Stoichiometric Calculations This Lesson Quick review of last


- Slides: 28

Stoichiometric Calculations

This Lesson • Quick review of last week • Stoichiometry • Balanced equations • Moles

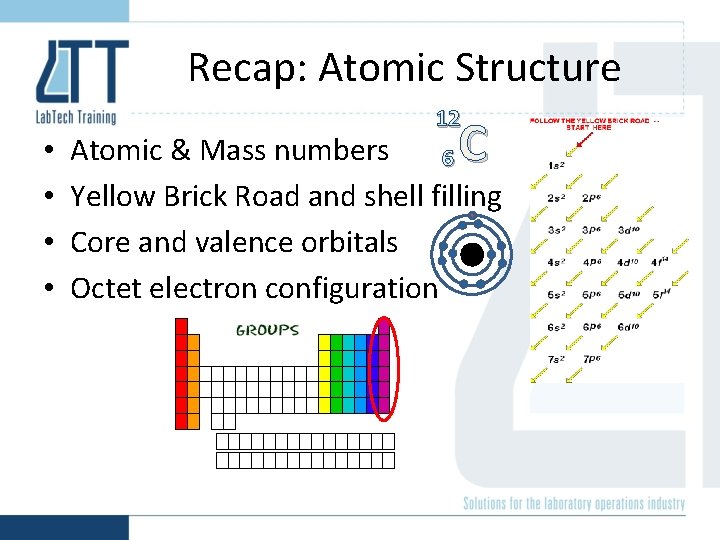
Recap: Atomic Structure • • 12 C Atomic & Mass numbers 6 Yellow Brick Road and shell filling Core and valence orbitals Octet electron configuration

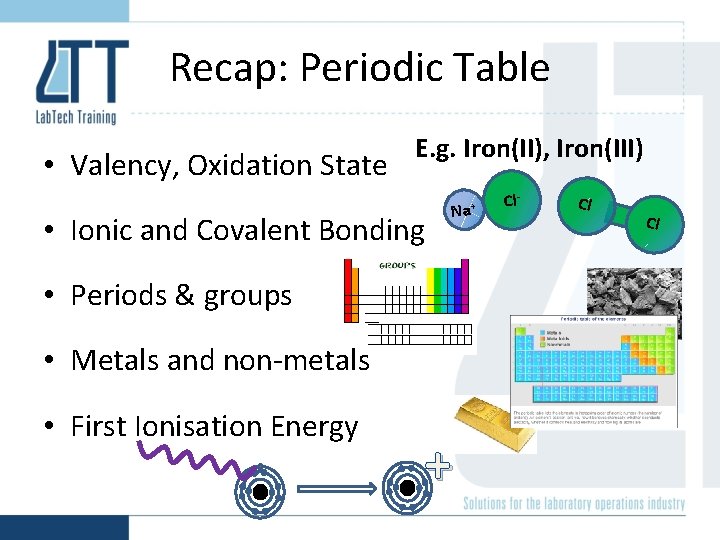
Recap: Periodic Table • Valency, Oxidation State E. g. Iron(II), Iron(III) • Ionic and Covalent Bonding Na+ • Periods & groups • Metals and non-metals • First Ionisation Energy + Cl- Cl Cl

Check 1. Is K a metal or non-metal? When it forms an ion, will it be a cation or anion? 2. Is Cl a metal or non-metal? When it forms an ion, will it be a cation or anion? 3. If K has a lower ionisation energy than Ca, which is more reactive? 4. Which groups do the following belong to: Fe, Rb, Br, He, Na? 5. What is the electron configuration of K? Which are core and which are valence electrons?

Stoichiometry is a branch of chemistry that deals with the relative quantities of reactants and products in chemical reactions. In a balanced chemical reaction, the relations among quantities of reactants and products typically form a ratio of positive integers. Stoichiometry can be used to determine quantities such as the amount of products (in mass, moles, volume, etc. ) that can be produced with given reactants. Stoichiometry is founded on the law of conservation of mass: the mass of the reactants equals the mass of the products.

Conservation of mass Since chemical reactions can neither create nor destroy matter, nor transmute one element into another, the amount of each element must be the same throughout the overall reaction. For example, the number of atoms of a given element X on the reactant side must equal the number of atoms of that element on the product side, whether or not all of those atoms are actually involved in a reaction. More generally, physics says that matter can be created and destroyed, but energy is conserved!

