26 7 Terpenes The Isoprene Rule Terpenes are






- Slides: 47

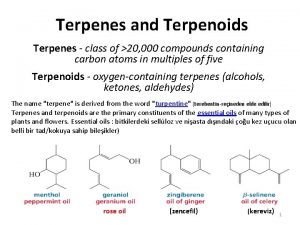
26. 7 Terpenes: The Isoprene Rule

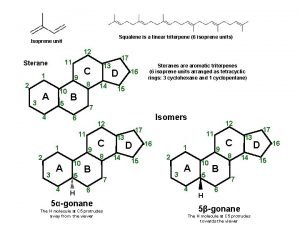
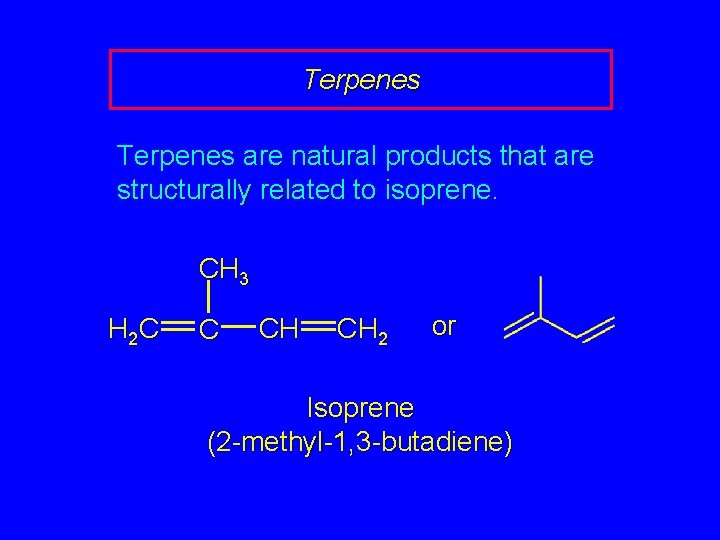
Terpenes are natural products that are structurally related to isoprene. CH 3 H 2 C C CH CH 2 or Isoprene (2 -methyl-1, 3 -butadiene)

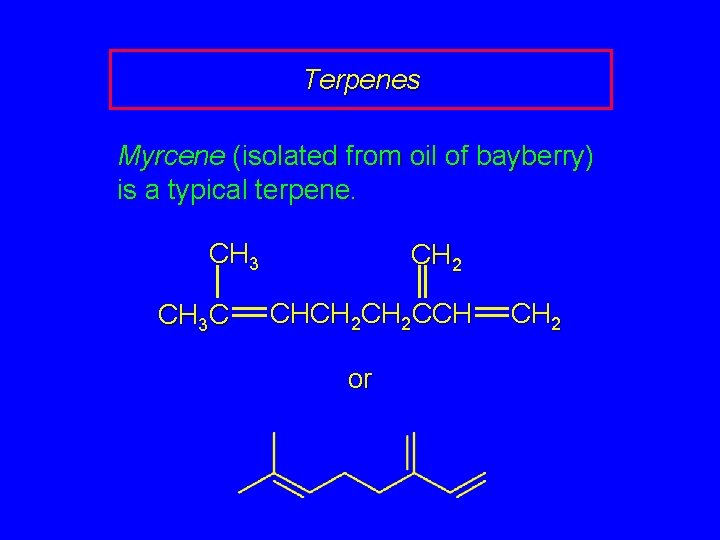
Terpenes Myrcene (isolated from oil of bayberry) is a typical terpene. CH 3 C CH 2 CHCH 2 CCH or CH 2

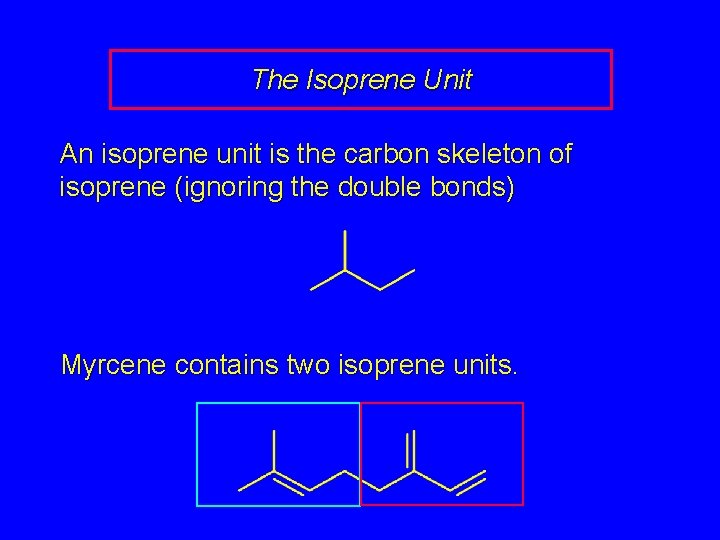
The Isoprene Unit An isoprene unit is the carbon skeleton of isoprene (ignoring the double bonds) Myrcene contains two isoprene units.

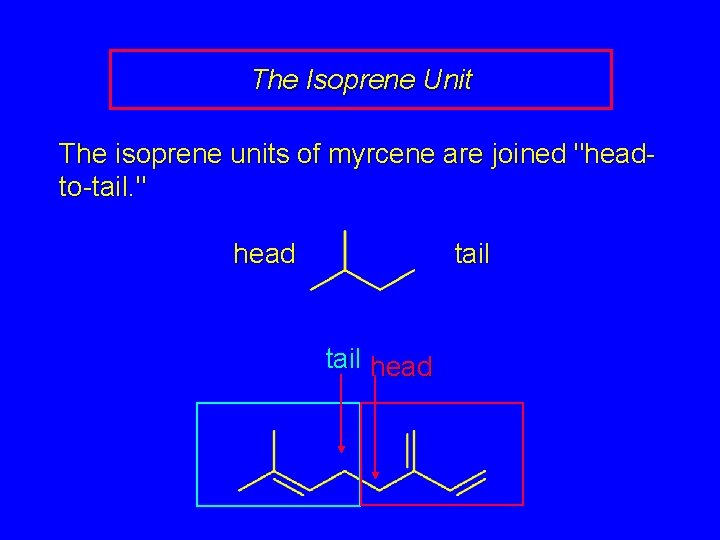
The Isoprene Unit The isoprene units of myrcene are joined "headto-tail. " head tail head

Table 26. 2 Classification of Terpenes Class Number of carbon atoms Monoterpene 10 Sesquiterpene 15 Diterpene 20 Sesterpene 25 Triterpene 30 Tetraterpene 40

