Stoichiometric Calculations 1 Review of Fundamental Concepts Formula



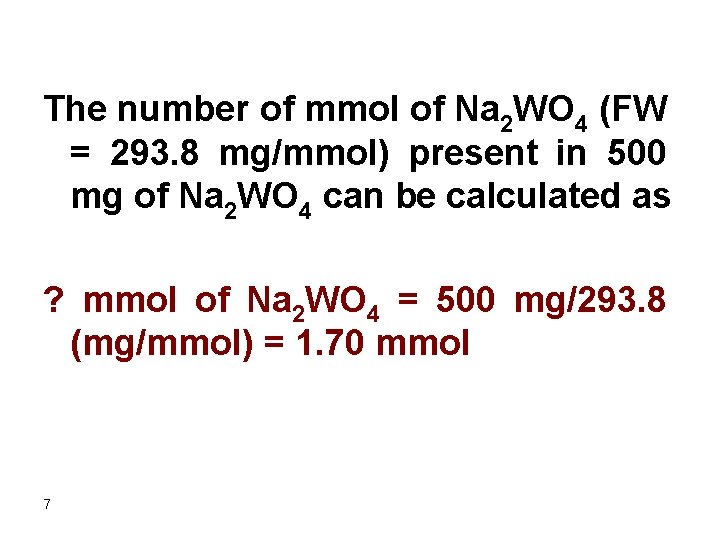










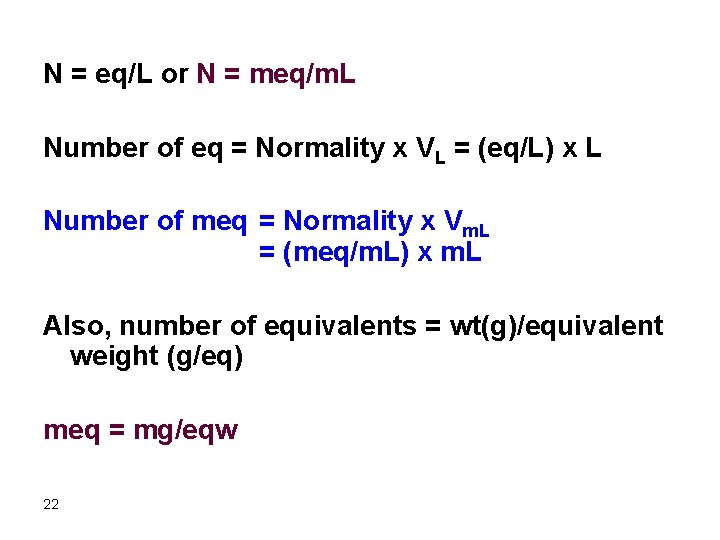













![For Solid Solutes in solid samples % (w/w) = [weight solute (g)/weight sample (g)] For Solid Solutes in solid samples % (w/w) = [weight solute (g)/weight sample (g)]](https://slidetodoc.com/presentation_image/5e30071740f2a8e6b2777a9320534eb9/image-50.jpg)

![For Liquid Solutes % (v/v) = [volume solute (m. L)/volume sample (m. L)] x For Liquid Solutes % (v/v) = [volume solute (m. L)/volume sample (m. L)] x](https://slidetodoc.com/presentation_image/5e30071740f2a8e6b2777a9320534eb9/image-52.jpg)













![[mg Fe/(55. 8 mg/mmol)] = 5 x (0. 0206 mmol/m. L) x 40. [mg Fe/(55. 8 mg/mmol)] = 5 x (0. 0206 mmol/m. L) x 40.](https://slidetodoc.com/presentation_image/5e30071740f2a8e6b2777a9320534eb9/image-78.jpg)





















- Slides: 119

Stoichiometric Calculations 1

Review of Fundamental Concepts Formula Weight It is assumed that you can calculate the formula or molecular weights of compounds from respective atomic weights of the elements forming these compounds. The formula weight (FW) of a substance is the sum of the atomic weights of the elements from which this substance is formed from. 2

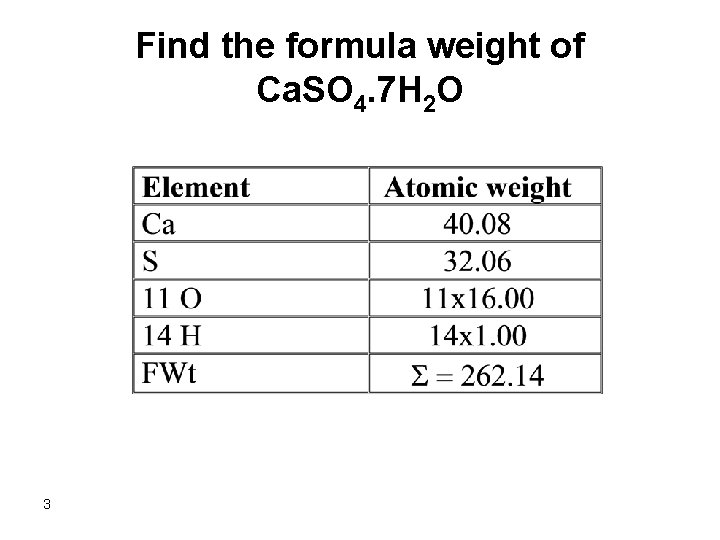
Find the formula weight of Ca. SO 4. 7 H 2 O 3

The Mole Concept 4

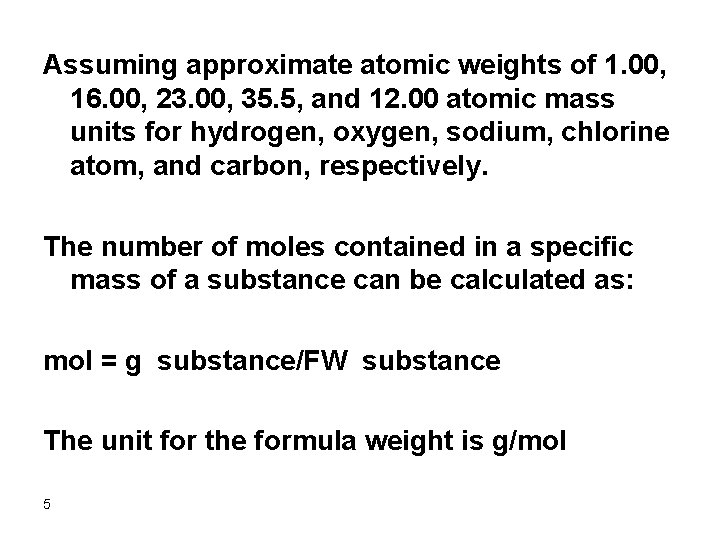
Assuming approximate atomic weights of 1. 00, 16. 00, 23. 00, 35. 5, and 12. 00 atomic mass units for hydrogen, oxygen, sodium, chlorine atom, and carbon, respectively. The number of moles contained in a specific mass of a substance can be calculated as: mol = g substance/FW substance The unit for the formula weight is g/mol 5

In the same manner, the number of mmol of a substance contained in a specific weight of the substance can be calculated as mmol = mol/1000 Or, mmol = mg substance/FW substance 6
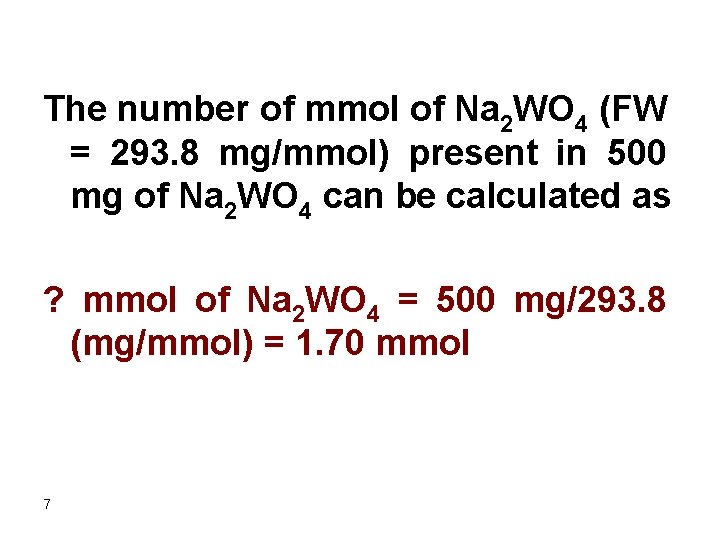
The number of mmol of Na 2 WO 4 (FW = 293. 8 mg/mmol) present in 500 mg of Na 2 WO 4 can be calculated as ? mmol of Na 2 WO 4 = 500 mg/293. 8 (mg/mmol) = 1. 70 mmol 7

The number of mg contained in 0. 25 mmol of Fe 2 O 3 (FW = 159. 7 mg/mmol) can be calculated as ? mg Fe 2 O 3 = 0. 25 mmol Fe 2 O 3 x 159. 7 (mg/mmol) = 39. 9 mg Therefore, either the number of mg of a substance can be obtained from its mmols or vice versa. 8

Calculations Involving Solutions Molarity of a solution can be defined as the number of moles of solute dissolved in 1 L of solution. This means that 1 mol of solute will be dissolved in some amount of water and the volume will be adjusted to 1 L. The amount of water may be less than 1 L as the final volume of solute and water is exactly 1 L. 9

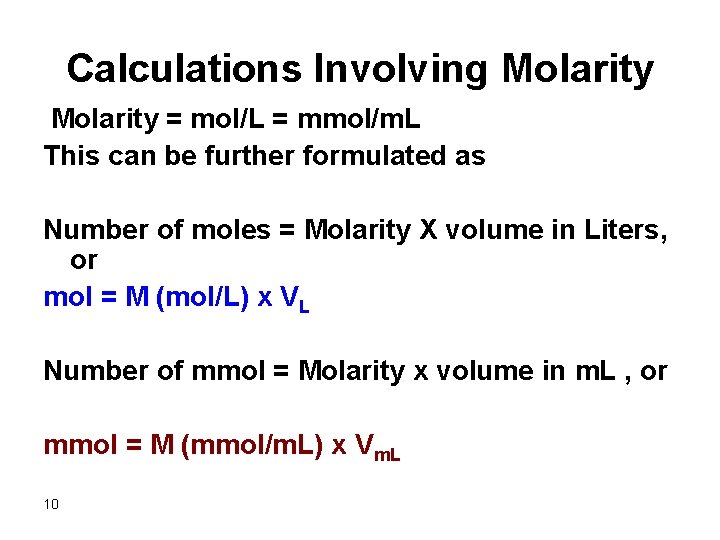
Calculations Involving Molarity = mol/L = mmol/m. L This can be further formulated as Number of moles = Molarity X volume in Liters, or mol = M (mol/L) x VL Number of mmol = Molarity x volume in m. L , or mmol = M (mmol/m. L) x Vm. L 10

Find the molarity of a solution resulting from dissolving 1. 26 g of Ag. NO 3 (FW = 169. 9 g/mol) in a total volume of 250 m. L solution. First find mmol Ag. NO 3 = 1. 26*103 mg Ag. NO 3 / 169. 9 mg/mmol = 7. 42 mmol Molarity = mmol/m. L M = 7. 42 mmol/250 m. L = 0. 0297 mmol/m. L 11

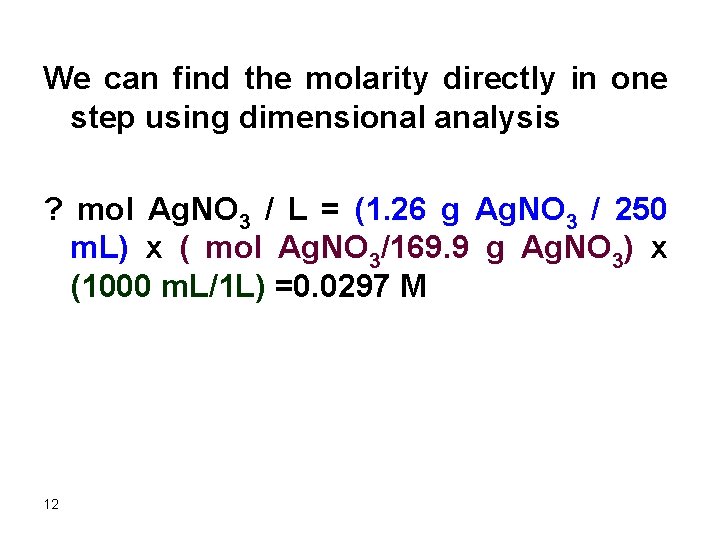
We can find the molarity directly in one step using dimensional analysis ? mol Ag. NO 3 / L = (1. 26 g Ag. NO 3 / 250 m. L) x ( mol Ag. NO 3/169. 9 g Ag. NO 3) x (1000 m. L/1 L) =0. 0297 M 12

Let us find the number of mg of Na. Cl per m. L of a 0. 25 M Na. Cl solution First we should be able to recognize the molarity as 0. 25 mol/L or 0. 25 mmol/m. L. Of course, the second term offers what we need directly ? mg Na. Cl in 1 m. L = (0. 25 mmol Na. Cl/m. L) x (58. 5 mg Na. Cl/mmol Na. Cl) = 14. 6 mg Na. Cl/m. L 13

Find the number of grams of Na 2 SO 4 required to prepare 500 m. L of 0. 1 M solution. First, we find mmoles needed from the relation mmol = M (mmol/m. L) x Vm. L mmol Na 2 SO 4 = 0. 1 mmol/m. L x 500 m. L = 50 mmol = mg substance/FW substance ? mg Na 2 SO 4 = 50 mmol x 142 mg/mmol = 7100 mg or 7. 1 g 14

One can use dimensional analysis to find the answer in one step as ? g Na 2 SO 4 = (0. 1 mol Na 2 SO 4/1000 m. L) x 500 m. L x (142 g Na 2 SO 4/mol) = 7. 1 g • 15