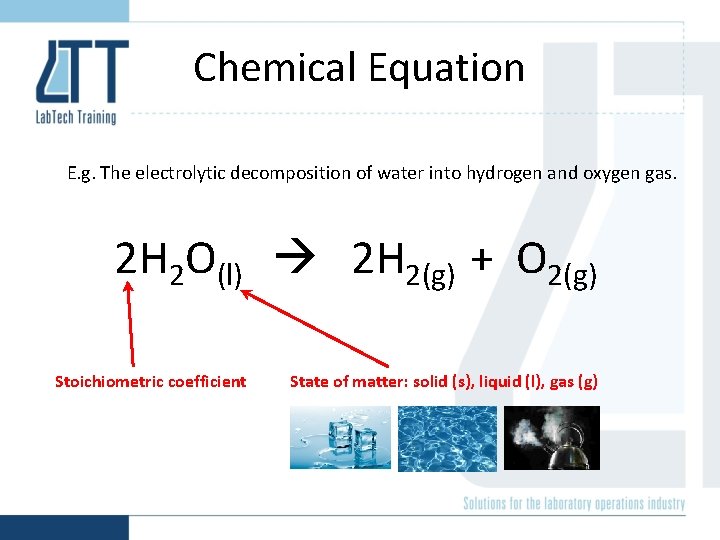
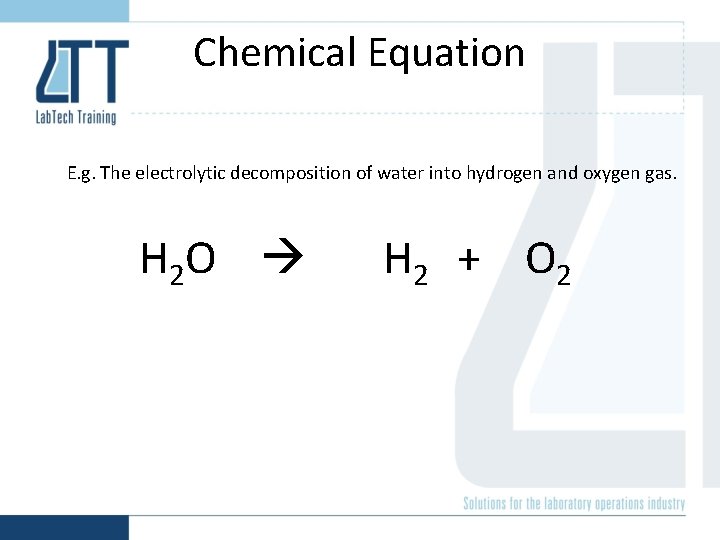
Electrolytic decomposition of water

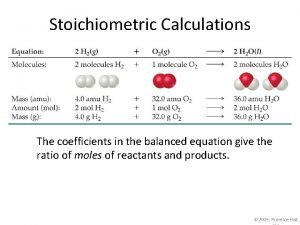
Chemical Equation E. g. The electrolytic decomposition of water into hydrogen and oxygen gas. 2 H 2 O(l) 2 H 2(g) + O 2(g) Stoichiometric coefficient State of matter: solid (s), liquid (l), gas (g)

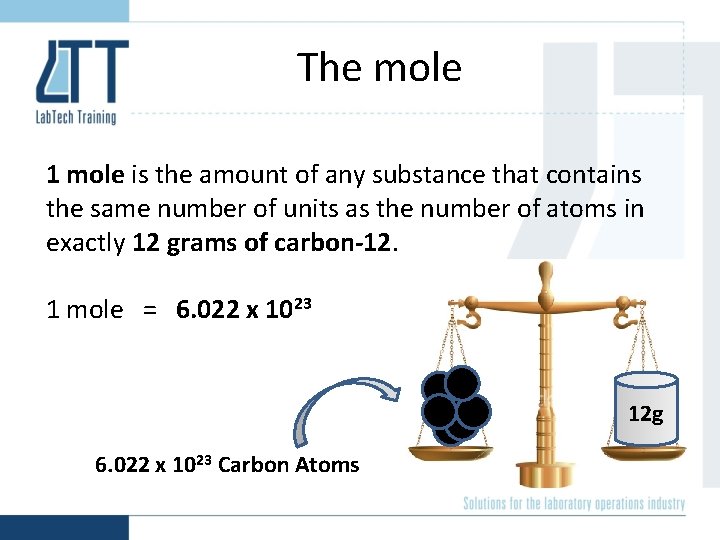
The mole 1 mole is the amount of any substance that contains the same number of units as the number of atoms in exactly 12 grams of carbon-12. 1 mole = 6. 022 x 1023 12 g 6. 022 x 1023 Carbon Atoms