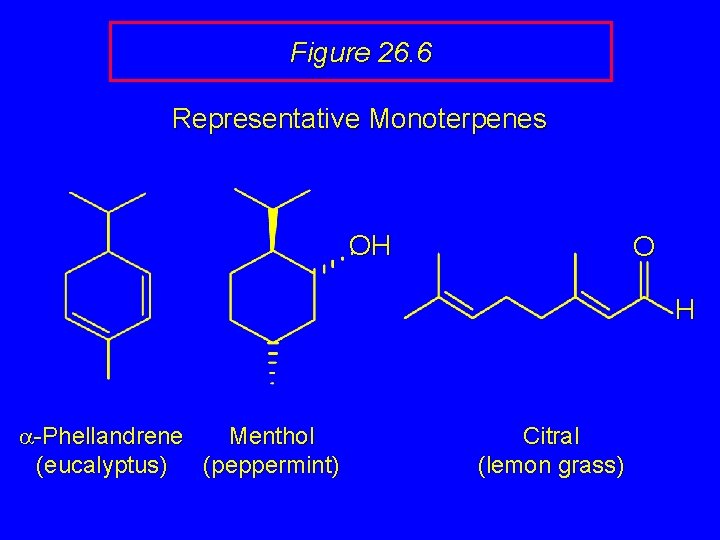
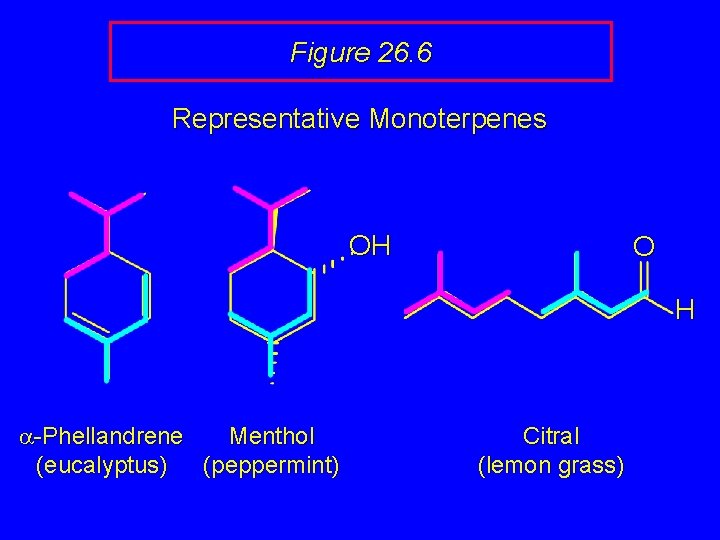
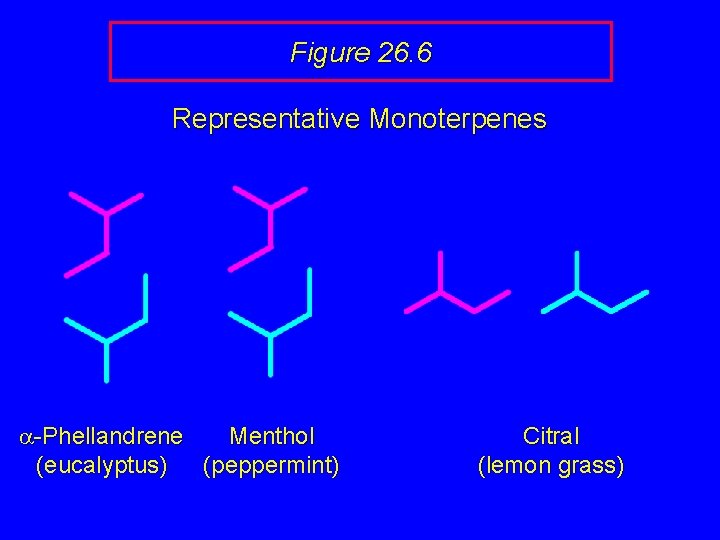
Figure 26. 6 Representative Monoterpenes OH O H a-Phellandrene Menthol (eucalyptus) (peppermint) Citral (lemon grass)

Figure 26. 6 Representative Monoterpenes OH O H a-Phellandrene Menthol (eucalyptus) (peppermint) Citral (lemon grass)

Figure 26. 6 Representative Monoterpenes a-Phellandrene Menthol (eucalyptus) (peppermint) Citral (lemon grass)

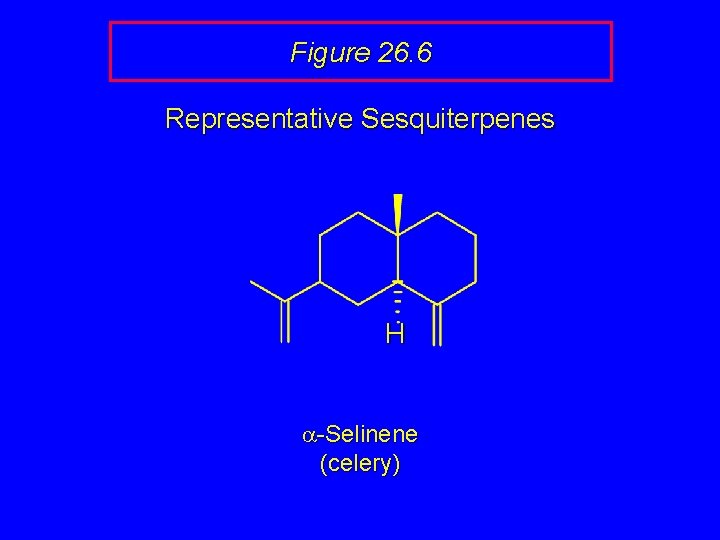
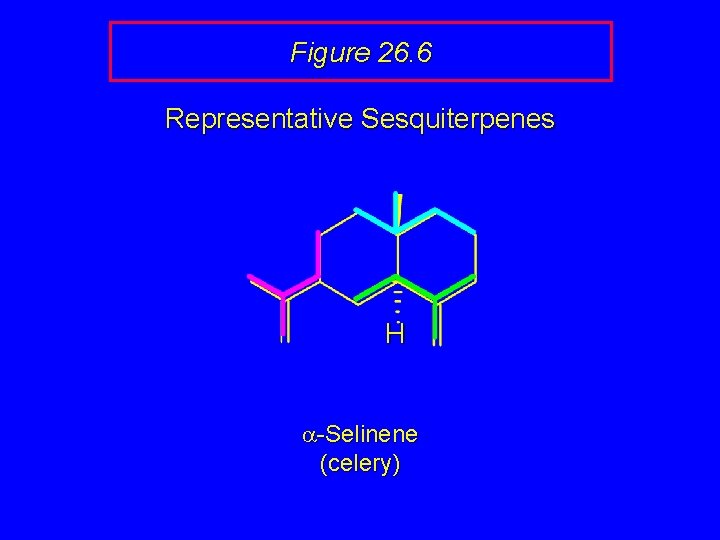
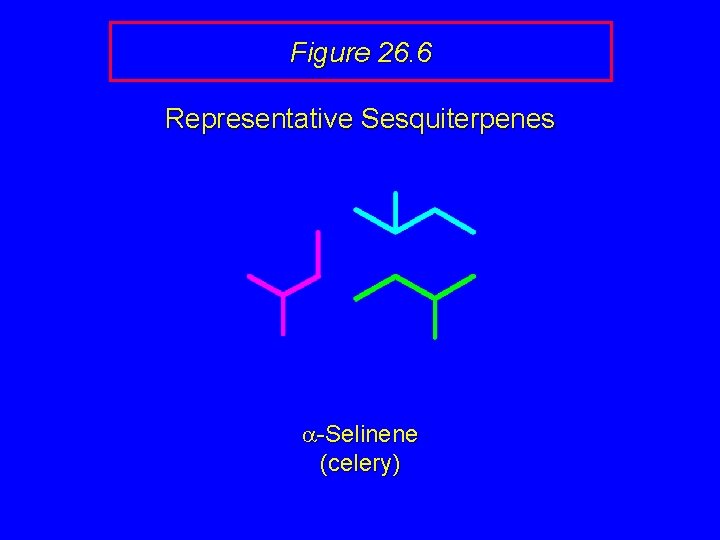
Figure 26. 6 Representative Sesquiterpenes H a-Selinene (celery)

Figure 26. 6 Representative Sesquiterpenes H a-Selinene (celery)

Figure 26. 6 Representative Sesquiterpenes a-Selinene (celery)