When two or more solutions are mixed, one can find the final concentration of each ion. However, you should always remember that the number of moles ( or mmoles ) is additive. For example: Find the molarity of K+ after mixing 100 m. L of 0. 25 M KCl with 200 m. L of 0. 1 M K 2 SO 4. 16

The idea here is to calculate the total mmol of K+ and divide it by volume in m. L. mmol K+ = mmol K+ from KCl + mmol K+ from K 2 SO 4 = 0. 25 mmol/ml x 100 m. L + 2 x 0. 1 mmol/m. L x 200 m. L = 65 mmol Molarity = 65 mmol/(200 + 100) m. L = 0. 22 M Note that the concentration of K+ in 0. 1 M K 2 SO 4 is 2 x 0. 1 M (i. e. 0. 2 M) 17

Lecture 9 Stoichiometric Calculations Normality Density Calculations 18

Normality We have previously talked about molarity as a method for expressing concentration. The second expression used to describe concentration of a solution is the normality. Normality can be defined as the number of equivalents of solute dissolved in 1 L of solution. Therefore, it is important for us to define what we mean by the number of equivalents, as well as the equivalent weight of a substance as a parallel term to formula weight. 19

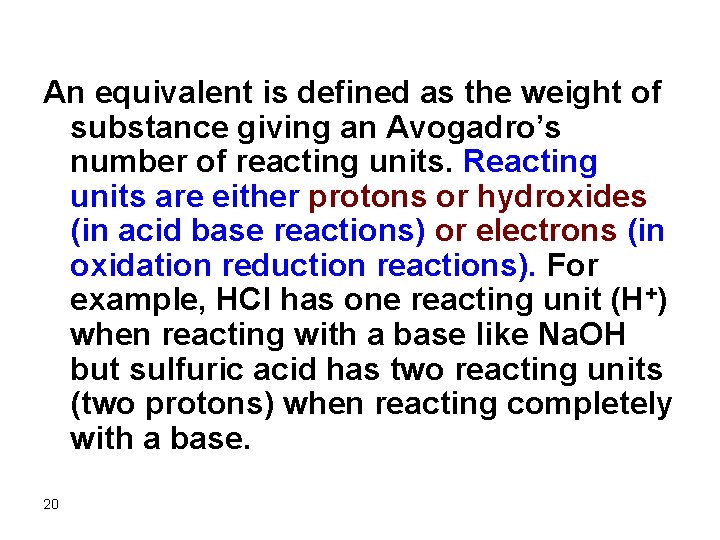
An equivalent is defined as the weight of substance giving an Avogadro’s number of reacting units. Reacting units are either protons or hydroxides (in acid base reactions) or electrons (in oxidation reduction reactions). For example, HCl has one reacting unit (H+) when reacting with a base like Na. OH but sulfuric acid has two reacting units (two protons) when reacting completely with a base. 20

Therefore, we say that the equivalent weight of HCl is equal to its formula weight and the equivalent weight of sulfuric acid is one half its formula weight. In the reaction where Mn(VII), in KMn. O 4, is reduced to Mn(II) five electrons are involved and the equivalent weight of KMn. O 4 is equal to its formula weight divided by 5. 21
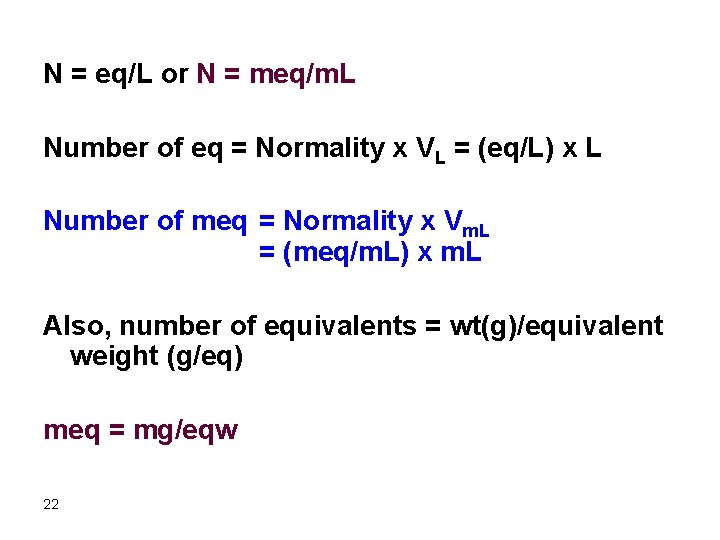
N = eq/L or N = meq/m. L Number of eq = Normality x VL = (eq/L) x L Number of meq = Normality x Vm. L = (meq/m. L) x m. L Also, number of equivalents = wt(g)/equivalent weight (g/eq) meq = mg/eqw 22

Equivalent weight = FW/n meq = mg/eqw substitute for eqw = FW/n gives: meq = mg/(FW/n), but mmol = mg/FW, therefore: meq = n * mmol dividing both sides by volume in m. L, we get: N = n M Where n is the number of reacting units ( protons, hydroxides, or electrons ) and if you are forming factors always remember that a mole contains n equivalents. The factor becomes (1 mol/n eq) or (n eq/1 mol). 23

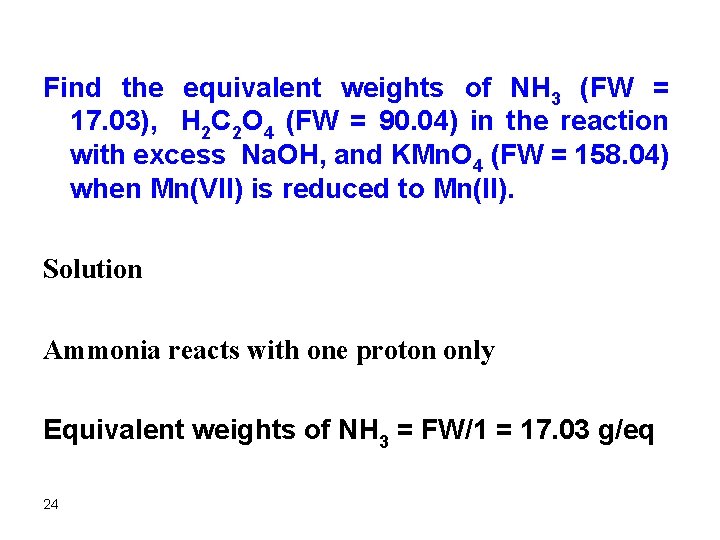
Find the equivalent weights of NH 3 (FW = 17. 03), H 2 C 2 O 4 (FW = 90. 04) in the reaction with excess Na. OH, and KMn. O 4 (FW = 158. 04) when Mn(VII) is reduced to Mn(II). Solution Ammonia reacts with one proton only Equivalent weights of NH 3 = FW/1 = 17. 03 g/eq 24

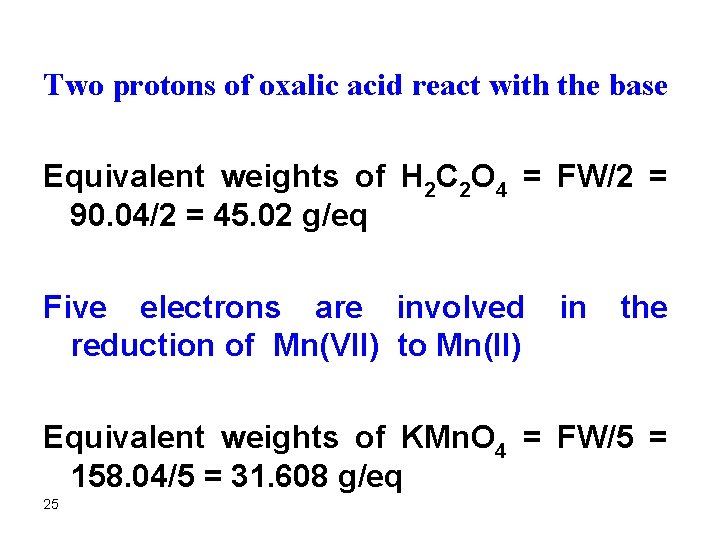
Two protons of oxalic acid react with the base Equivalent weights of H 2 C 2 O 4 = FW/2 = 90. 04/2 = 45. 02 g/eq Five electrons are involved in the reduction of Mn(VII) to Mn(II) Equivalent weights of KMn. O 4 = FW/5 = 158. 04/5 = 31. 608 g/eq 25

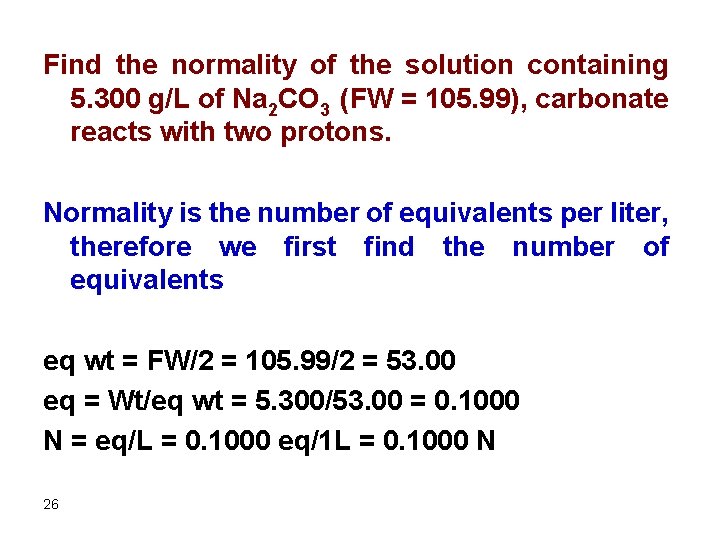
Find the normality of the solution containing 5. 300 g/L of Na 2 CO 3 (FW = 105. 99), carbonate reacts with two protons. Normality is the number of equivalents per liter, therefore we first find the number of equivalents eq wt = FW/2 = 105. 99/2 = 53. 00 eq = Wt/eq wt = 5. 300/53. 00 = 0. 1000 N = eq/L = 0. 1000 eq/1 L = 0. 1000 N 26

The problem can be worked out simply as below ? eq Na 2 CO 3 /L = (5. 300 g Na 2 CO 3 /L ) x (1 mol Na 2 CO 3 /105. 99 g Na 2 CO 3 ) x (2 eq Na 2 CO 3 /1 mol Na 2 CO 3) = 0. 1 N A further option is to find the number of moles first followed by multiplying the result by 2 to obtain the number of equivalents. 27

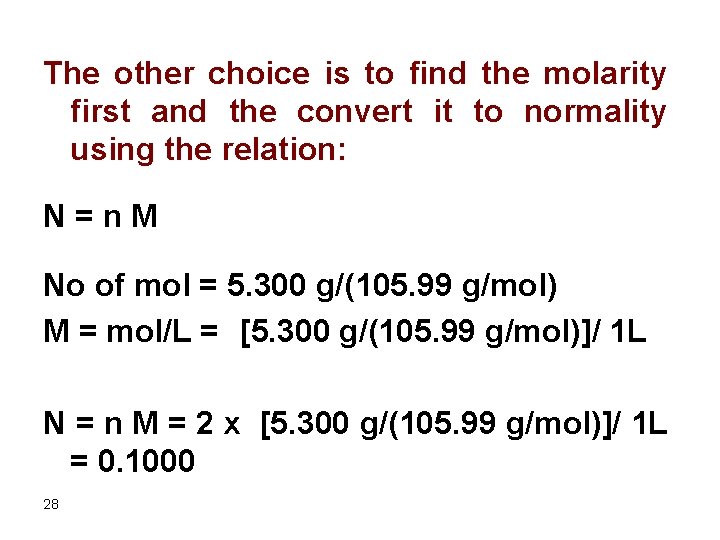
The other choice is to find the molarity first and the convert it to normality using the relation: N = n M No of mol = 5. 300 g/(105. 99 g/mol) M = mol/L = [5. 300 g/(105. 99 g/mol)]/ 1 L N = n M = 2 x [5. 300 g/(105. 99 g/mol)]/ 1 L = 0. 1000 28

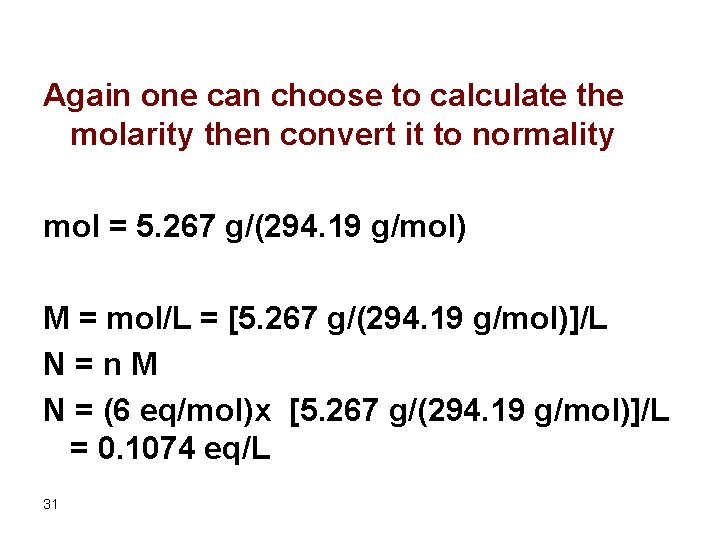
Find the normality of the solution containing 5. 267 g/L K 2 Cr 2 O 7 (FW = 294. 19) if Cr 6+ is reduced to Cr 3+. The same as the previous example N = eq/L, therefore we should find the number of eq where eq = wt/eq wt, therefore we should find the equivalent weight; where eq wt = FW/n. Here; each Contributes three electrons and since the dichromate contains two Cr atoms we have 6 reacting units 29