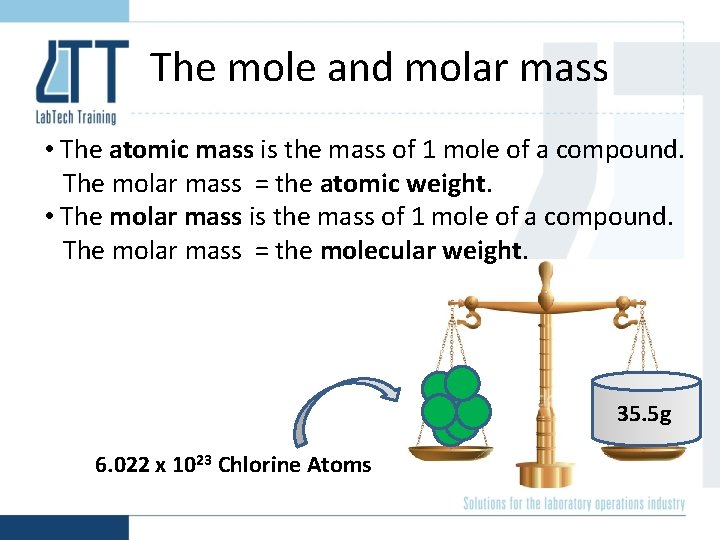
The mole and molar mass • The atomic mass is the mass of 1 mole of a compound. The molar mass = the atomic weight. • The molar mass is the mass of 1 mole of a compound. The molar mass = the molecular weight. 35. 5 g 6. 022 x 1023 Chlorine Atoms

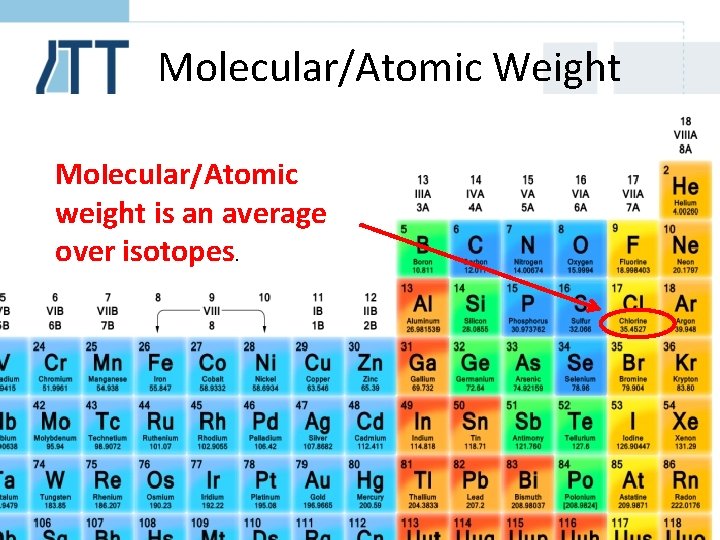
Molecular/Atomic Weight Molecular/Atomic weight is an average over isotopes.

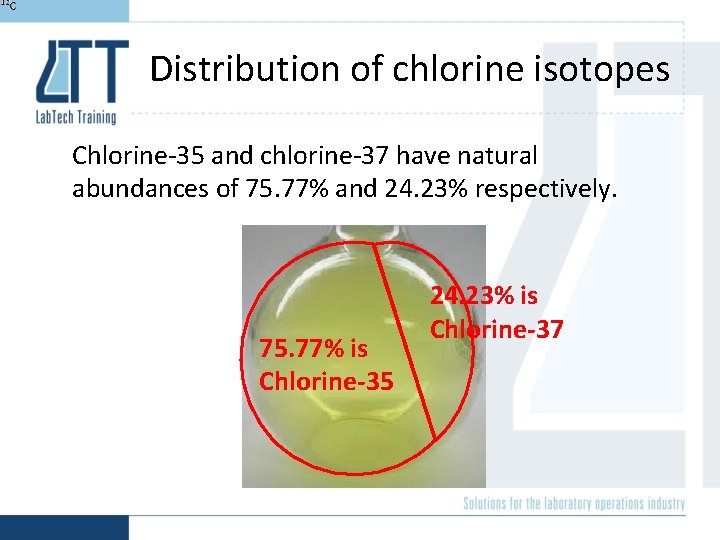
Distribution of chlorine isotopes Chlorine-35 and chlorine-37 have natural abundances of 75. 77% and 24. 23% respectively. 75. 77% is Chlorine-35 24. 23% is Chlorine-37