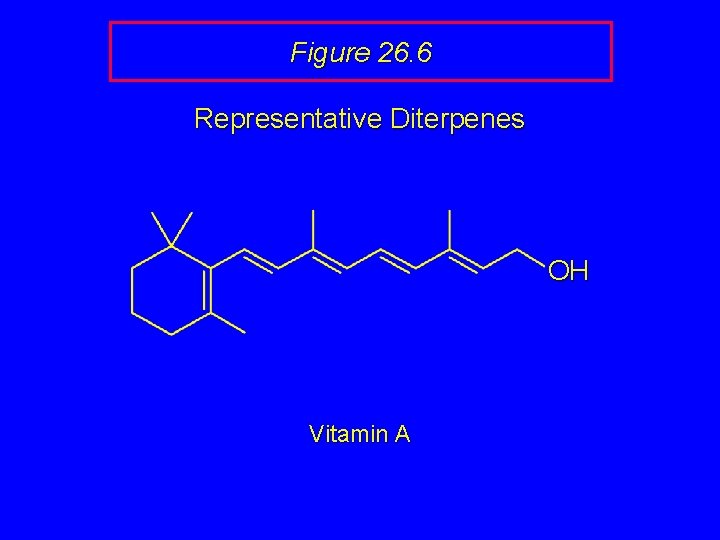
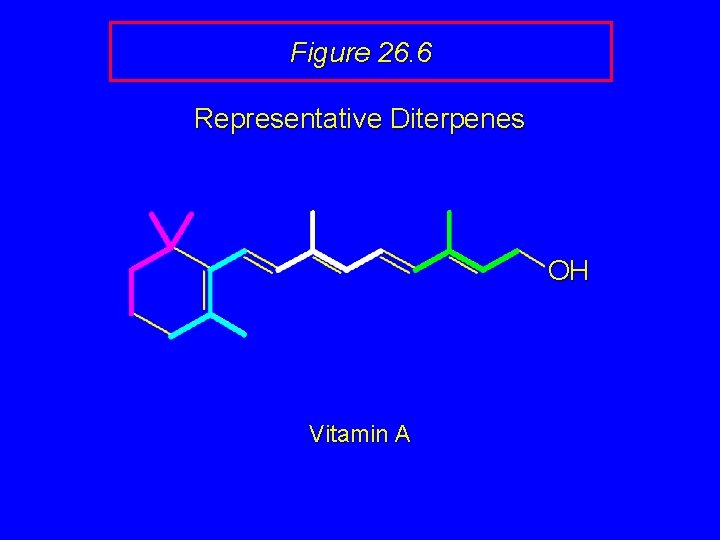
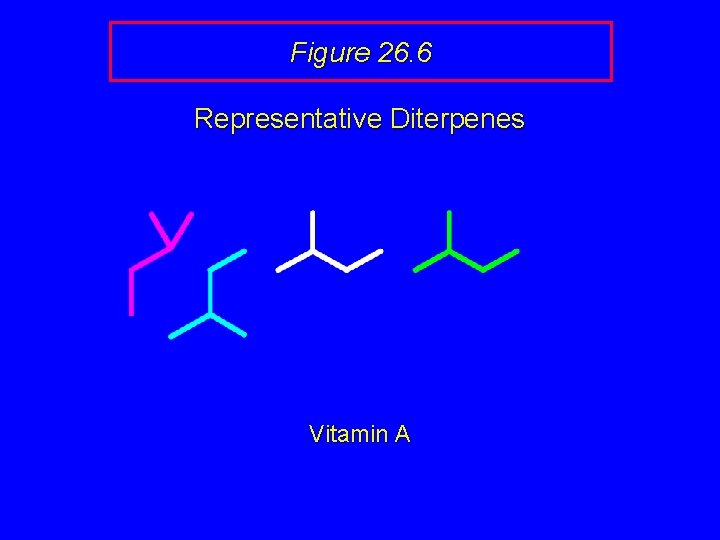
Figure 26. 6 Representative Diterpenes OH Vitamin A

Figure 26. 6 Representative Diterpenes OH Vitamin A

Figure 26. 6 Representative Diterpenes Vitamin A

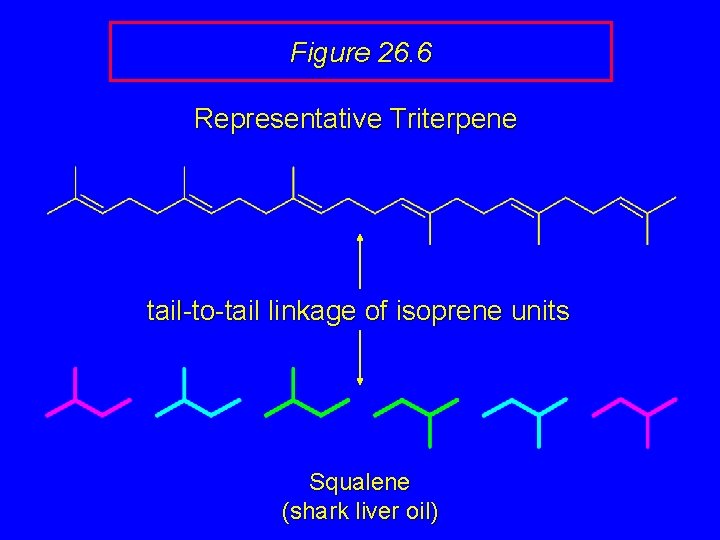
Figure 26. 6 Representative Triterpene tail-to-tail linkage of isoprene units Squalene (shark liver oil)

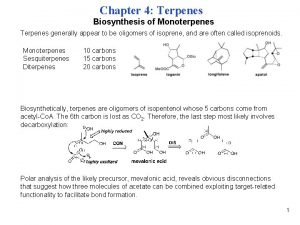
26. 8 Isopentenyl Pyrophosphate: The Biological Isoprene Unit

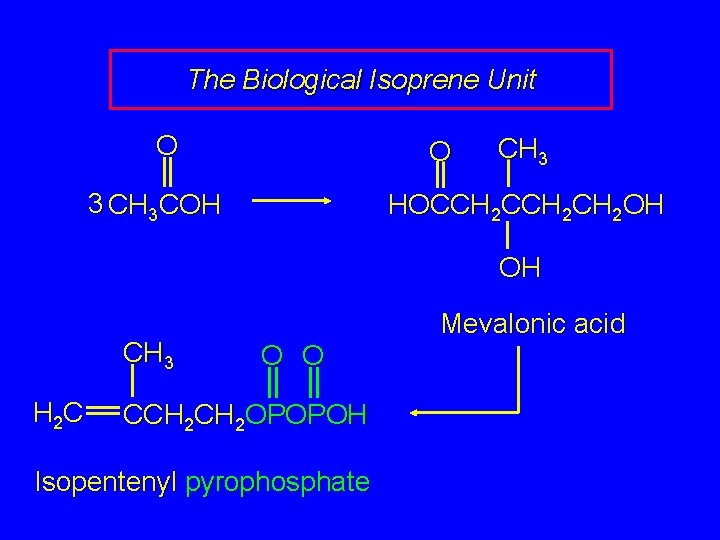
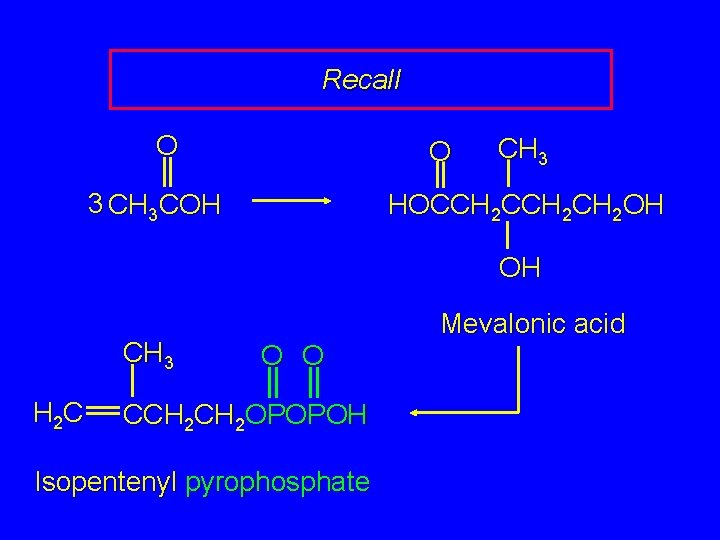
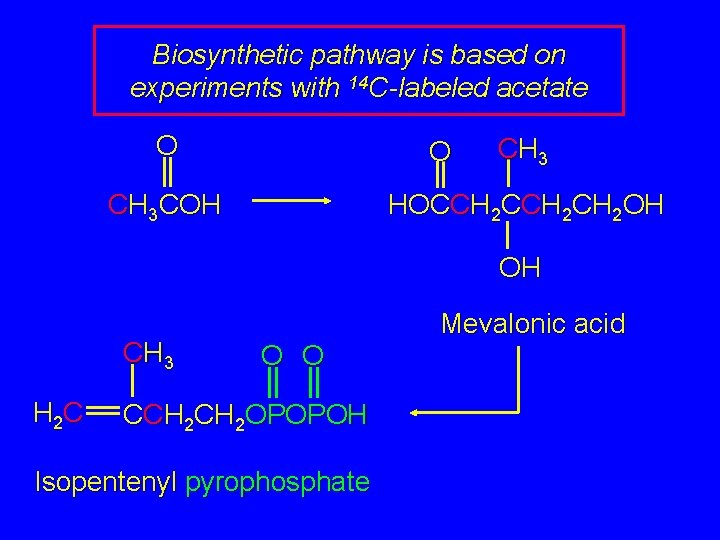
The Biological Isoprene Unit The isoprene units in terpenes do not come from isoprene. They come from isopentenyl pyrophosphate. Isopentenyl pyrophosphate (5 carbons) comes from acetate (2 carbons) via mevalonate (6 carbons).