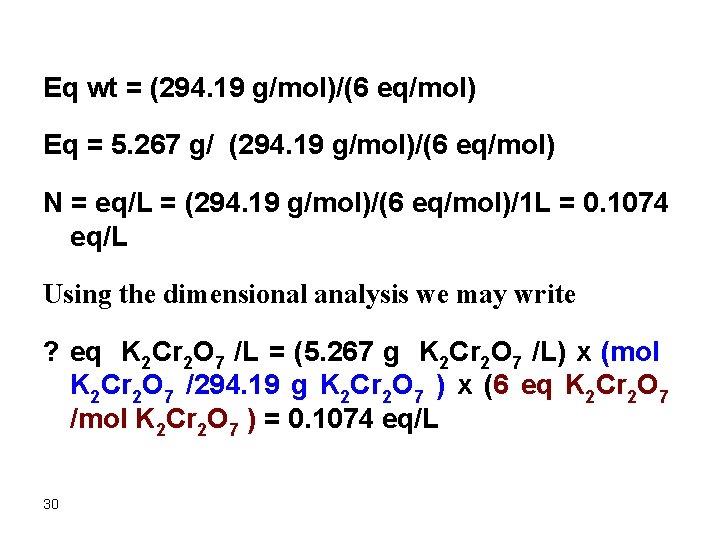
Eq wt = (294. 19 g/mol)/(6 eq/mol) Eq = 5. 267 g/ (294. 19 g/mol)/(6 eq/mol) N = eq/L = (294. 19 g/mol)/(6 eq/mol)/1 L = 0. 1074 eq/L Using the dimensional analysis we may write ? eq K 2 Cr 2 O 7 /L = (5. 267 g K 2 Cr 2 O 7 /L) x (mol K 2 Cr 2 O 7 /294. 19 g K 2 Cr 2 O 7 ) x (6 eq K 2 Cr 2 O 7 /mol K 2 Cr 2 O 7 ) = 0. 1074 eq/L 30

Again one can choose to calculate the molarity then convert it to normality mol = 5. 267 g/(294. 19 g/mol) M = mol/L = [5. 267 g/(294. 19 g/mol)]/L N = n M N = (6 eq/mol)x [5. 267 g/(294. 19 g/mol)]/L = 0. 1074 eq/L 31

Density Calculations In this section, you will learn how to find the molarity of solution from two pieces of information (density and percentage). Usually the calculation is simple and can be done using several procedures. Look at the examples below: 32

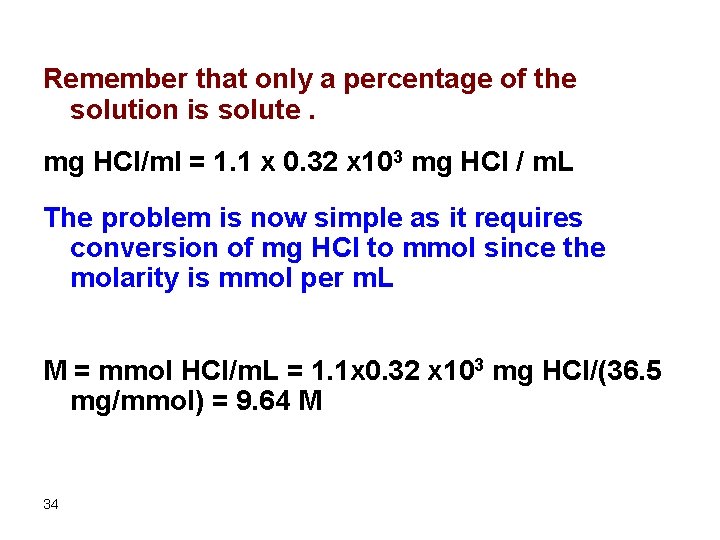
Example What volume of concentrated HCl (FW = 36. 5 g/mol, 32%, density = 1. 1 g/m. L) are required to prepare 500 m. L of 2. 0 M solution. Always start with the density and find how many grams of solute in each m. L of solution. Density = g solution/m. L 33

Remember that only a percentage of the solution is solute. mg HCl/ml = 1. 1 x 0. 32 x 103 mg HCl / m. L The problem is now simple as it requires conversion of mg HCl to mmol since the molarity is mmol per m. L M = mmol HCl/m. L = 1. 1 x 0. 32 x 103 mg HCl/(36. 5 mg/mmol) = 9. 64 M 34

Now, we can calculate the volume required from the relation Mi. Vi (before dilution) = Mf. Vf (after dilution) 9. 64 x Vm. L= 2. 0 x 500 m. L Vm. L = 10. 4 m. L This means that 10. 4 m. L of the concentrated HCl should be added to distilled water and the volume should then be adjusted to 500 m. L 35

How many m. L of concentrated H 2 SO 4 (FW = 98. 1 g/mol, 94%, d = 1. 831 g/m. L) are required to prepare 1 L of 0. 100 M solution? mg H 2 SO 4 / m. L = 1. 831*0. 94*103 mg /m. L Now we only need to convert mg to mmol M = mmol/m. L = [(1. 831 x 0. 94 x 103 mg) / (98. 1 mg/mmol)] / m. L = 17. 5 M 36

To find the volume required to prepare the solution Mi. Vi (before dilution) = Mf. Vf (after dilution) 17. 5 x Vm. L = 0. 100 x 1000 m. L Vm. L = 5. 71 m. L which should be added to distilled water and then adjusted to 1 L. 37

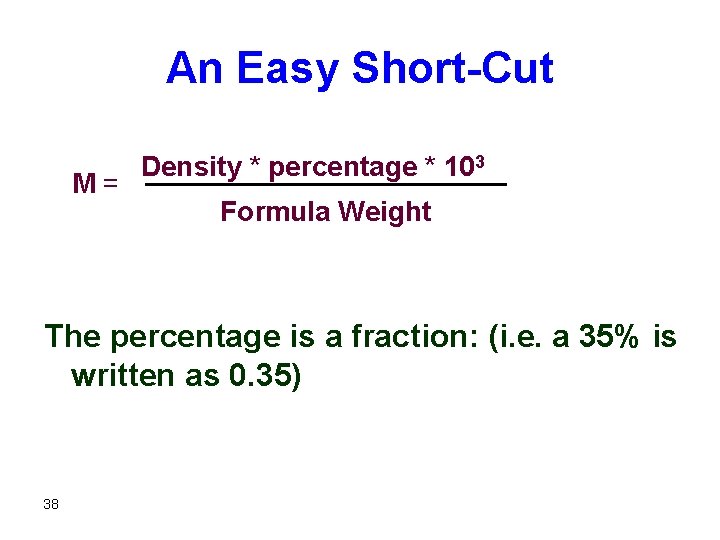
An Easy Short-Cut M = Density * percentage * 103 Formula Weight The percentage is a fraction: (i. e. a 35% is written as 0. 35) 38

Analytical Versus Equilibrium Concentration When we prepare a solution by weighing a specific amount of solute and dissolve it in a specific volume of solution, we get a solution with specific concentration. This concentration is referred to as analytical concentration. However, the concentration in solution may be different from the analytical concentration, especially when partially dissociating substances are used. 39

An example would be clear if we consider preparing 0. 1 M acetic acid (weak acid) by dissolving 0. 1 mol of the acid in 1 L solution. Now, we have an analytical concentration of acetic acid (HOAc) equals 0. 1 M. But what is the actual equilibrium concentration of HOAc? We have HOAc = H+ + OAc. The analytical concentration ( CHOAc ) = 0. 1 M CHOAc = [HOAc]undissociated + [OAc-] The equilibrium concentration = [HOAc]undissociated. 40

For good electrolytes which are 100% dissociated in water the analytical and equilibrium concentrations can be calculated for the ions, rather than the whole species. For example, a 1. 0 M Ca. Cl 2 in water results in 0 M Ca. Cl 2, 1. 0 M Ca 2+, and 2. 0 M Cl- since all calcium chloride dissociates in solution. For species x we express the analytical concentration as Cx and the equilibrium concentration as [x]. 41

Lecture 10 Stoichiometric Calculations, cont…. . Expressing Concentrations 42

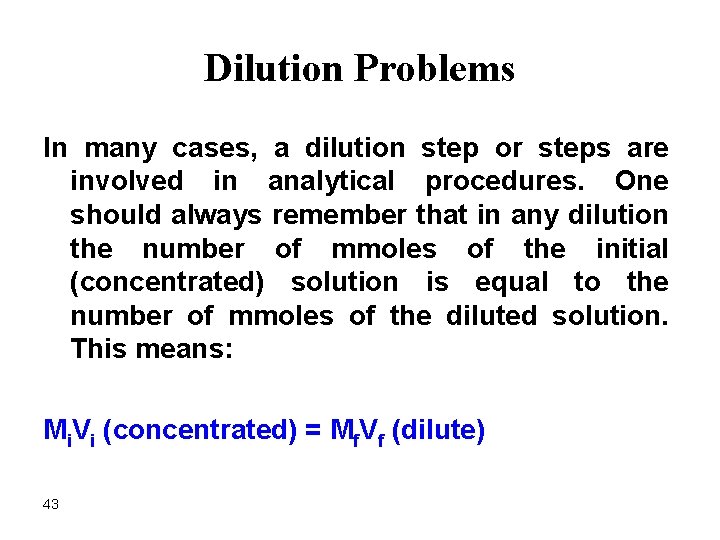
Dilution Problems In many cases, a dilution step or steps are involved in analytical procedures. One should always remember that in any dilution the number of mmoles of the initial (concentrated) solution is equal to the number of mmoles of the diluted solution. This means: Mi. Vi (concentrated) = Mf. Vf (dilute) 43

Prepare 200 m. L of 0. 12 M KNO 3 solution from 0. 48 M solution. Mi. Vi (concentrated) = Mf. Vf (dilute) 0. 48 x Vm. L = 0. 12 x 200 Vm. L = (0. 12 x 200)/0. 48 = 50 m. L Therefore, 50 m. L of 0. 48 M KNO 3 should be diluted to 200 m. L to obtain 0. 12 M solution 44

A 5. 0 g Mn sample was dissolved in 100 m. L water. If the percentage of Mn (At wt = 55 g/mol) in the sample is about 5%. What volume is needed to prepare 100 m. L of approximately 3. 0 x 10 -3 M solution. First we find approximate mol Mn in the sample = 5. 0 x (5/100) g Mn/(55 g/mol) = 4. 5 x 10 -3 mol Molarity of Mn solution = (4. 5 x 10 -3 x 103 mmol)/100 m. L = 4. 5 x 10 -2 M 45

The problem can now be solved easily using the dilution relation Mi. Vi (concentrated) = Mf. Vf (dilute) 4. 5 x 10 -2 x Vm. L = 3. 0 x 10 -3 x 100 Vm. L = (3. 0 x 10 -3 x 100)/4. 5 x 10 -2 = 6. 7 m. L Therefore, about 6. 7 m. L of the Mn sample should be diluted to obtain an approximate concentration of 3. 0 x 10 -3 M solution. 46

What volume of 0. 4 M Ba(OH)2 should be added to 50 m. L of 0. 30 M Na. OH in order to obtain a solution that is 0. 5 M in OH-. We have to be able to see that the mmol OH- coming from Ba(OH)2 and Na. OH will equal the number of mmol of OH- in the final solution, which is mmol OH- from Ba(OH)2 + mmol OH- from Na. OH = mmol OH- in final solution 47

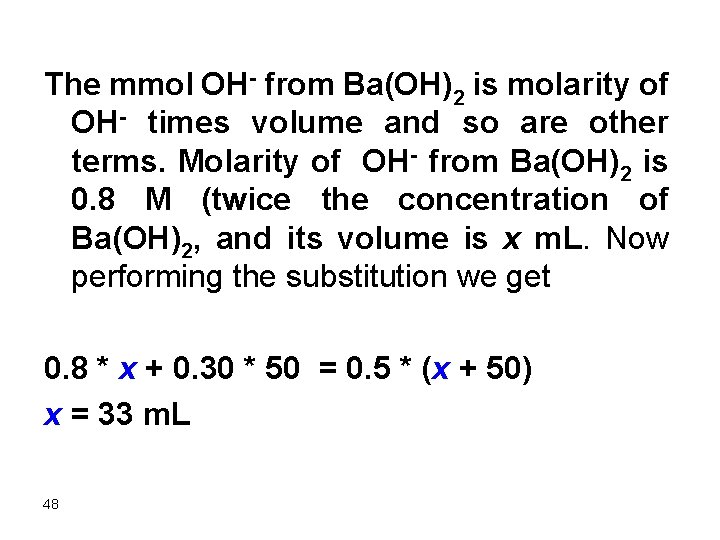
The mmol OH- from Ba(OH)2 is molarity of OH- times volume and so are other terms. Molarity of OH- from Ba(OH)2 is 0. 8 M (twice the concentration of Ba(OH)2, and its volume is x m. L. Now performing the substitution we get 0. 8 * x + 0. 30 * 50 = 0. 5 * (x + 50) x = 33 m. L 48

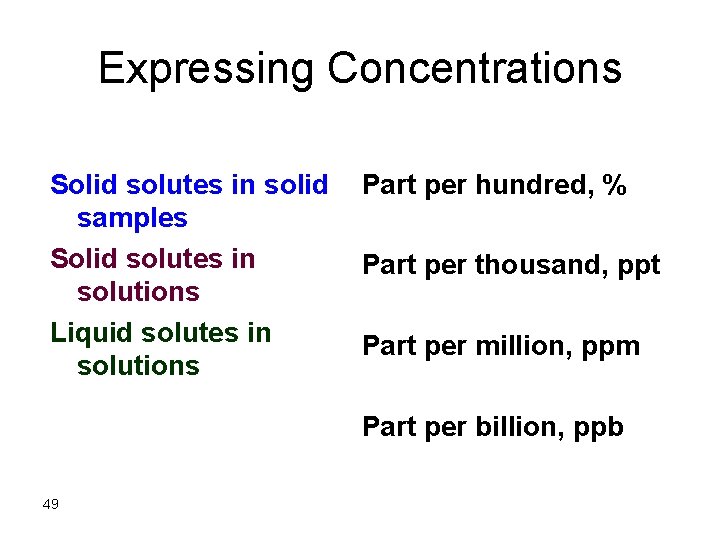
Expressing Concentrations Solid solutes in solid Part per hundred, % samples Solid solutes in Part per thousand, ppt solutions Liquid solutes in Part per million, ppm solutions Part per billion, ppb 49
![For Solid Solutes in solid samples ww weight solute gweight sample g For Solid Solutes in solid samples % (w/w) = [weight solute (g)/weight sample (g)]](https://slidetodoc.com/presentation_image/5e30071740f2a8e6b2777a9320534eb9/image-50.jpg)
For Solid Solutes in solid samples % (w/w) = [weight solute (g)/weight sample (g)] x 100 ppt (w/w) = [weight solute (g)/weight sample (g)] x 1000 ppm (w/w) = [weight solute (g)/weight sample (g)] x 106 ppb (w/w) = [weight solute (g)/weight sample (g)] x 109 A ppm can be represented by several terms like the one above, (mg solute/kg sample), ( g solute/106 g sample), etc. . 50