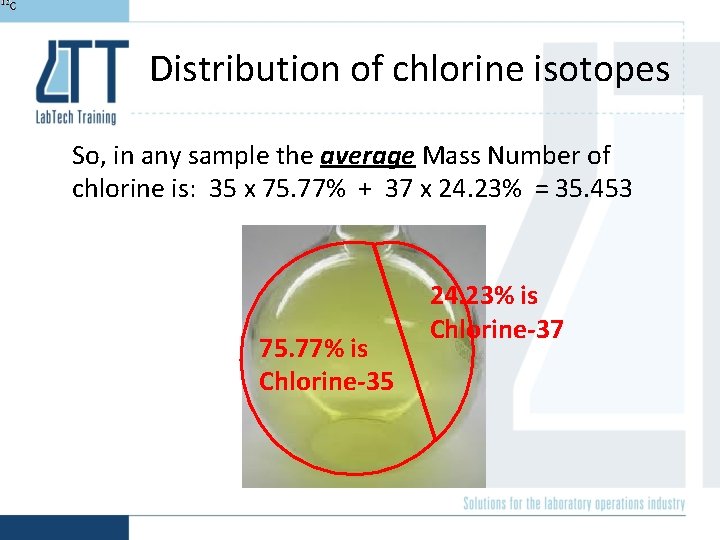
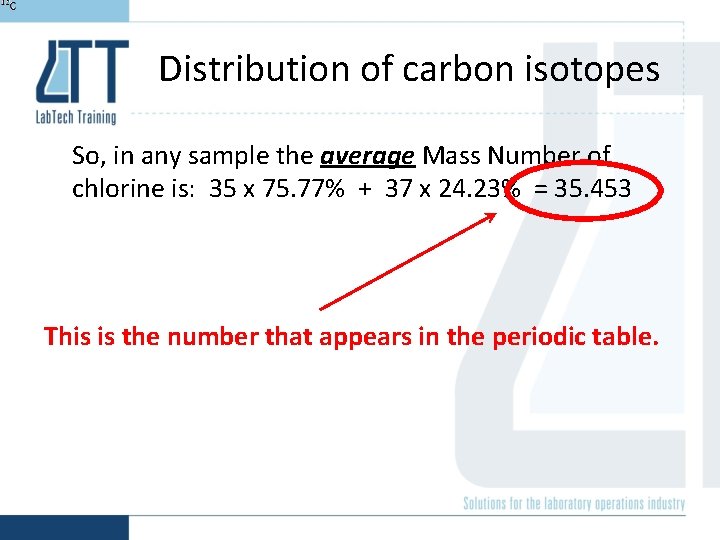
Distribution of chlorine isotopes So, in any sample the average Mass Number of chlorine is: 35 x 75. 77% + 37 x 24. 23% = 35. 453 75. 77% is Chlorine-35 24. 23% is Chlorine-37

Distribution of carbon isotopes So, in any sample the average Mass Number of chlorine is: 35 x 75. 77% + 37 x 24. 23% = 35. 453 This is the number that appears in the periodic table.

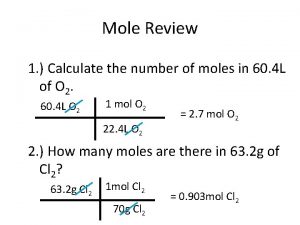
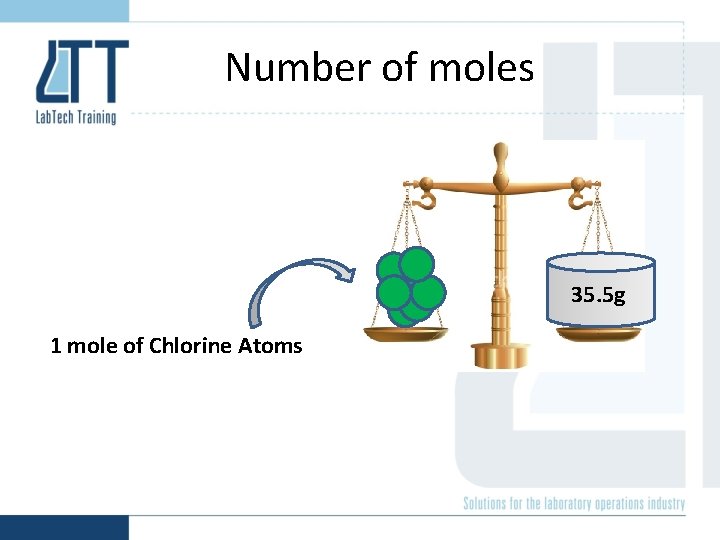
Number of moles 35. 5 g 1 mole of Chlorine Atoms