The Biological Isoprene Unit O O 3 CH 3 COH CH 3 HOCCH 2 CH 2 OH OH CH 3 H 2 C Mevalonic acid O O CCH 2 OPOPOH Isopentenyl pyrophosphate

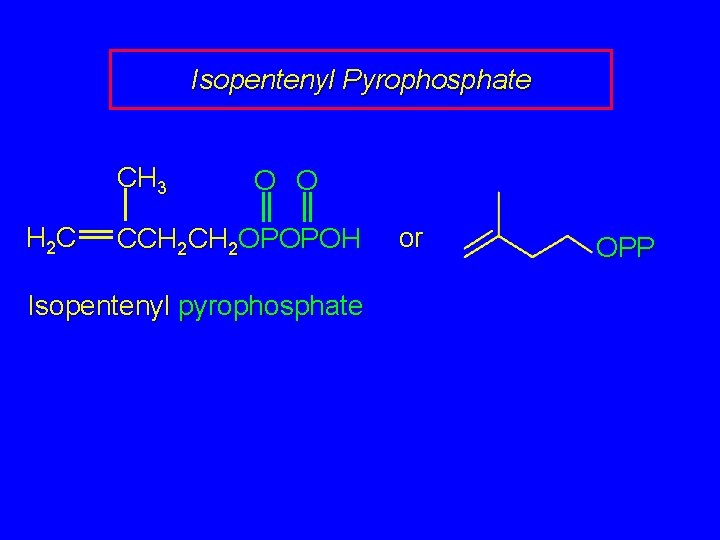
Isopentenyl Pyrophosphate CH 3 H 2 C O O CCH 2 OPOPOH Isopentenyl pyrophosphate or OPP

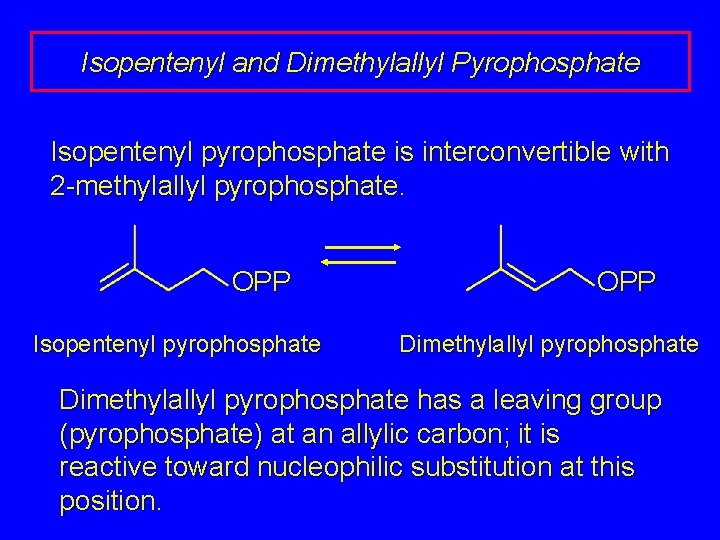
Isopentenyl and Dimethylallyl Pyrophosphate Isopentenyl pyrophosphate is interconvertible with 2 -methylallyl pyrophosphate. OPP Isopentenyl pyrophosphate OPP Dimethylallyl pyrophosphate has a leaving group (pyrophosphate) at an allylic carbon; it is reactive toward nucleophilic substitution at this position.

26. 9 Carbon-Carbon Bond Formation in Terpene Biosynthesis

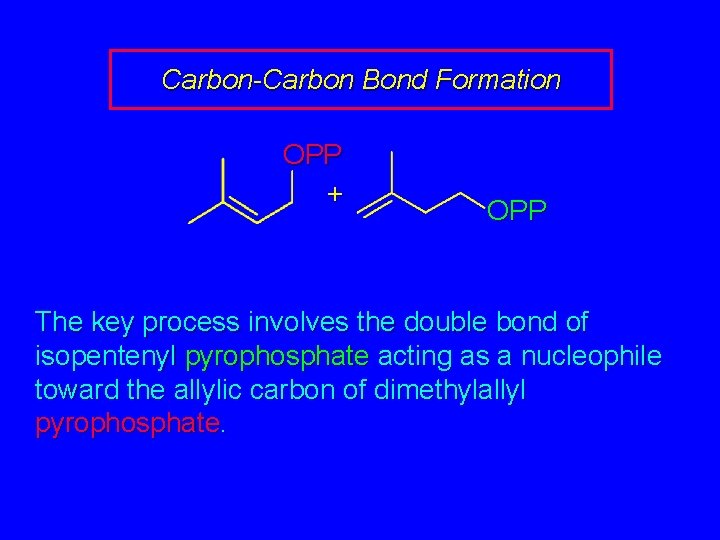
Carbon-Carbon Bond Formation OPP + OPP The key process involves the double bond of isopentenyl pyrophosphate acting as a nucleophile toward the allylic carbon of dimethylallyl pyrophosphate.

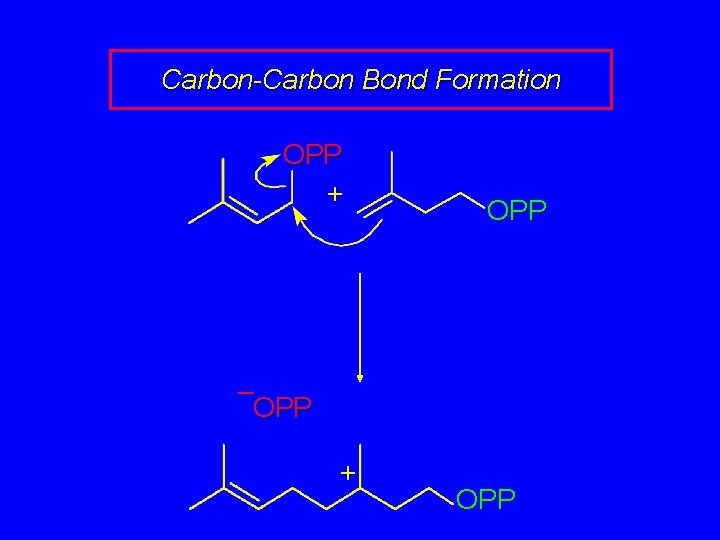
Carbon-Carbon Bond Formation OPP + OPP – OPP + OPP