If the solute is dissolved in solution we have % (w/v) = [weight solute (g)/volume sample (m. L)] x 100 ppt (w/v) = [weight solute (g)/volume sample (m. L)] x 1000 ppm (w/v) = [weight solute (g)/volume sample (m. L)] x 106 ppb (w/v) = [weight solute (g)/volume sample (m. L)] x 109 Also a ppm can be expressed as above or as (g solute/106 m. L solution), (mg solute/L solution), or (mg/m. L), etc. . 51
![For Liquid Solutes vv volume solute m Lvolume sample m L x For Liquid Solutes % (v/v) = [volume solute (m. L)/volume sample (m. L)] x](https://slidetodoc.com/presentation_image/5e30071740f2a8e6b2777a9320534eb9/image-52.jpg)
For Liquid Solutes % (v/v) = [volume solute (m. L)/volume sample (m. L)] x 100 ppt (v/v) = [volume solute (m. L)/volume sample (m. L)] x 1000 ppm (v/v) = [volume solute (m. L)/volume sample (m. L)] x 106 ppb (v/v) = [volume solute (m. L)/volume sample (m. L)] x 109 A ppm can be expressed as above or as (m. L/L), (m. L/103 L), etc. . 52

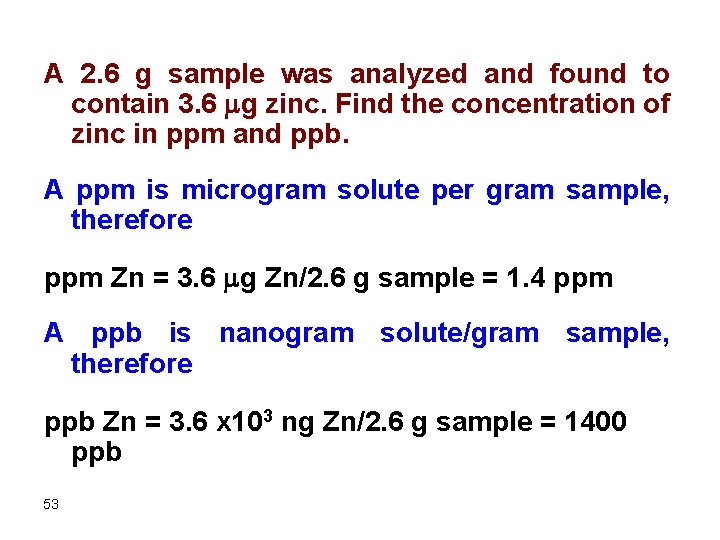
A 2. 6 g sample was analyzed and found to contain 3. 6 mg zinc. Find the concentration of zinc in ppm and ppb. A ppm is microgram solute per gram sample, therefore ppm Zn = 3. 6 mg Zn/2. 6 g sample = 1. 4 ppm A ppb is nanogram solute/gram sample, therefore ppb Zn = 3. 6 x 103 ng Zn/2. 6 g sample = 1400 ppb 53

A 25. 0 m. L sample was found to contain 26. 7 mg glucose. Express the concentration as ppm and mg/d. L glucose. Solution A ppm is defined as mg/m. L, therefore ppm = 26. 7 mg/(25. 0 x 10 -3 m. L) = 1. 07 x 103 ppm 54

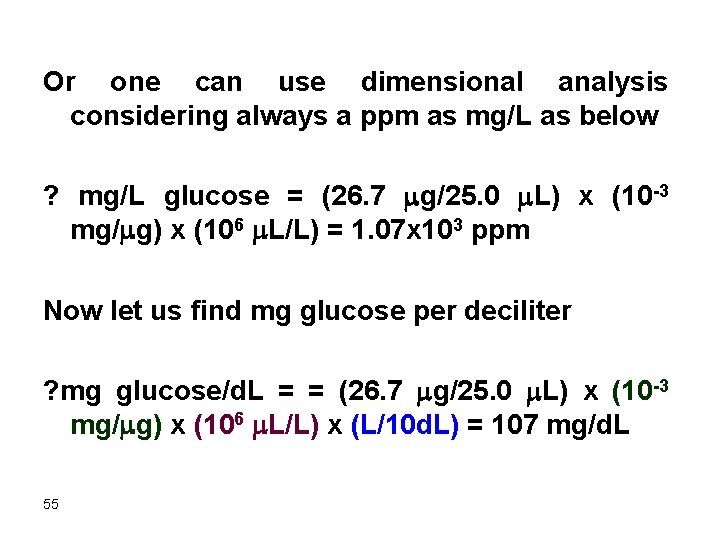
Or one can use dimensional analysis considering always a ppm as mg/L as below ? mg/L glucose = (26. 7 mg/25. 0 m. L) x (10 -3 mg/mg) x (106 m. L/L) = 1. 07 x 103 ppm Now let us find mg glucose per deciliter ? mg glucose/d. L = = (26. 7 mg/25. 0 m. L) x (10 -3 mg/mg) x (106 m. L/L) x (L/10 d. L) = 107 mg/d. L 55

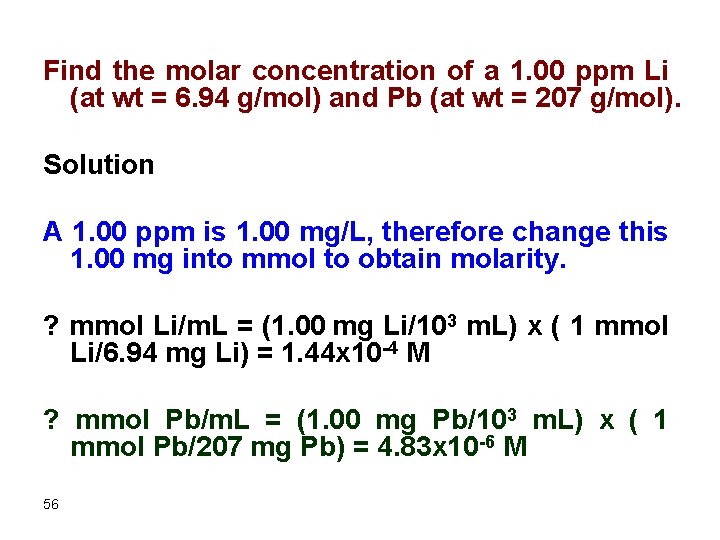
Find the molar concentration of a 1. 00 ppm Li (at wt = 6. 94 g/mol) and Pb (at wt = 207 g/mol). Solution A 1. 00 ppm is 1. 00 mg/L, therefore change this 1. 00 mg into mmol to obtain molarity. ? mmol Li/m. L = (1. 00 mg Li/103 m. L) x ( 1 mmol Li/6. 94 mg Li) = 1. 44 x 10 -4 M ? mmol Pb/m. L = (1. 00 mg Pb/103 m. L) x ( 1 mmol Pb/207 mg Pb) = 4. 83 x 10 -6 M 56

Find the number of mg Na 2 CO 3 (FW = 106 g/mol) required to prepare 500 m. L of 9. 20 ppm Na solution. The idea is to find mg sodium ( 23. 0 mg/mmol) required and then get the mmoles sodium and relate it to mmoles sodium carbonate followed by calculation of the weight of sodium carbonate. ? mg Na = 9. 20 mg/L x 0. 5 L = 4. 6 mg Na mmol Na = 4. 60 mg Na/23. 0 mg/mmol Na 2 CO 3 = 2 Na+ 57

mmol Na 2 CO 3 = 1/2 * mmol Na = 4. 60/46. 0 mmol = 0. 100 ? mg Na 2 CO 3 = 0. 100 mmol Na 2 CO 3 x (106 mg Na 2 CO 3/ mmol Na 2 CO 3) = 10. 6 mg One can work such a problem in one step as below ? mg Na 2 CO 3 = (9. 2 mg Na/1000 m. L) x 500 m. L x (1 mmol Na/23. 0 mg Na) x (1 mmol Na 2 CO 3/2 mmol Na) x (106 mg Na 2 CO 3/1 mmol Na 2 CO 3) = 10. 6 mg 58

Lecture 11 Stoichiometric Calculations, cont…. . Volumetric Analysis 59

Stoichiometric Calculations: Volumetric Analysis In this section we look at calculations involved in titration processes as well as general quantitative reactions. In a volumetric titration, an analyte of unknown concentration is titrated with a standard in presence of a suitable indicator. For a reaction to be used in titration the following characteristics should be satisfied: 60

1. The stoichiometry of the reaction should be exactly known. This means that we should know the number of moles of A reacting with 1 mole of B. 2. The reaction should be rapid and reaction between A and B should occur immediately and instantly after addition of each drop of titrant (the solution in the burette). 3. There should be no side reactions. A reacts with B only. 61

4. The reaction should be quantitative. A reacts completely with B. 5. There should exist a suitable indicator which has distinct color change. 6. There should be very good agreement between the equivalence point (theoretical) and the end point (experimental). This means that Both points should occur at the same volume of titrant or at most a very close volume. 62

Standard solutions A standard solution is a solution of known and exactly defined concentration. Usually standards are classified as either primary standards or secondary standards. There are not too many secondary standards available to analysts and standardization of other substances is necessary to prepare secondary standards. A primary standard should have the following properties: 63

1. Should have a purity of at least 99. 98% 2. Stable to drying, a necessary step to expel adsorbed water molecules before weighing 3. Should have high formula weight as the uncertainty in weight is decreased when weight is increased 4. Should be non hygroscopic 5. Should possess the same properties as that required for a titration 64

Remember!! Na. OH and HCl are not primary standards and therefore should be standardized using a primary or secondary standard. Na. OH absorbs CO 2 from air, highly hygroscopic, and usually of low purity. HCl and other acid in solution are not standards as the percentage written on the reagent bottle is a claimed value and should not be taken as guaranteed. 65

Molarity Volumetric Calculations Volumetric calculations involving molarity are rather simple. The way this information is presented in the text is not very helpful. Therefore, disregard and forget about all equations and relations listed in rectangles in the text, you will not need it. What you really need is to use the stoichiometry of the reaction to find how many mmol of A as compared to the number of mmoles of B. 66

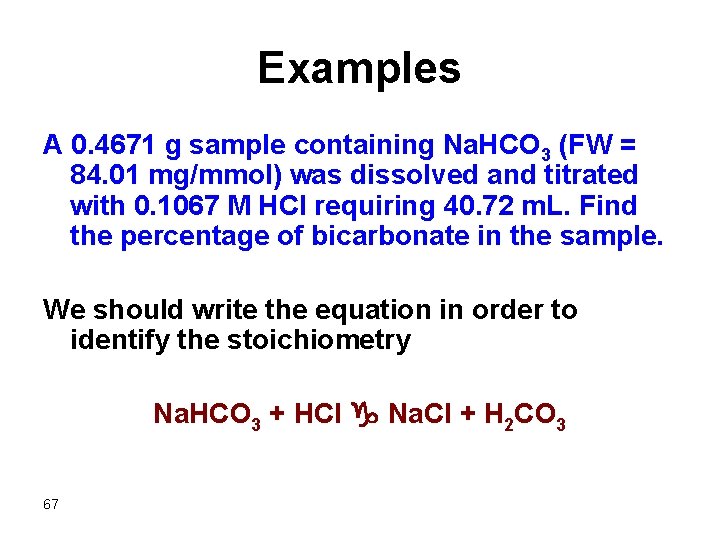
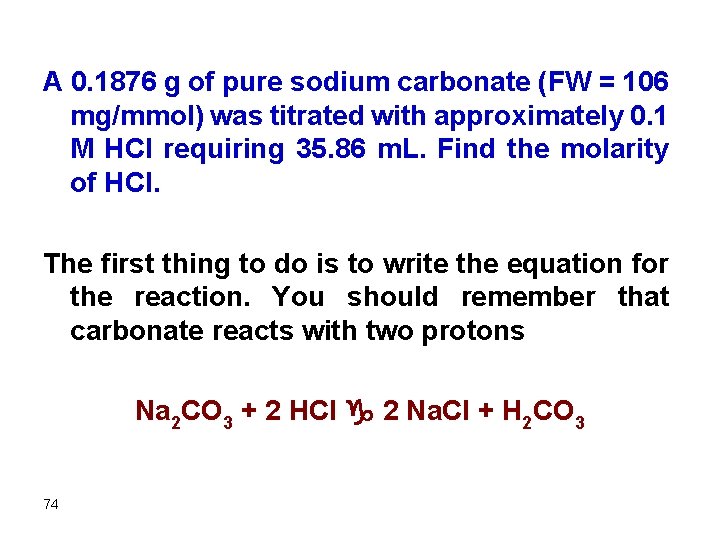
Examples A 0. 4671 g sample containing Na. HCO 3 (FW = 84. 01 mg/mmol) was dissolved and titrated with 0. 1067 M HCl requiring 40. 72 m. L. Find the percentage of bicarbonate in the sample. We should write the equation in order to identify the stoichiometry 67 Na. HCO 3 + HCl g Na. Cl + H 2 CO 3