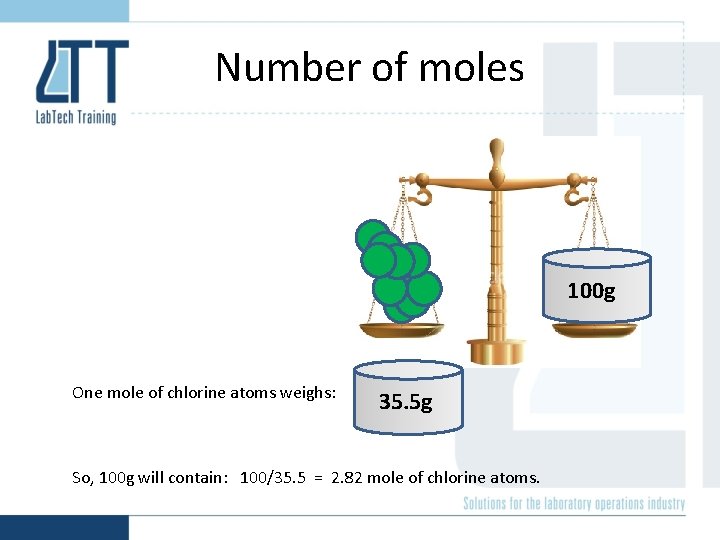
Number of moles 100 g One mole of chlorine atoms weighs: 35. 5 g So, 100 g will contain: 100/35. 5 = 2. 82 mole of chlorine atoms.

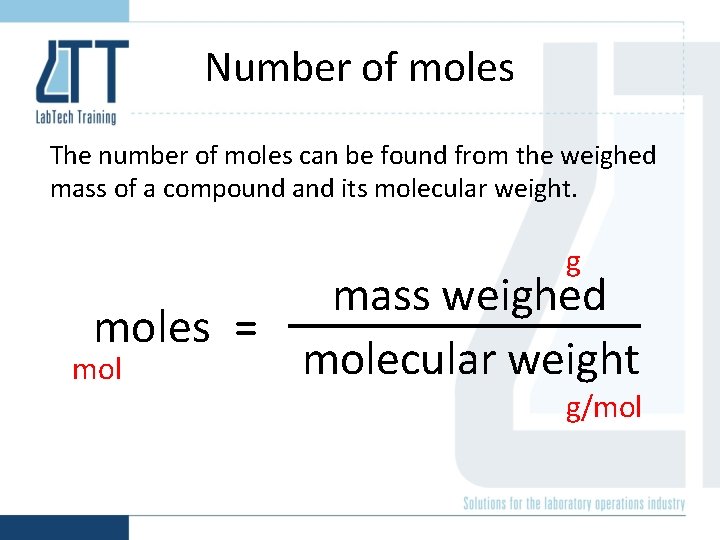
Number of moles The number of moles can be found from the weighed mass of a compound and its molecular weight. g mass weighed moles = molecular weight mol g/mol