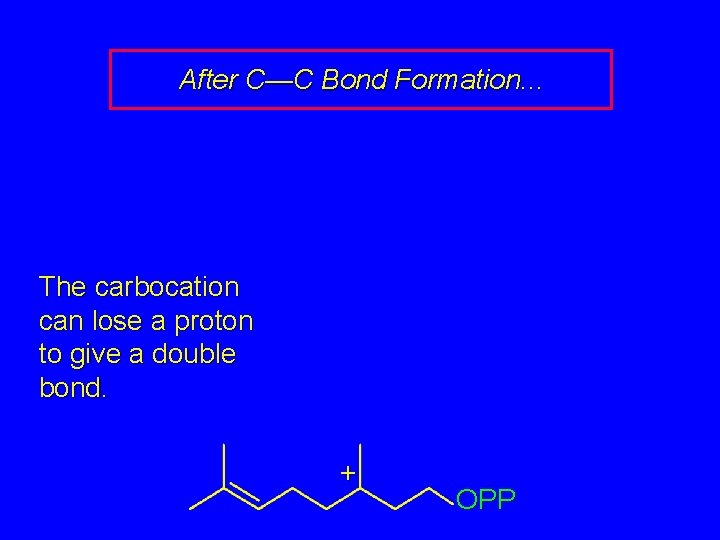
After C—C Bond Formation. . . The carbocation can lose a proton to give a double bond. + OPP

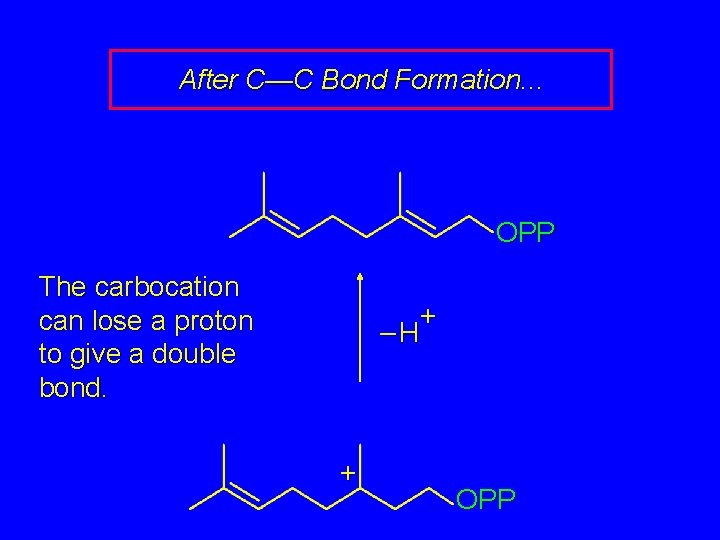
After C—C Bond Formation. . . OPP The carbocation can lose a proton to give a double bond. + –H + OPP

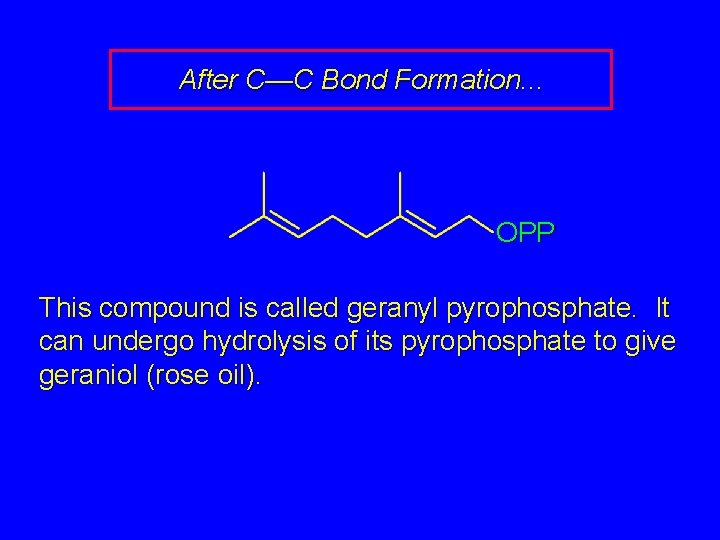
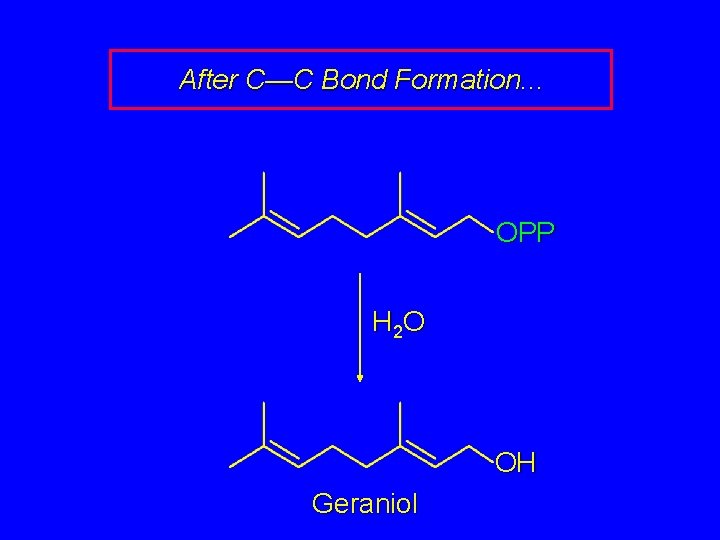
After C—C Bond Formation. . . OPP This compound is called geranyl pyrophosphate. It can undergo hydrolysis of its pyrophosphate to give geraniol (rose oil).

After C—C Bond Formation. . . OPP H 2 O OH Geraniol

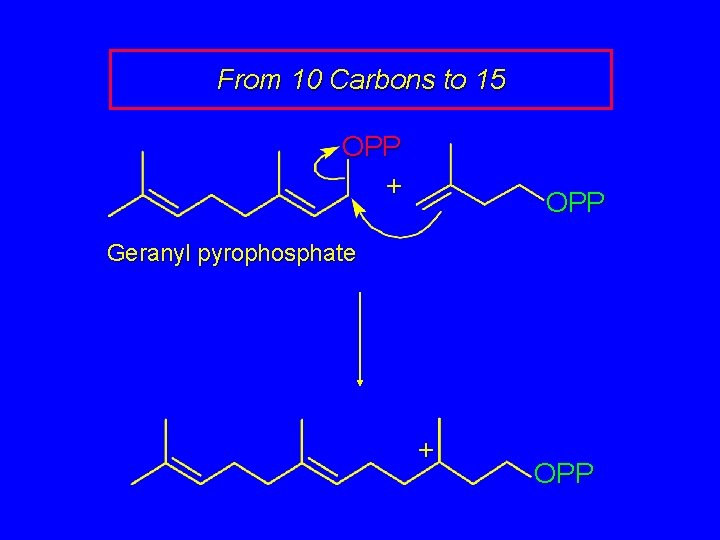
From 10 Carbons to 15 OPP + OPP Geranyl pyrophosphate + OPP