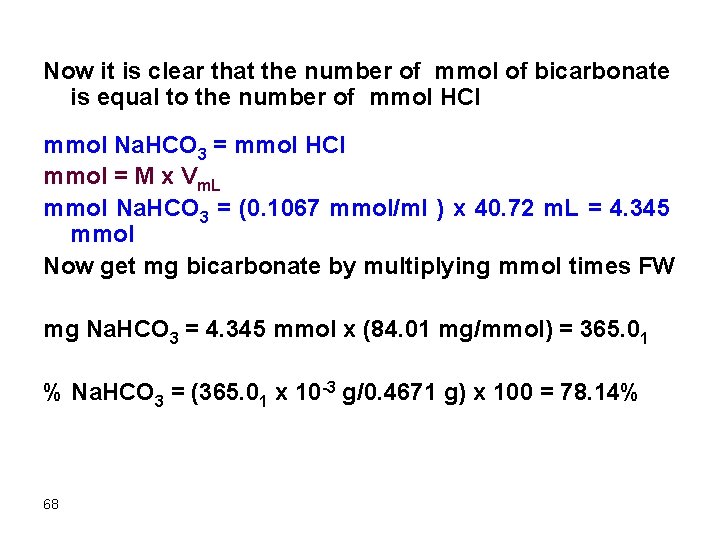
Now it is clear that the number of mmol of bicarbonate is equal to the number of mmol HCl mmol Na. HCO 3 = mmol HCl mmol = M x Vm. L mmol Na. HCO 3 = (0. 1067 mmol/ml ) x 40. 72 m. L = 4. 345 mmol Now get mg bicarbonate by multiplying mmol times FW mg Na. HCO 3 = 4. 345 mmol x (84. 01 mg/mmol) = 365. 01 % Na. HCO 3 = (365. 01 x 10 -3 g/0. 4671 g) x 100 = 78. 14% 68

We can use dimensional analysis to calculate the mg Na. HCO 3 directly then get the percentage as above. ? mg Na. HCO 3 = (0. 1067 mmol HCl/ml) x 40. 72 m. L x (mmol Na. HCO 3/mmol HCl) x (84. 01 mg Na. HCO 3/ mmol Na. HCO 3) = 365. 0 mg 69

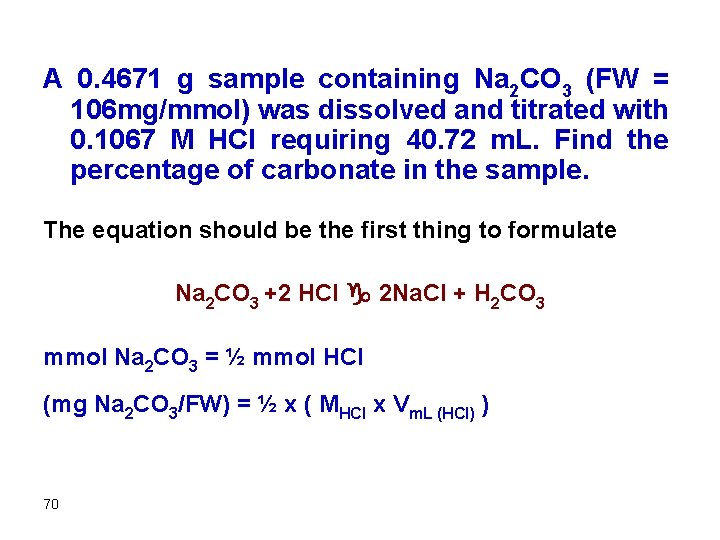
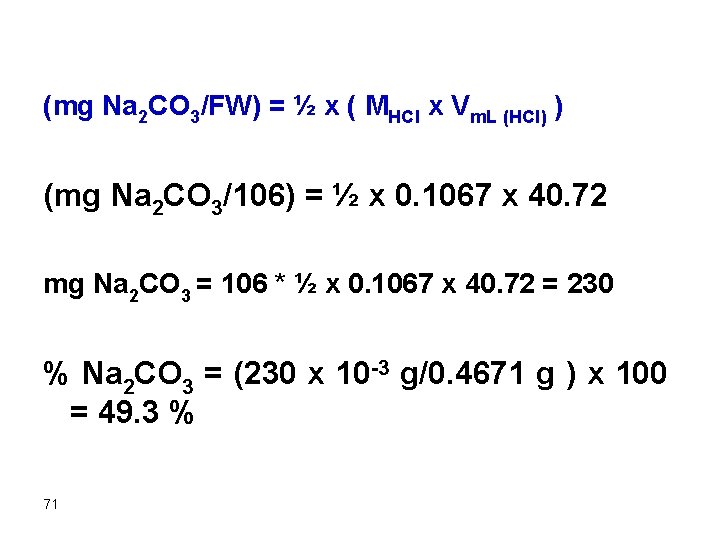
A 0. 4671 g sample containing Na 2 CO 3 (FW = 106 mg/mmol) was dissolved and titrated with 0. 1067 M HCl requiring 40. 72 m. L. Find the percentage of carbonate in the sample. The equation should be the first thing to formulate Na 2 CO 3 +2 HCl g 2 Na. Cl + H 2 CO 3 mmol Na 2 CO 3 = ½ mmol HCl (mg Na 2 CO 3/FW) = ½ x ( MHCl x Vm. L (HCl) ) 70

(mg Na 2 CO 3/FW) = ½ x ( MHCl x Vm. L (HCl) ) (mg Na 2 CO 3/106) = ½ x 0. 1067 x 40. 72 mg Na 2 CO 3 = 106 * ½ x 0. 1067 x 40. 72 = 230 % Na 2 CO 3 = (230 x 10 -3 g/0. 4671 g ) x 100 = 49. 3 % 71

How many m. L of 0. 25 M Na. OH will react with 5. 0 m. L of 0. 10 M H 2 SO 4 + 2 Na. OH g Na 2 SO 4 + 2 H 2 O mmol Na. OH = 2 mmol H 2 SO 4 MNa. OH x Vm. L(Na. OH) = 2 {M(H 2 SO 4) x Vm. L(H 2 SO 4)} 0. 25 x Vm. L = 2 x 0. 10 x 5. 0 Vm. L = 4. 0 m. L 72

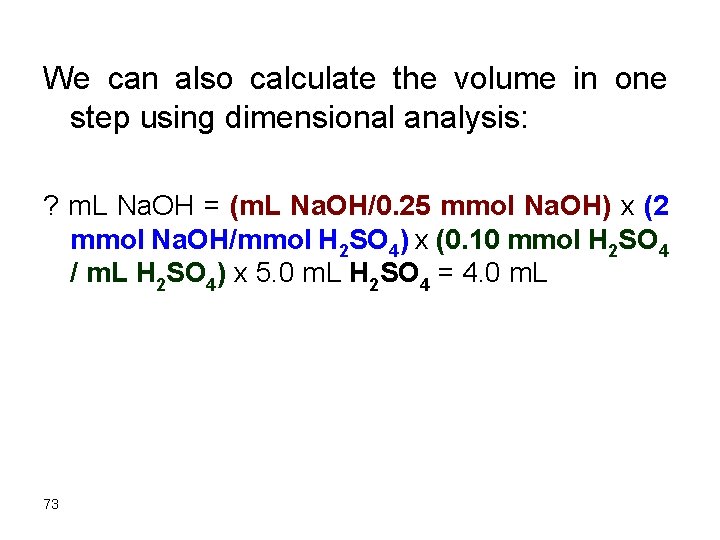
We can also calculate the volume in one step using dimensional analysis: ? m. L Na. OH = (m. L Na. OH/0. 25 mmol Na. OH) x (2 mmol Na. OH/mmol H 2 SO 4) x (0. 10 mmol H 2 SO 4 / m. L H 2 SO 4) x 5. 0 m. L H 2 SO 4 = 4. 0 m. L 73

A 0. 1876 g of pure sodium carbonate (FW = 106 mg/mmol) was titrated with approximately 0. 1 M HCl requiring 35. 86 m. L. Find the molarity of HCl. The first thing to do is to write the equation for the reaction. You should remember that carbonate reacts with two protons Na 2 CO 3 + 2 HCl g 2 Na. Cl + H 2 CO 3 74

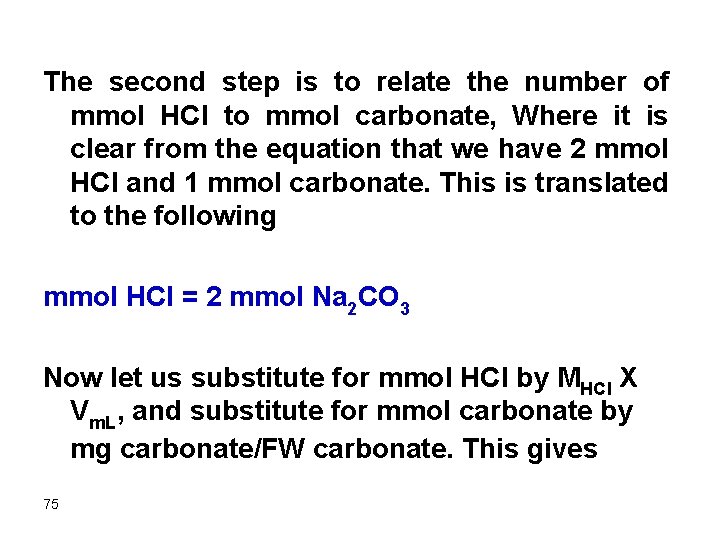
The second step is to relate the number of mmol HCl to mmol carbonate, Where it is clear from the equation that we have 2 mmol HCl and 1 mmol carbonate. This is translated to the following mmol HCl = 2 mmol Na 2 CO 3 Now let us substitute for mmol HCl by MHCl X Vm. L, and substitute for mmol carbonate by mg carbonate/FW carbonate. This gives 75

MHCl x 35. 86 = 2 * 187. 6 mg/ (106 mg/mmol) MHCl = 0. 09872 M The same result can be obtained using dimensional analysis in one single step: ? mmol HCl/m. L = (187. 6 mg Na 2 CO 3 /35. 86 m. L HCl) x ( mmol Na 2 CO 3/106 mg Na 2 CO 3) x (2 mmol HCl/mmol Na 2 CO 3) = 0. 09872 M 76

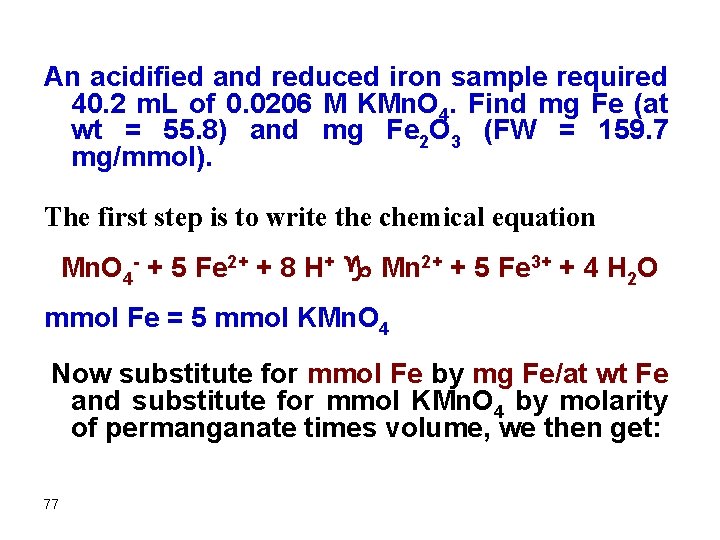
An acidified and reduced iron sample required 40. 2 m. L of 0. 0206 M KMn. O 4. Find mg Fe (at wt = 55. 8) and mg Fe 2 O 3 (FW = 159. 7 mg/mmol). The first step is to write the chemical equation Mn. O 4 - + 5 Fe 2+ + 8 H+ g Mn 2+ + 5 Fe 3+ + 4 H 2 O mmol Fe = 5 mmol KMn. O 4 Now substitute for mmol Fe by mg Fe/at wt Fe and substitute for mmol KMn. O 4 by molarity of permanganate times volume, we then get: 77
![mg Fe55 8 mgmmol 5 x 0 0206 mmolm L x 40 [mg Fe/(55. 8 mg/mmol)] = 5 x (0. 0206 mmol/m. L) x 40.](https://slidetodoc.com/presentation_image/5e30071740f2a8e6b2777a9320534eb9/image-78.jpg)
[mg Fe/(55. 8 mg/mmol)] = 5 x (0. 0206 mmol/m. L) x 40. 2 m. L mg Fe = 231 mg This can also be done in a single step as follows: ? mg Fe = (0. 0206 mmol KMn. O 4 /m. L) x 40. 2 m. L x (5 mmol Fe/mmol KMn. O 4) x (55. 8 mg Fe/mmol Fe) = 231 mg 78

To calculate the mg Fe 2 O 3 we set the following: 2 Fe g Fe 2 O 3 mmol Fe = 2 mmol Fe 2 O 3 Substitute for mmol Fe in equation 1 2* [mg Fe 2 O 3/ (159. 7 mg/mmol)] = 5 x (0. 0206 mmol/m. L) x 40. 2 m. L mg Fe 2 O 3 = 331 mg The last step can also be done using dimensional analysis as follows: ? mg Fe 2 O 3 = (0. 0206 mmol KMn. O 4 /m. L) x 40. 2 m. L x (5 mmol Fe/mmol KMn. O 4) x (mmol Fe 2 O 3 /2 mmol Fe) x ( 159. 7 mg Fe 2 O 3/mmol Fe 2 O 3) = 331 mg 79