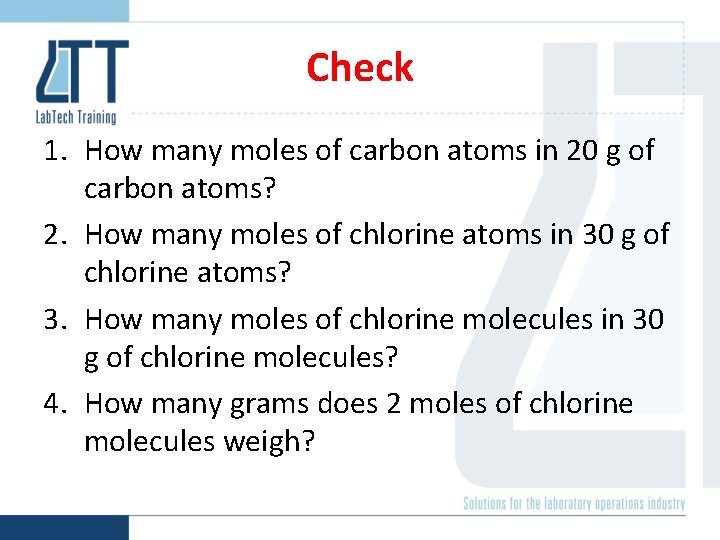
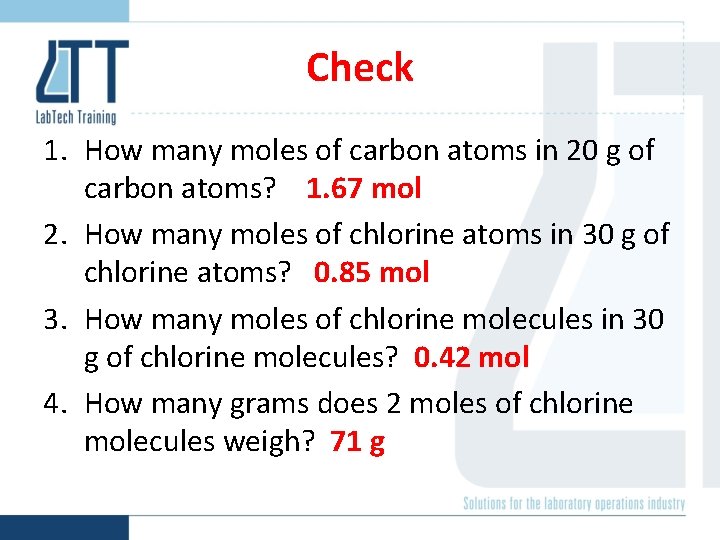
Check 1. How many moles of carbon atoms in 20 g of carbon atoms? 2. How many moles of chlorine atoms in 30 g of chlorine atoms? 3. How many moles of chlorine molecules in 30 g of chlorine molecules? 4. How many grams does 2 moles of chlorine molecules weigh?

Check 1. How many moles of carbon atoms in 20 g of carbon atoms? 1. 67 mol 2. How many moles of chlorine atoms in 30 g of chlorine atoms? 0. 85 mol 3. How many moles of chlorine molecules in 30 g of chlorine molecules? 0. 42 mol 4. How many grams does 2 moles of chlorine molecules weigh? 71 g

Chemical Equation E. g. The electrolytic decomposition of water into hydrogen and oxygen gas. H 2 O H 2 + O 2

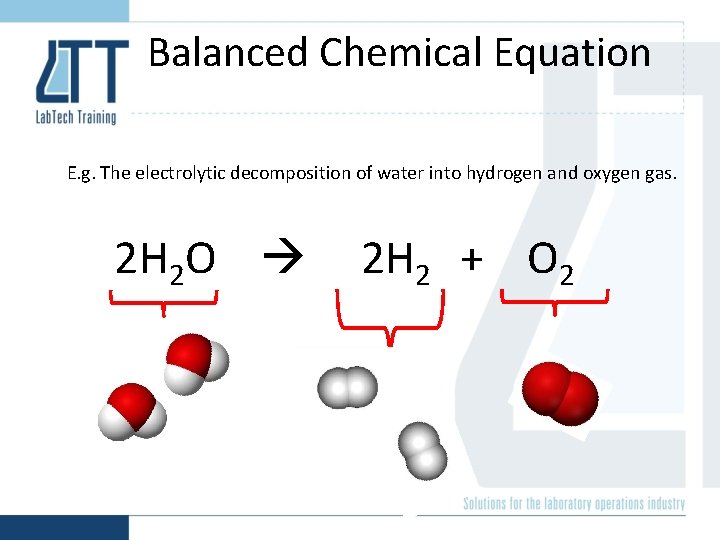
Balanced Chemical Equation E. g. The electrolytic decomposition of water into hydrogen and oxygen gas. 2 H 2 O 2 H 2 + O 2