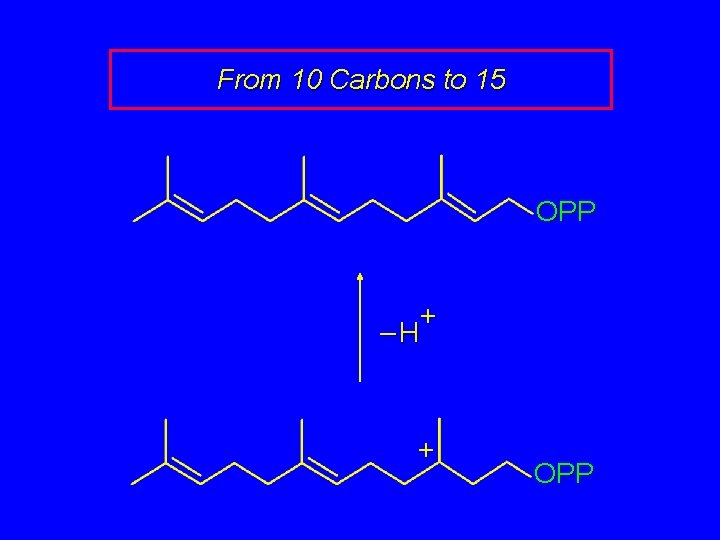
From 10 Carbons to 15 OPP + –H + OPP

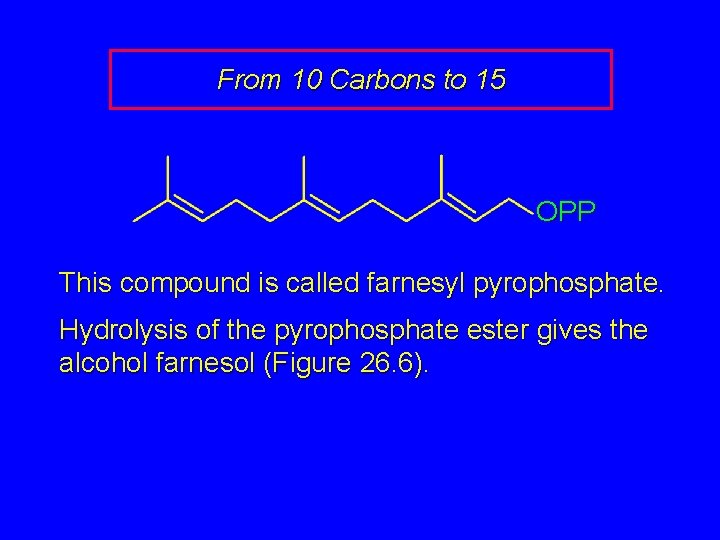
From 10 Carbons to 15 OPP This compound is called farnesyl pyrophosphate. Hydrolysis of the pyrophosphate ester gives the alcohol farnesol (Figure 26. 6).

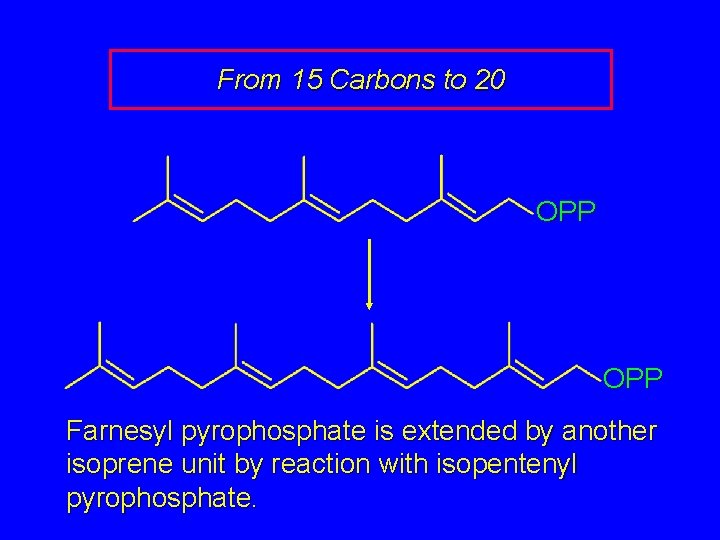
From 15 Carbons to 20 OPP Farnesyl pyrophosphate is extended by another isoprene unit by reaction with isopentenyl pyrophosphate.

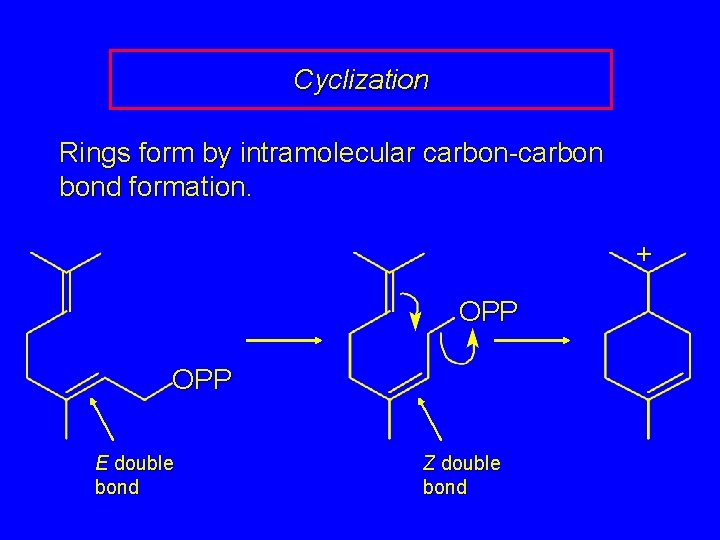
Cyclization Rings form by intramolecular carbon-carbon bond formation. + OPP E double bond Z double bond

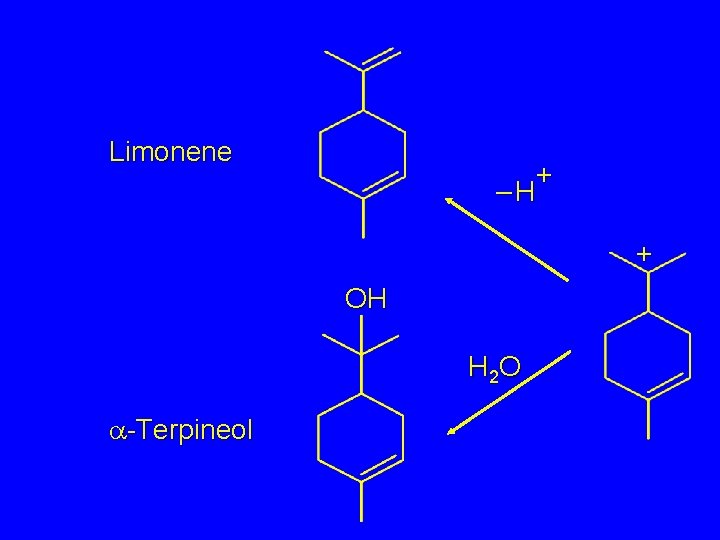
Limonene + –H + OH H 2 O a-Terpineol

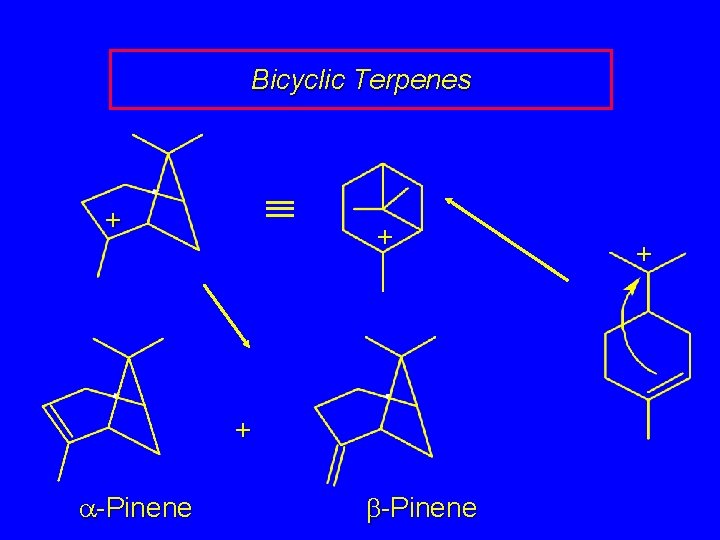
Bicyclic Terpenes + + + a-Pinene b-Pinene +

26. 10 The Pathway from Acetate to Isopentenyl Pyrophosphate

Recall O O 3 CH 3 COH CH 3 HOCCH 2 CH 2 OH OH CH 3 H 2 C Mevalonic acid O O CCH 2 OPOPOH Isopentenyl pyrophosphate