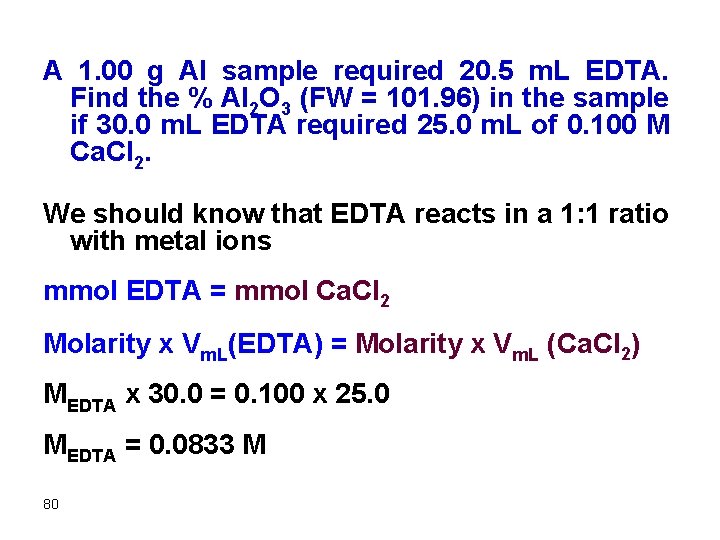
A 1. 00 g Al sample required 20. 5 m. L EDTA. Find the % Al 2 O 3 (FW = 101. 96) in the sample if 30. 0 m. L EDTA required 25. 0 m. L of 0. 100 M Ca. Cl 2. We should know that EDTA reacts in a 1: 1 ratio with metal ions mmol EDTA = mmol Ca. Cl 2 Molarity x Vm. L(EDTA) = Molarity x Vm. L (Ca. Cl 2) MEDTA x 30. 0 = 0. 100 x 25. 0 MEDTA = 0. 0833 M 80

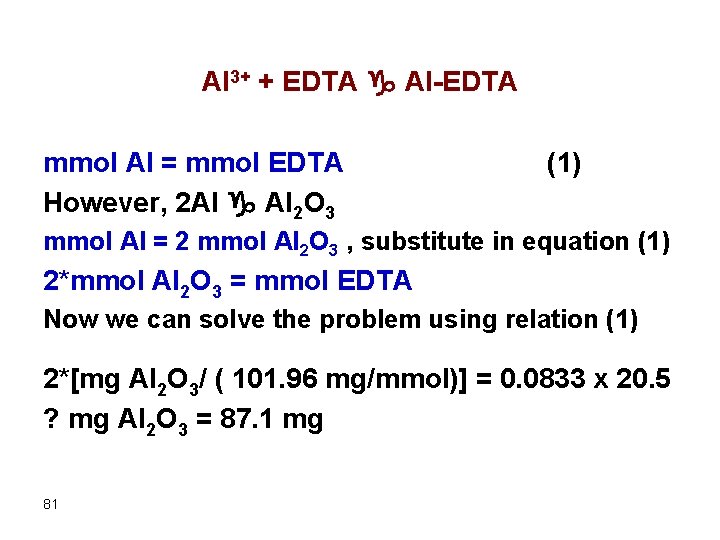
Al 3+ + EDTA g Al-EDTA mmol Al = mmol EDTA However, 2 Al g Al 2 O 3 (1) mmol Al = 2 mmol Al 2 O 3 , substitute in equation (1) 2*mmol Al 2 O 3 = mmol EDTA Now we can solve the problem using relation (1) 2*[mg Al 2 O 3/ ( 101. 96 mg/mmol)] = 0. 0833 x 20. 5 ? mg Al 2 O 3 = 87. 1 mg 81

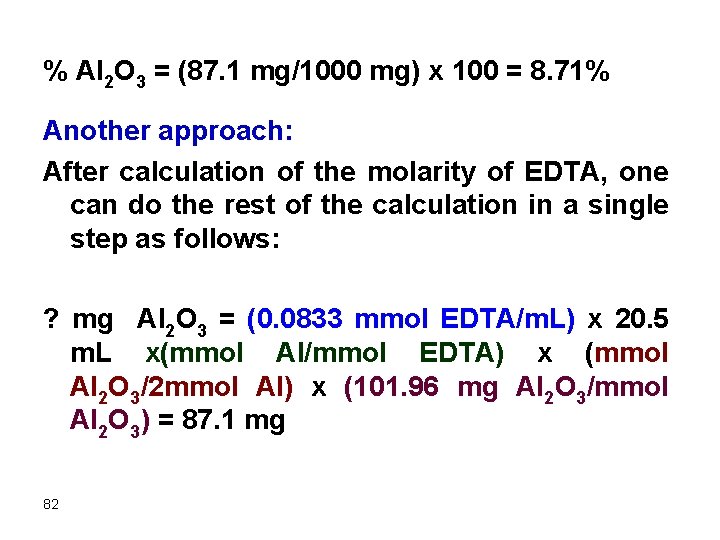
% Al 2 O 3 = (87. 1 mg/1000 mg) x 100 = 8. 71% Another approach: After calculation of the molarity of EDTA, one can do the rest of the calculation in a single step as follows: ? mg Al 2 O 3 = (0. 0833 mmol EDTA/m. L) x 20. 5 m. L x(mmol Al/mmol EDTA) x (mmol Al 2 O 3/2 mmol Al) x (101. 96 mg Al 2 O 3/mmol Al 2 O 3) = 87. 1 mg 82

Lecture 12 Stoichiometric Calculations, Cont…. . Back Titrations

We have seen in previous sections that correct solution of any problem involving reactions between two substances requires setting up two important relations: 1. Writing a balanced chemical equation representing stoichiometric relationships. 2. Formulating a relationship between the number of mmol of substance A and mmol of substance B. 3. The last step in the calculation is to substitute for the mmol A or B by either one of the following according to given information: mmol = M x Vm. L mmol = mg/FW 84

Find the volume of 0. 100 M KMn. O 4 that will react with 50. 0 m. L of 0. 200 M H 2 O 2 according to the following equation: 5 H 2 O 2 + 2 KMn. O 4 + 6 H+ g 2 Mn 2+ + 5 O 2 + 8 H 2 O Solution We have the equation ready therefore the following step is to formulate the relationship between the number of moles of the two reactants. We always start with the one we want to calculate, that is 85

mmol KMn. O 4 = 2/5 mmol H 2 O 2 It is clear that we should substitute M x Vm. L for mmol in both substances as this information is given to us. 0. 100 x Vm. L = (2/5) x 0. 200 x 50. 0 Vm. L = 40. 0 m. L KMn. O 4 86

Find the volume of 0. 100 M KMn. O 4 that will react with 50. 0 m. L of 0. 200 M Mn. SO 4 according to the following equation: 3 Mn. SO 4 + 2 KMn. O 4 + 4 OH- g 5 Mn. O 2 + 2 H 2 O + 2 K+ + 3 SO 42 Again, we have the equation ready therefore we turn to formulating a relationship for the number of mmol for both substances. mmol KMn. O 4 = 2/3 mmol Mn. SO 4 87

mmol KMn. O 4 = 2/3 mmol Mn. SO 4 It is clear that we should substitute M x Vm. L for mmol in both substances as this information is given to us. 0. 100 x Vm. L = 2/3 x 0. 200 x 50. 0 Vm. L = 66. 7 m. L KMn. O 4 88

Find the volume of 0. 100 M KMn. O 4 that will react with 500 mg of H 2 C 2 O 4 (FW = 90. 0 mg/mmol) according to the following equation: 5 H 2 C 2 O 4 + 2 KMn. O 4 + 6 H+ g 10 CO 2 + 2 Mn 2++ 8 H 2 O + 2 K+ Solution We have the equation ready therefore we turn to formulating a relationship for the number of mmol for both substances. mmol KMn. O 4 = 2/5 mmol H 2 C 2 O 4 89

mmol KMn. O 4 = 2/5 mmol H 2 C 2 O 4 It is clear that we should substitute M x Vm. L for mmol permanganate while substitute for mmol oxalic acid by mg/FW. This gives: 0. 100 x Vm. L = 2/5 x 500 mg / (90. 0 mg/mmol) Vm. L = 22. 2 m. L KMn. O 4 90

Back-Titrations In this technique, an accurately known amount of a reagent is added to analyte in such a way that some excess of the added reagent is left. This excess is then titrated to determine its amount and thus: mmol reagent taken = mmol reagent reacted with analyte + mmol reagent titrated Therefore, the analyte can be determined since we know mmol reagent added and mmol reagent titrated. 91

mmol reagent reacted = mmol reagent taken – mmol reagent titrated Finally, the number of mmol reagent reacted can be related to the number of mmol analyte from the stoichiometry of the reaction between the two substances. 92


Why Do We Use Back-Titrations? Back-titrations are important especially in some situations like: 1. When the titration reaction is slow. Addition of an excess reagent will force the reaction to proceed faster. 2. When the titration reaction lacks a good indicator. We will see details of this later. 3. When the analyte is not very stable. Addition of excess reagent will finish the analyte instantly thus overcoming stability problems. 94

Examples A 2. 63 g Cr(III) sample was dissolved analyzed by addition of 5. 00 m. L of 0. 0103 M EDTA. The excess EDTA required 1. 32 m. L of 0. 0112 M Zn(II). Calculate % Cr. Cl 3 (FW=158. 4 mg/mmol) in the sample. Solution We should remember that EDTA reacts in a 1: 1 ratio. The first step in the calculation is to find the mmol EDTA reacted from the relation: mmol EDTA reacted = mmol EDTA taken – mmol EDTA titrated 95

We substitute for both mmol EDTA taken and mmol EDTA titrated by M x Vm. L for each. Also since EDTA reacts in a 1: 1 ratio, we can state the following: mmol EDTA reacted = mmol Cr. Cl 3. mmol EDTA titrated = mmol Zn(II). Therefore, we now have the following reformulated relation: mmol Cr. Cl 3 = mmol EDTA taken – mmol Zn(II) Substitution gives: 96

mmol Cr. Cl 3 = 0. 0103 x 5. 00 – 0. 0112 x 1. 32 mmol Cr. Cl 3 = 0. 0367 mmol We can then find the number of mg Cr. Cl 3 by multiplying mmol times FW. mg Cr. Cl 3 = 0. 0367 mmol x 158. 4 mg/mmol = 5. 81 mg % Cr. Cl 3 = (5. 81 mg/2. 63 x 103 mg) x 100 = 0. 221% 97

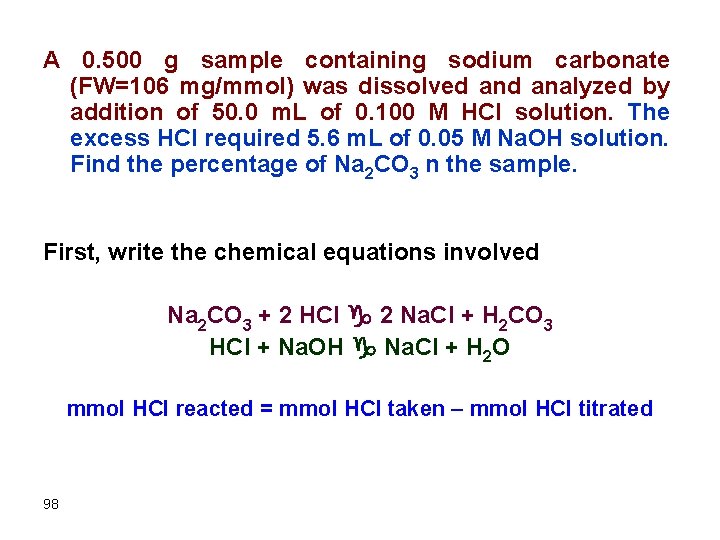
A 0. 500 g sample containing sodium carbonate (FW=106 mg/mmol) was dissolved analyzed by addition of 50. 0 m. L of 0. 100 M HCl solution. The excess HCl required 5. 6 m. L of 0. 05 M Na. OH solution. Find the percentage of Na 2 CO 3 n the sample. First, write the chemical equations involved Na 2 CO 3 + 2 HCl g 2 Na. Cl + H 2 CO 3 HCl + Na. OH g Na. Cl + H 2 O mmol HCl reacted = mmol HCl taken – mmol HCl titrated 98

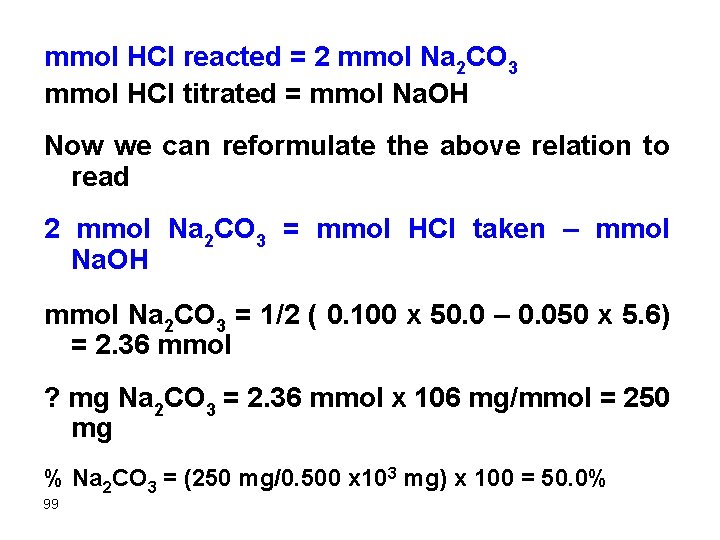
mmol HCl reacted = 2 mmol Na 2 CO 3 mmol HCl titrated = mmol Na. OH Now we can reformulate the above relation to read 2 mmol Na 2 CO 3 = mmol HCl taken – mmol Na. OH mmol Na 2 CO 3 = 1/2 ( 0. 100 x 50. 0 – 0. 050 x 5. 6) = 2. 36 mmol ? mg Na 2 CO 3 = 2. 36 mmol x 106 mg/mmol = 250 mg % Na 2 CO 3 = (250 mg/0. 500 x 103 mg) x 100 = 50. 0% 99

