Include the roman numeral indicating the charge of the metal behind the name of the cation. n Then write the anion as instructed above. n

Examples: n n Pb(NO 3)2 = Lead (II) Nitrate Cu. Cl 2 = Copper (II) Chloride Fe(OH)2 = Iron (II) Hydroxide Fe. Cl 3 = Iron (III) Chloride

Names and Formulas 14. chlorite 15. perchlorate 16. hypochlorite 17. chloride 18. chlorate

Names and Formulas 19. Sodium Iodide 20. Calcium Chloride 21. Potassium Sulfide 22. Magnesium Oxide 23. Lithium Sulfate 24. Ammonium Bromide 25. Calcium Nitride

Names and Formulas 26. Cessium Phosphide 27. Potassium Bromate 28. Magnesium Hypochlorite 29. Lithium Oxide 30. Beryllium Phosphate 31. Ammonium Carbonate

Names and Formulas 32. Sodium Bromate 33. Iron (III) Oxide 34. Iron (II) Iodate

Names and Formulas 35. Be 3 N 2 36. KCl. O 3 37. Cu 2 O 38. Mg. SO 3 39. (NH 4)2 S 40. Ca(IO 3)2