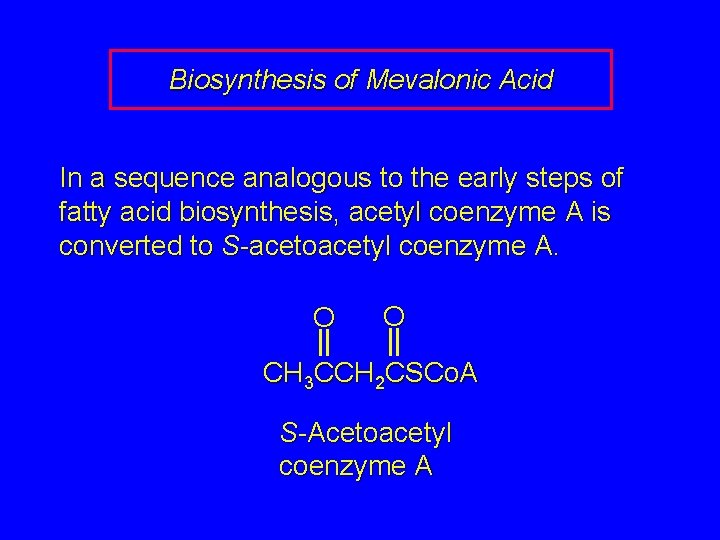
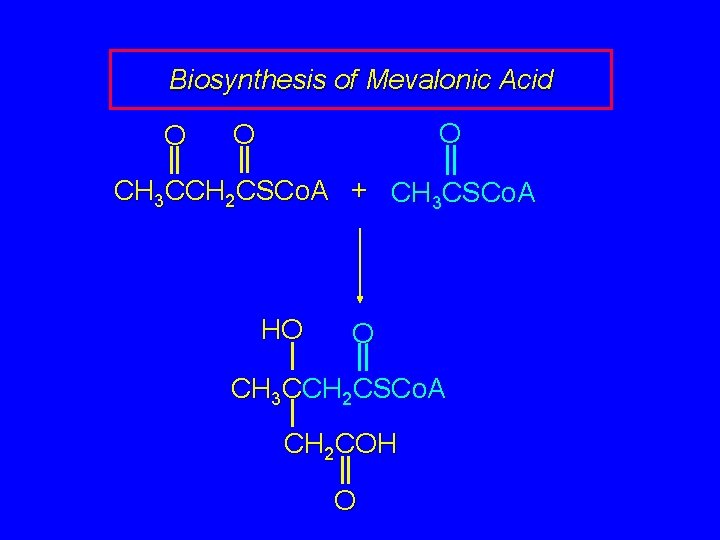
Biosynthesis of Mevalonic Acid In a sequence analogous to the early steps of fatty acid biosynthesis, acetyl coenzyme A is converted to S-acetoacetyl coenzyme A. O O CH 3 CCH 2 CSCo. A S-Acetoacetyl coenzyme A

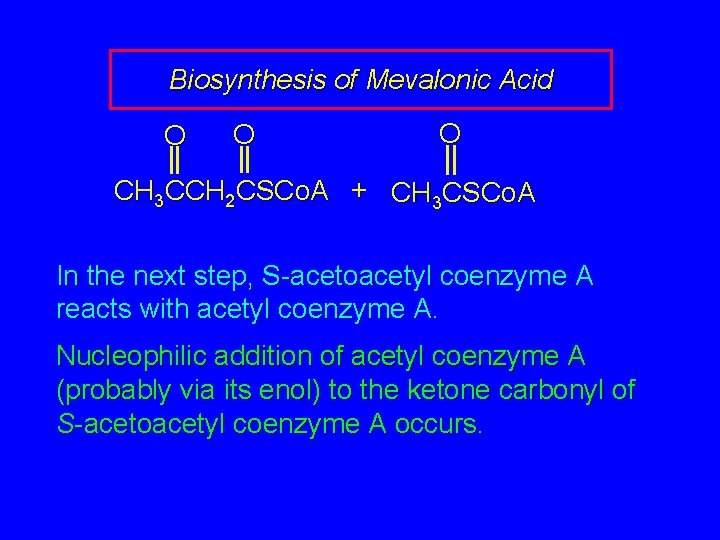
Biosynthesis of Mevalonic Acid O O O CH 3 CCH 2 CSCo. A + CH 3 CSCo. A In the next step, S-acetoacetyl coenzyme A reacts with acetyl coenzyme A. Nucleophilic addition of acetyl coenzyme A (probably via its enol) to the ketone carbonyl of S-acetoacetyl coenzyme A occurs.

Biosynthesis of Mevalonic Acid O O O CH 3 CCH 2 CSCo. A + CH 3 CSCo. A HO O CH 3 CCH 2 CSCo. A CH 2 COH O

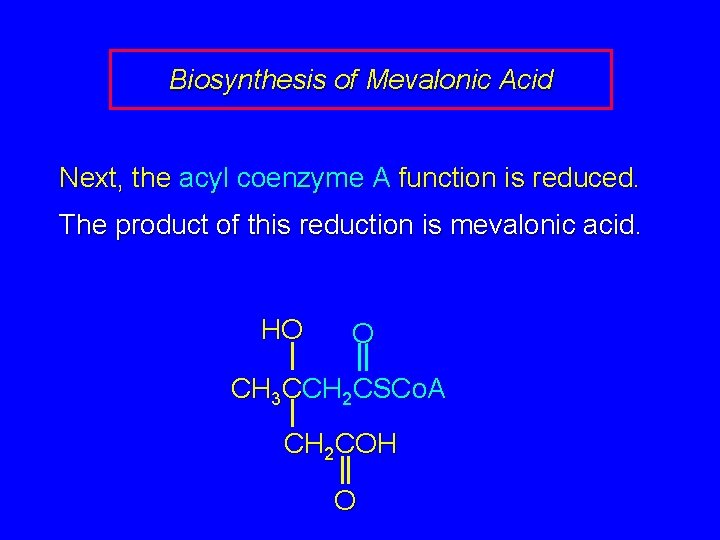
Biosynthesis of Mevalonic Acid Next, the acyl coenzyme A function is reduced. The product of this reduction is mevalonic acid. HO O CH 3 CCH 2 CSCo. A CH 2 COH O

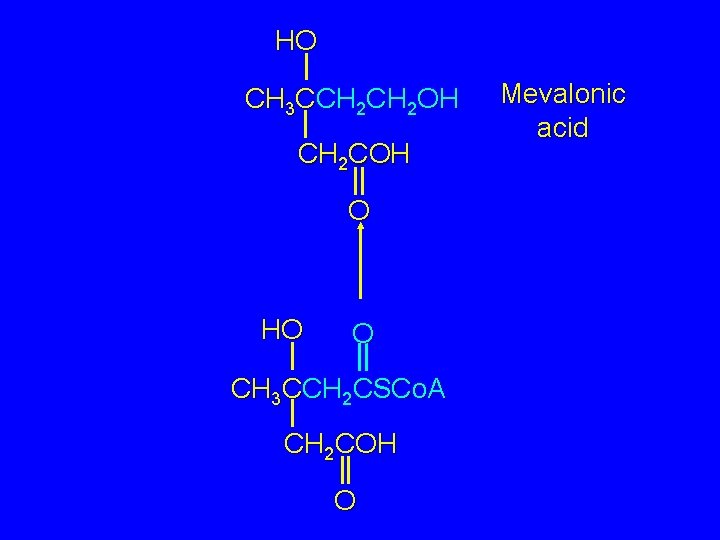
HO CH 3 CCH 2 OH CH 2 COH O HO O CH 3 CCH 2 CSCo. A CH 2 COH O Mevalonic acid