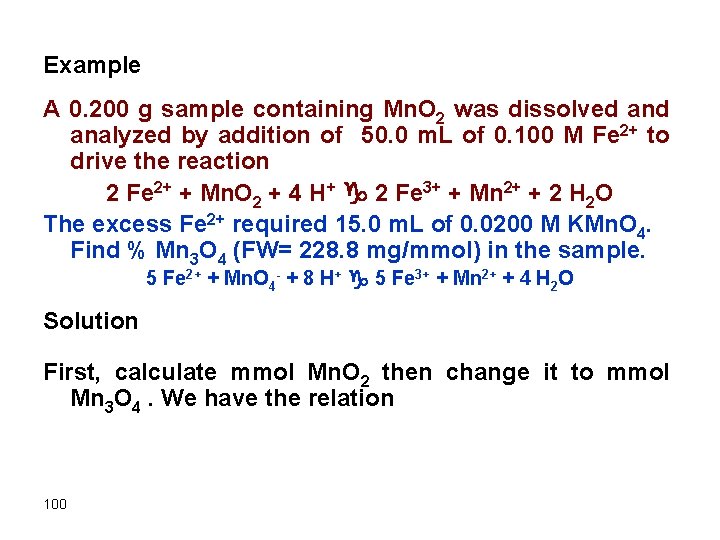
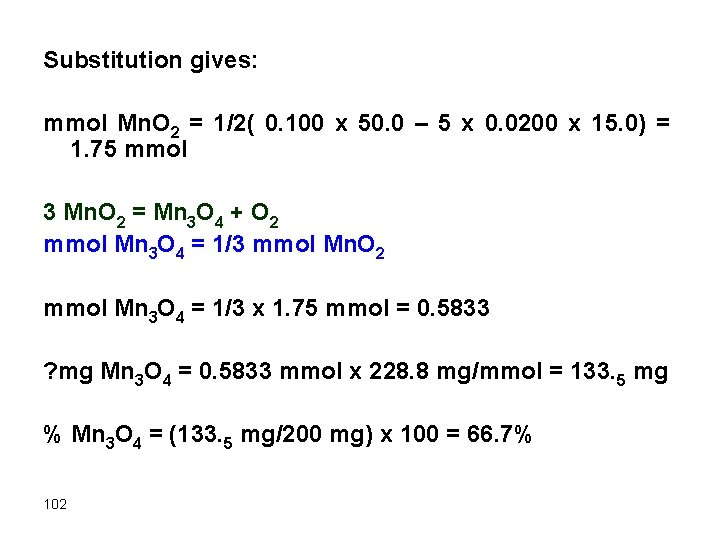
Example A 0. 200 g sample containing Mn. O 2 was dissolved analyzed by addition of 50. 0 m. L of 0. 100 M Fe 2+ to drive the reaction 2 Fe 2+ + Mn. O 2 + 4 H+ g 2 Fe 3+ + Mn 2+ + 2 H 2 O The excess Fe 2+ required 15. 0 m. L of 0. 0200 M KMn. O 4. Find % Mn 3 O 4 (FW= 228. 8 mg/mmol) in the sample. 5 Fe 2+ + Mn. O 4 - + 8 H+ g 5 Fe 3+ + Mn 2+ + 4 H 2 O Solution First, calculate mmol Mn. O 2 then change it to mmol Mn 3 O 4. We have the relation 100

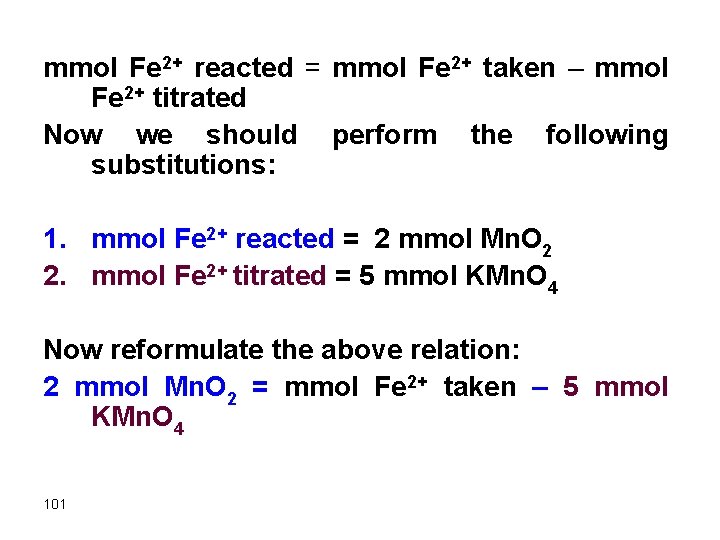
mmol Fe 2+ reacted = mmol Fe 2+ taken – mmol Fe 2+ titrated Now we should perform the following substitutions: 1. mmol Fe 2+ reacted = 2 mmol Mn. O 2 2. mmol Fe 2+ titrated = 5 mmol KMn. O 4 Now reformulate the above relation: 2 mmol Mn. O 2 = mmol Fe 2+ taken – 5 mmol KMn. O 4 101

Substitution gives: mmol Mn. O 2 = 1/2( 0. 100 x 50. 0 – 5 x 0. 0200 x 15. 0) = 1. 75 mmol 3 Mn. O 2 = Mn 3 O 4 + O 2 mmol Mn 3 O 4 = 1/3 mmol Mn. O 2 mmol Mn 3 O 4 = 1/3 x 1. 75 mmol = 0. 5833 ? mg Mn 3 O 4 = 0. 5833 mmol x 228. 8 mg/mmol = 133. 5 mg % Mn 3 O 4 = (133. 5 mg/200 mg) x 100 = 66. 7% 102

Normality volumetric Calculations We have seen previously that solving volumetric problems required setting up a relation between the number of mmoles or reacting species. In case of normality, calculation is easier as we always have the number of milli equivalents (meq) of substance A is equal to meq of substance B, regardless of the stoichiometry in the chemical equation. Of course this is because the number of moles involved in the reaction is accounted for in the calculation of meqs. Therefore, the first step in a calculation using normalities is to write down the relation meq A = meq B 103

The next step is to substitute for meq by one of the following relations meq = N x Vm. L meq = mg/eq wt meq = n x mmol We should remember from previous lectures that: eq wt = FW/n N = n x M Therefore, change from molarity or number of moles to normality or number of equivalents using the above relations. 104

Examples A 0. 4671 g sample containing sodium bicarbonate was titrated with HCl requiring 40. 72 m. L. The acid was standardized by titrating 0. 1876 g of sodium carbonate (FW = 106 mg/mmol) requiring 37. 86 m. L of the acid. Find the percentage of Na. HCO 3 (FW=84. 0 mg/mmol) in the sample. This problem was solved previously using molarity. The point here is to use normalities in order to practice and learn how to use the meq concept. First, let us find the normality of the acid from reaction with carbonate (reacts with two protons as you know). 105

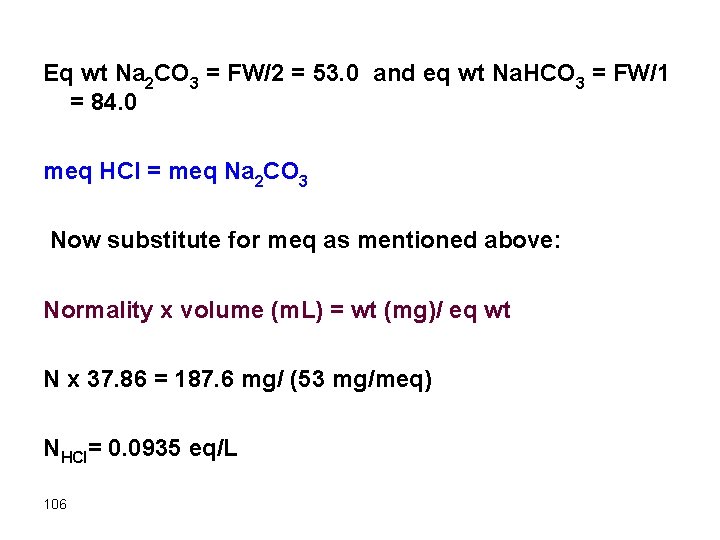
Eq wt Na 2 CO 3 = FW/2 = 53. 0 and eq wt Na. HCO 3 = FW/1 = 84. 0 meq HCl = meq Na 2 CO 3 Now substitute for meq as mentioned above: Normality x volume (m. L) = wt (mg)/ eq wt N x 37. 86 = 187. 6 mg/ (53 mg/meq) NHCl= 0. 0935 eq/L 106

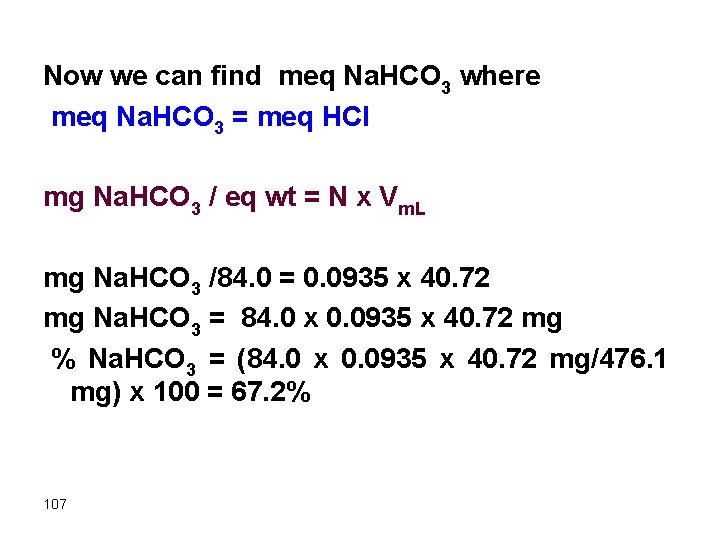
Now we can find meq Na. HCO 3 where meq Na. HCO 3 = meq HCl mg Na. HCO 3 / eq wt = N x Vm. L mg Na. HCO 3 /84. 0 = 0. 0935 x 40. 72 mg Na. HCO 3 = 84. 0 x 0. 0935 x 40. 72 mg % Na. HCO 3 = (84. 0 x 0. 0935 x 40. 72 mg/476. 1 mg) x 100 = 67. 2% 107

Lecture 13 Stoichiometric Calculations, cont…. Back to Normality Calculations

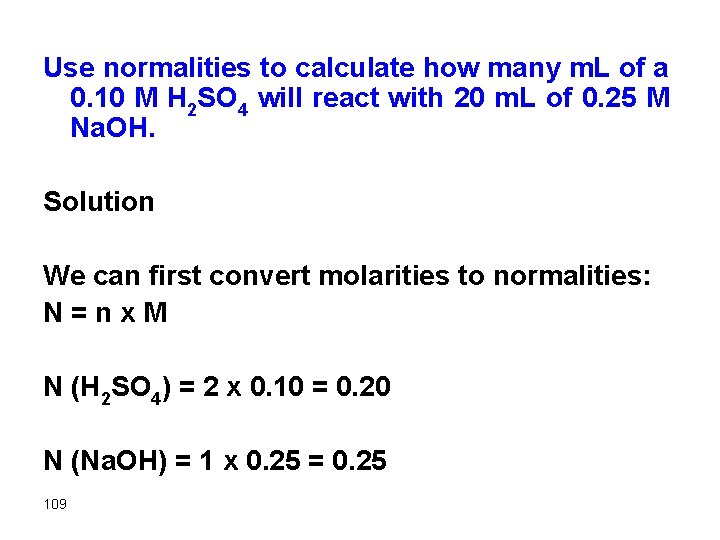
Use normalities to calculate how many m. L of a 0. 10 M H 2 SO 4 will react with 20 m. L of 0. 25 M Na. OH. Solution We can first convert molarities to normalities: N = n x M N (H 2 SO 4) = 2 x 0. 10 = 0. 20 N (Na. OH) = 1 x 0. 25 = 0. 25 109

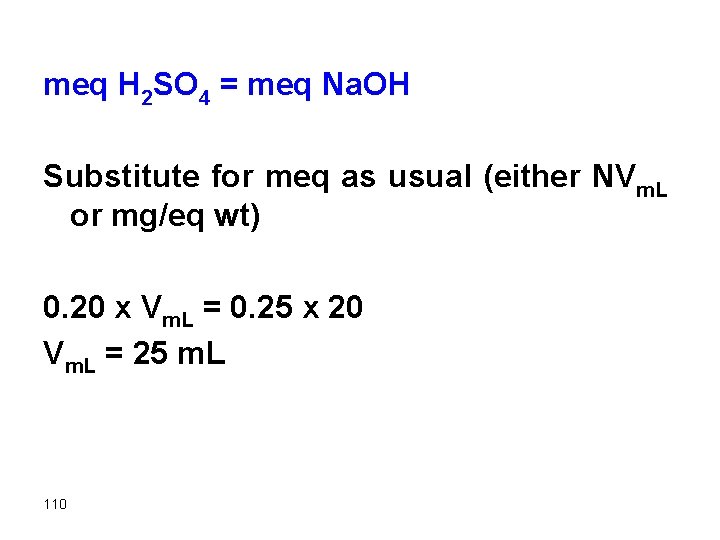
meq H 2 SO 4 = meq Na. OH Substitute for meq as usual (either NVm. L or mg/eq wt) 0. 20 x Vm. L = 0. 25 x 20 Vm. L = 25 m. L 110

Example Find the normality of sodium carbonate (FW = 106) in a solution containing 0. 212 g carbonate in 100 m. L solution if: The carbonate is used as a monobasic base. The carbonate is used as a dibasic base. Solution If carbonate is a monobasic base then eq wt = FW/1 = 106 mg/meq 111

To find the normality of the solution we find the weight per m. L and then convert the weight per m. L to meq/m. L. We have 212 mg/100 m. L which means 2. 12 mg/m. L. Now the point is how many meq per 2. 12 mg sodium carbonate. meq = mg/eq wt = 2. 12/106 = 0. 02 meq Then the normality is 0. 02 N If carbonate is to be used as a dibasic salt then the eq wt = FW/2 = 106/2 = 53 mg/meq. 112