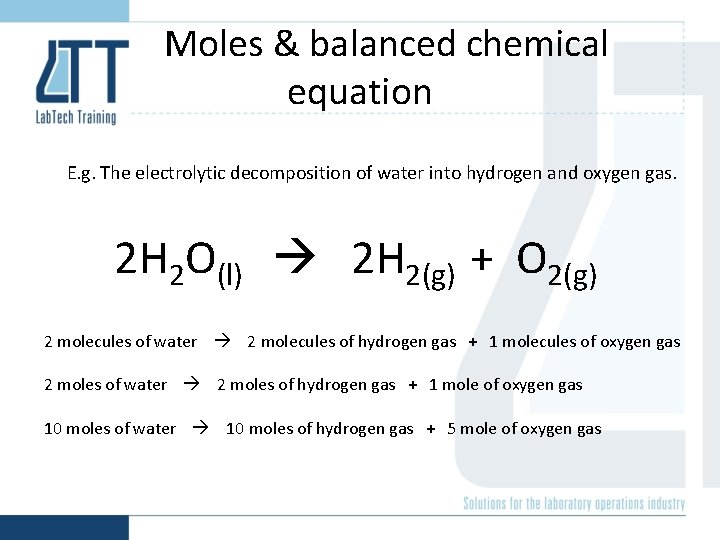
Moles & balanced chemical equation E. g. The electrolytic decomposition of water into hydrogen and oxygen gas. 2 H 2 O(l) 2 H 2(g) + O 2(g) 2 molecules of water 2 molecules of hydrogen gas + 1 molecules of oxygen gas 2 moles of water 2 moles of hydrogen gas + 1 mole of oxygen gas 10 moles of water 10 moles of hydrogen gas + 5 mole of oxygen gas

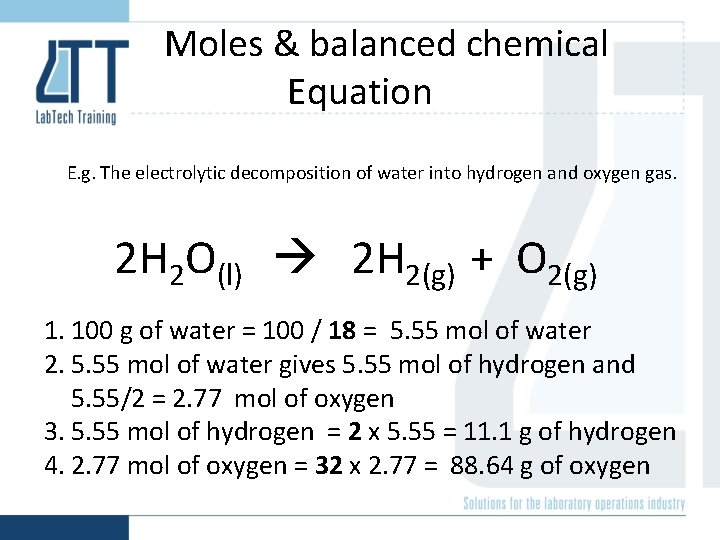
Moles & balanced chemical Equation E. g. The electrolytic decomposition of water into hydrogen and oxygen gas. 2 H 2 O(l) 2 H 2(g) + O 2(g) 1. 100 g of water = 100 / 18 = 5. 55 mol of water 2. 5. 55 mol of water gives 5. 55 mol of hydrogen and 5. 55/2 = 2. 77 mol of oxygen 3. 5. 55 mol of hydrogen = 2 x 5. 55 = 11. 1 g of hydrogen 4. 2. 77 mol of oxygen = 32 x 2. 77 = 88. 64 g of oxygen

Moles & balanced chemical rk o w Equation n an e c g r e y e x w t o a E. g. The electrolytic decomposition of watern into hydrogen and oxygen gas. d o w i n f t a o a n u t e q n g e u o o r 2 H 2 O(l)ced y 2 H + O d m 2(g) a n h n a i l h awater =u 100 c / 18 rt=a 5. 55 mol of water 1. 100 g of b e a m c 2. t 5. 55 mol of water gives 5. 55 mol of hydrogen and h a w i o m of oxygen h 5. 55/2 = W t 2. 77 romol u mol toffhydrogen = 2 x 5. 55 = 11. 1 g of hydrogen 3. o 5. 55 e g 4. 2. 77 e mol of oxygen = 32 x 2. 77 = 88. 64 g of oxygen w