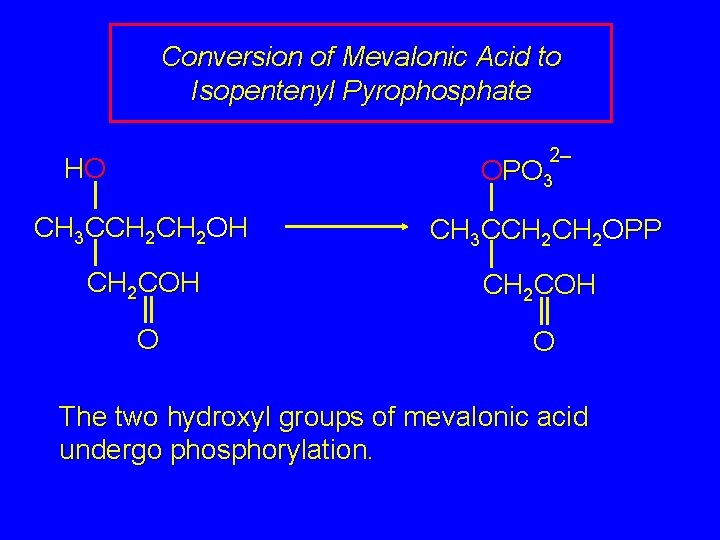
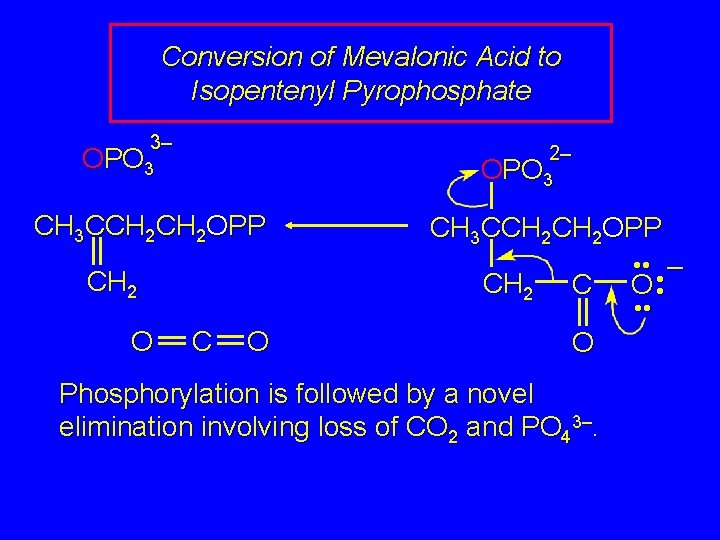
Conversion of Mevalonic Acid to Isopentenyl Pyrophosphate 2– HO OPO 3 CH 3 CCH 2 OH CH 3 CCH 2 OPP CH 2 COH O O The two hydroxyl groups of mevalonic acid undergo phosphorylation.

Conversion of Mevalonic Acid to Isopentenyl Pyrophosphate 3– 2– OPO 3 CH 3 CCH 2 OPP CH 2 O CH 3 CCH 2 OPP CH 2 C O Phosphorylation is followed by a novel elimination involving loss of CO 2 and PO 43–. • • – O • •

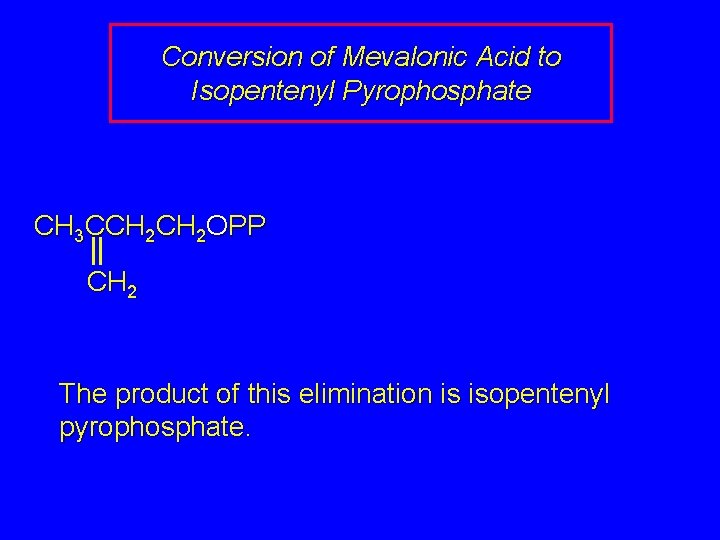
Conversion of Mevalonic Acid to Isopentenyl Pyrophosphate CH 3 CCH 2 OPP CH 2 The product of this elimination is isopentenyl pyrophosphate.

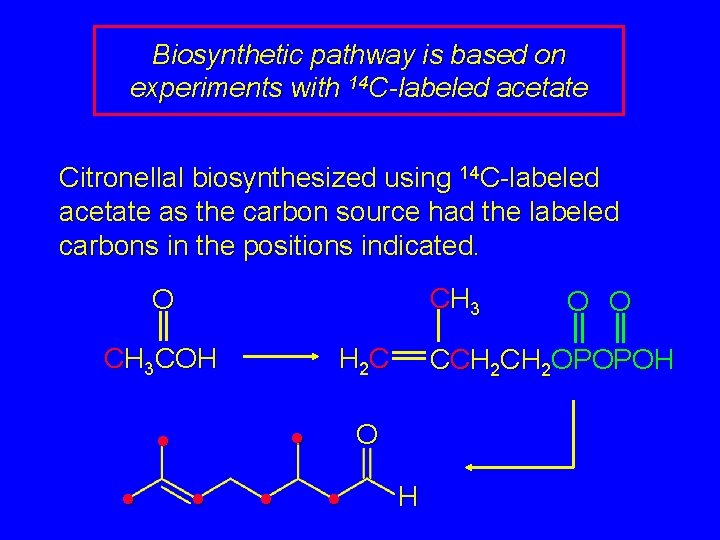
Biosynthetic pathway is based on experiments with 14 C-labeled acetate O O CH 3 COH CH 3 HOCCH 2 CH 2 OH OH CH 3 H 2 C Mevalonic acid O O CCH 2 OPOPOH Isopentenyl pyrophosphate

Biosynthetic pathway is based on experiments with 14 C-labeled acetate Citronellal biosynthesized using 14 C-labeled acetate as the carbon source had the labeled carbons in the positions indicated. CH 3 O CH 3 COH H 2 C • • • CCH 2 OPOPOH O • O O H
 Isoprene units examples
Isoprene units examples Antigentest åre
Antigentest åre Isoprene rule
Isoprene rule Isoprene terpene
Isoprene terpene Irregular terpene
Irregular terpene Steroid
Steroid Terpenes steroids prostaglandins
Terpenes steroids prostaglandins Ecuelle a piquer
Ecuelle a piquer Isoprene
Isoprene How many double bonds preset in the triterpene squalene?
How many double bonds preset in the triterpene squalene? Achönberg
Achönberg Phản ứng thế ankan
Phản ứng thế ankan Các môn thể thao bắt đầu bằng tiếng bóng
Các môn thể thao bắt đầu bằng tiếng bóng Sự nuôi và dạy con của hổ
Sự nuôi và dạy con của hổ điện thế nghỉ
điện thế nghỉ Một số thể thơ truyền thống
Một số thể thơ truyền thống Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Gấu đi như thế nào
Gấu đi như thế nào Bảng số nguyên tố lớn hơn 1000
Bảng số nguyên tố lớn hơn 1000 Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Tia chieu sa te
Tia chieu sa te Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Sơ đồ cơ thể người
Sơ đồ cơ thể người Tư thế ngồi viết
Tư thế ngồi viết Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 Thang điểm glasgow
Thang điểm glasgow Tư thế ngồi viết
Tư thế ngồi viết ưu thế lai là gì
ưu thế lai là gì Thẻ vin
Thẻ vin Cái miệng nó xinh thế
Cái miệng nó xinh thế Thể thơ truyền thống
Thể thơ truyền thống Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Diễn thế sinh thái là
Diễn thế sinh thái là Slidetodoc
Slidetodoc Thứ tự các dấu thăng giáng ở hóa biểu
Thứ tự các dấu thăng giáng ở hóa biểu Phép trừ bù
Phép trừ bù Alleluia hat len nguoi oi
Alleluia hat len nguoi oi Hổ sinh sản vào mùa nào
Hổ sinh sản vào mùa nào đại từ thay thế
đại từ thay thế Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Công thức tính độ biến thiên đông lượng
Công thức tính độ biến thiên đông lượng