To find the normality of the solution we find the weight per m. L and then convert the weight per m. L to meq/m. L. We have 212 mg/100 m. L which means 2. 12 mg/m. L. Now the point is how many meq per 2. 12 mg sodium carbonate. meq = mg/eq wt = 2. 12/53 = 0. 04 meq Then normality will be 0. 04 N 113

How many mg of I 2 (FW = 254 mg/mmol) should you weigh to prepare 250 m. L of 0. 100 N solution in the reaction: I 2 + 2 e g 2 ISolution To find the number of mg I 2 to be weighed and dissolved we get the meq required. We have: meq = N x Vm. L meq = 0. 100 x 250 = 25. 0 meq mg I 2 = meq x eq wt = meq x FW/2 = 25. 0 x 254/2 = 3. 18 x 103 mg 114

Find the normality of the solution containing 0. 25 g/L H 2 C 2 O 4 (FW = 90. 0 mg/mmol). Oxalic acid reacts as a diacidic substance. Solution Since oxalic acid acts as a diacidic substance then its eq wt = FW/2 = 90. 0/2 = 45. 0 mg/meq 115

We convert the mg acid per m. L to meq acid per m. L We have 0. 25 mg acid/m. L meq acid = 0. 25 mg/45. 0 mg/meq = 0. 0056 meq Therefore, the normality is 0. 0056 meq/m. L 116

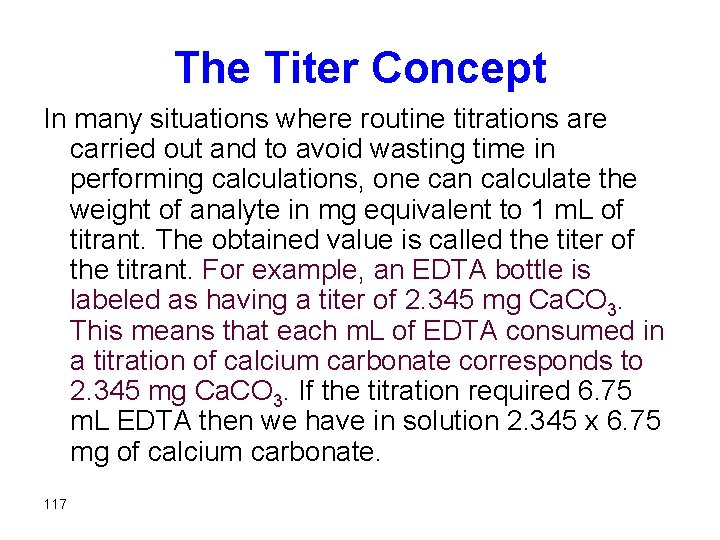
The Titer Concept In many situations where routine titrations are carried out and to avoid wasting time in performing calculations, one can calculate the weight of analyte in mg equivalent to 1 m. L of titrant. The obtained value is called the titer of the titrant. For example, an EDTA bottle is labeled as having a titer of 2. 345 mg Ca. CO 3. This means that each m. L of EDTA consumed in a titration of calcium carbonate corresponds to 2. 345 mg Ca. CO 3. If the titration required 6. 75 m. L EDTA then we have in solution 2. 345 x 6. 75 mg of calcium carbonate. 117

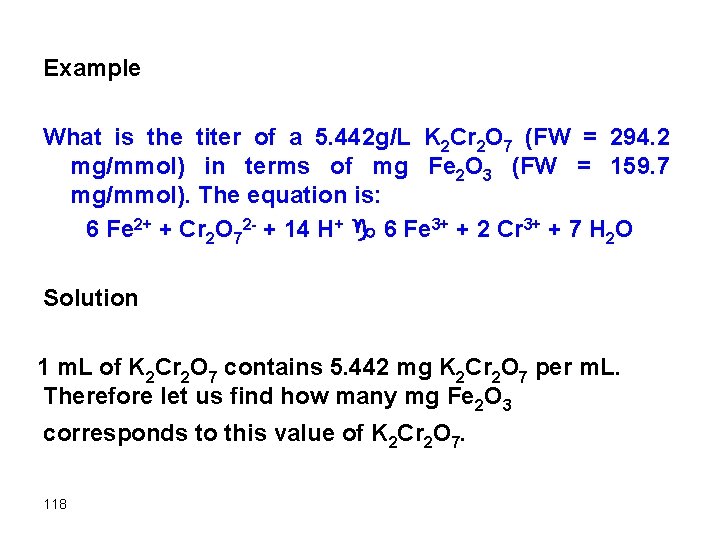
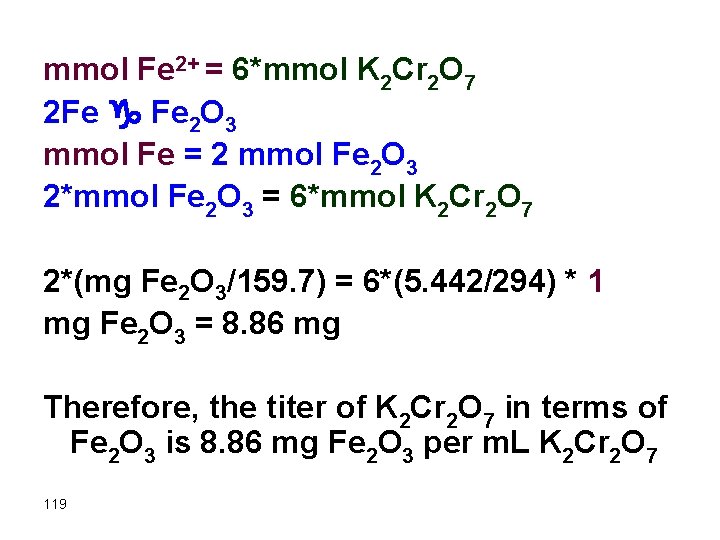
Example What is the titer of a 5. 442 g/L K 2 Cr 2 O 7 (FW = 294. 2 mg/mmol) in terms of mg Fe 2 O 3 (FW = 159. 7 mg/mmol). The equation is: 6 Fe 2+ + Cr 2 O 72 - + 14 H+ g 6 Fe 3+ + 2 Cr 3+ + 7 H 2 O Solution 1 m. L of K 2 Cr 2 O 7 contains 5. 442 mg K 2 Cr 2 O 7 per m. L. Therefore let us find how many mg Fe 2 O 3 corresponds to this value of K 2 Cr 2 O 7. 118

mmol Fe 2+ = 6*mmol K 2 Cr 2 O 7 2 Fe g Fe 2 O 3 mmol Fe = 2 mmol Fe 2 O 3 2*mmol Fe 2 O 3 = 6*mmol K 2 Cr 2 O 7 2*(mg Fe 2 O 3/159. 7) = 6*(5. 442/294) * 1 mg Fe 2 O 3 = 8. 86 mg Therefore, the titer of K 2 Cr 2 O 7 in terms of Fe 2 O 3 is 8. 86 mg Fe 2 O 3 per m. L K 2 Cr 2 O 7 119
 Mass to mass formula
Mass to mass formula Defining stoichiometry
Defining stoichiometry Stoichiometry and stoichiometric calculations
Stoichiometry and stoichiometric calculations Unit 1 review fundamental economic concepts answers
Unit 1 review fundamental economic concepts answers Types of connections in steel structures
Types of connections in steel structures What is stoichiometry
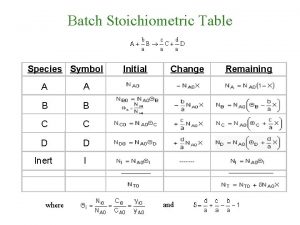
What is stoichiometry Stoichiometric table
Stoichiometric table Stoichiometric table for flow system
Stoichiometric table for flow system Stoichiometric gasoline
Stoichiometric gasoline Stoichiometric table for flow system
Stoichiometric table for flow system Me 322
Me 322 Stoichiometric table
Stoichiometric table Equivalence point definition
Equivalence point definition Stoichiometric table for flow system
Stoichiometric table for flow system Symbolaab
Symbolaab Mstockx
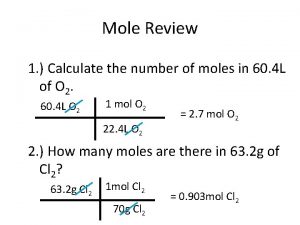
Mstockx Mole ratio examples
Mole ratio examples Stoichiometric table for reversible reaction
Stoichiometric table for reversible reaction Fundamental concepts in video
Fundamental concepts in video Basic concepts of simulation
Basic concepts of simulation What are the basic concepts of managerial economics
What are the basic concepts of managerial economics Sociolinguistics concept
Sociolinguistics concept Four fundamental oop concepts
Four fundamental oop concepts Chapter p prerequisites fundamental concepts of algebra
Chapter p prerequisites fundamental concepts of algebra Chapter p prerequisites fundamental concepts of algebra
Chapter p prerequisites fundamental concepts of algebra Chapter p prerequisites
Chapter p prerequisites Occbio
Occbio Fundamental and powerful concepts
Fundamental and powerful concepts Discounting principle in economics
Discounting principle in economics Fundamental concepts in video
Fundamental concepts in video Dosage calculation formulas
Dosage calculation formulas Oral medication calculation formula
Oral medication calculation formula Dose calculation formula for child
Dose calculation formula for child Dosage on hand formula
Dosage on hand formula Bloomberg 101
Bloomberg 101 Chapter review motion part a vocabulary review answer key
Chapter review motion part a vocabulary review answer key Writ of certiorari ap gov example
Writ of certiorari ap gov example Nader amin-salehi
Nader amin-salehi Prisma diagram example
Prisma diagram example Narrative review vs systematic review
Narrative review vs systematic review Presiune hidrostatica
Presiune hidrostatica From a club of 24 members a president
From a club of 24 members a president Permutations
Permutations Prinsip menghitung
Prinsip menghitung Deviation
Deviation Indexing head parts
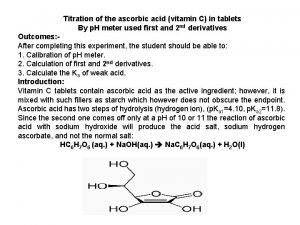
Indexing head parts Vitamin c titration calculations
Vitamin c titration calculations Activity sheet 2: stock market calculations answer key
Activity sheet 2: stock market calculations answer key What is a level loop in surveying
What is a level loop in surveying Thinking algebraically answer key
Thinking algebraically answer key The calculations of quantities in chemical reactions
The calculations of quantities in chemical reactions What is molar enthalpy
What is molar enthalpy Magnitude calculations
Magnitude calculations Sap calculations chatham
Sap calculations chatham Condensed q formula for 4 inch hose
Condensed q formula for 4 inch hose Difference between drying and evaporation
Difference between drying and evaporation Cocurrent flow heat exchanger
Cocurrent flow heat exchanger Percentage strength w/w
Percentage strength w/w Pharmaceutical calculations percentage strength
Pharmaceutical calculations percentage strength Tpn calculations
Tpn calculations Drip rate calculation formula
Drip rate calculation formula Mixer sizing calculations
Mixer sizing calculations How to calculate tariffs grade 10
How to calculate tariffs grade 10 How to do tpn calculations
How to do tpn calculations Sap calculations islington
Sap calculations islington Fire sprinkler hydraulic calculation example
Fire sprinkler hydraulic calculation example Chapter 2 measurements and calculations
Chapter 2 measurements and calculations Rate of diffusion of gas
Rate of diffusion of gas Reverse calculations to check answers
Reverse calculations to check answers How do you calculate change in enthalpy
How do you calculate change in enthalpy Electric machine design training
Electric machine design training Truss roof details
Truss roof details Windshield wiper mechanism design
Windshield wiper mechanism design 22,4 l/mol
22,4 l/mol Parenteral medication examples
Parenteral medication examples Double acting cylinder with cushioning symbol
Double acting cylinder with cushioning symbol Difference between endpoint and kinetic method
Difference between endpoint and kinetic method Sterile compounding calculations
Sterile compounding calculations Precipitation titrations
Precipitation titrations Alligation pharmacy
Alligation pharmacy Statically determinate truss
Statically determinate truss Biochemical calculations examples
Biochemical calculations examples Time of death estimations worksheet
Time of death estimations worksheet 555 timer equations
555 timer equations Colligative properties example problems
Colligative properties example problems Normal calculations in reverse
Normal calculations in reverse Bod formula
Bod formula Calculation of gravimetric analysis
Calculation of gravimetric analysis Pharmacy compounding calculations
Pharmacy compounding calculations Percentage calculations worksheet
Percentage calculations worksheet Wetflag calculation
Wetflag calculation Real gdp adalah
Real gdp adalah Sustical
Sustical Ieee battery sizing calculations
Ieee battery sizing calculations Gravimetric analysis calculations
Gravimetric analysis calculations Calculate iv push rate
Calculate iv push rate Energy calculations oxford
Energy calculations oxford Cooling curve calculations
Cooling curve calculations Fault tree analysis probability calculations
Fault tree analysis probability calculations Dr nabil khouri
Dr nabil khouri How to do enthalpy change
How to do enthalpy change Dimensional analysis calculator
Dimensional analysis calculator What is a 1:2 dilution
What is a 1:2 dilution Zip line slope and sag
Zip line slope and sag Conversions and calculations chapter 6
Conversions and calculations chapter 6 Steady state current formula
Steady state current formula Sap calculations rayleigh
Sap calculations rayleigh Kinetic energy pyramid
Kinetic energy pyramid Titration calculations
Titration calculations Bond energy calculations worksheet
Bond energy calculations worksheet Virtual loss of gm formula
Virtual loss of gm formula Specific heat of water
Specific heat of water Sfd bmd diagram
Sfd bmd diagram Cantilevered roof truss
Cantilevered roof truss Reverse percentage calculator excel
Reverse percentage calculator excel Evaporation heat transfer
Evaporation heat transfer Pharmaceutical measurements
Pharmaceutical measurements Amoxicillin dosage chart by weight
Amoxicillin dosage chart by weight Pediatric dosage calculations for nurses
Pediatric dosage calculations for nurses Measurements and calculations chapter 2 test
Measurements and calculations chapter 2 test