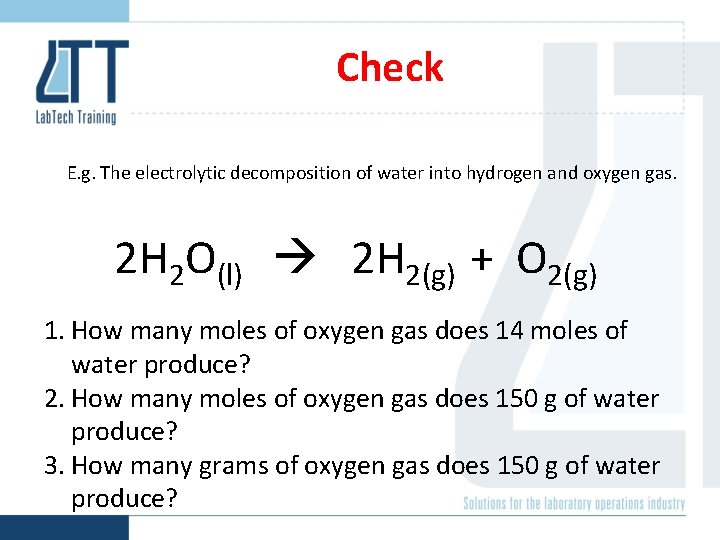
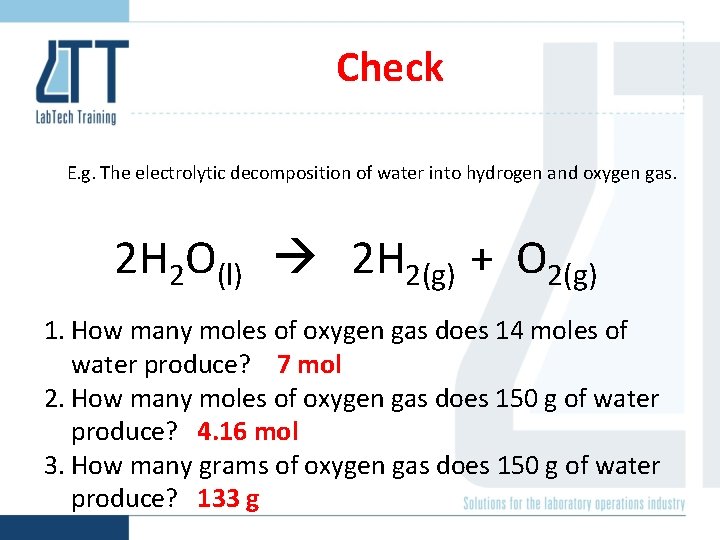
Check E. g. The electrolytic decomposition of water into hydrogen and oxygen gas. 2 H 2 O(l) 2 H 2(g) + O 2(g) 1. How many moles of oxygen gas does 14 moles of water produce? 2. How many moles of oxygen gas does 150 g of water produce? 3. How many grams of oxygen gas does 150 g of water produce?

Check E. g. The electrolytic decomposition of water into hydrogen and oxygen gas. 2 H 2 O(l) 2 H 2(g) + O 2(g) 1. How many moles of oxygen gas does 14 moles of water produce? 7 mol 2. How many moles of oxygen gas does 150 g of water produce? 4. 16 mol 3. How many grams of oxygen gas does 150 g of water produce? 133 g

Summary • Stoichiometry • Balanced equations • Moles
 Mass to mass stoichiometry formula
Mass to mass stoichiometry formula All stoichiometric calculations begin with a
All stoichiometric calculations begin with a Stoichiometry and stoichiometric calculations
Stoichiometry and stoichiometric calculations Structural steel connection calculations calculations
Structural steel connection calculations calculations Quick find algorithm
Quick find algorithm Collision forces quick check
Collision forces quick check What is stoichiometric
What is stoichiometric Stoichiometry table method
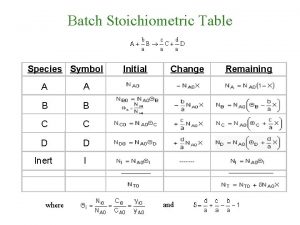
Stoichiometry table method Stoichiometric table for flow system
Stoichiometric table for flow system Stoichiometric gasoline
Stoichiometric gasoline Stoichiometric table for flow system
Stoichiometric table for flow system Stoichiometric air fuel ratio
Stoichiometric air fuel ratio Symbolaab
Symbolaab Stoichiometric point definition
Stoichiometric point definition Stoichiometric table for flow system
Stoichiometric table for flow system Stoichiometric
Stoichiometric Stoichiometry
Stoichiometry Stoichiometry formulas
Stoichiometry formulas Stoichiometric table for reversible reaction
Stoichiometric table for reversible reaction Iop conjugations
Iop conjugations P = mc
P = mc Python quick review
Python quick review Lesson 4 gravity and motion lesson review
Lesson 4 gravity and motion lesson review Chapter review motion part a vocabulary review answer key
Chapter review motion part a vocabulary review answer key Uncontrollable spending ap gov
Uncontrollable spending ap gov Narrative review vs systematic review
Narrative review vs systematic review What is inclusion and exclusion criteria
What is inclusion and exclusion criteria Narrative review vs systematic review
Narrative review vs systematic review The last lesson introduction
The last lesson introduction