Lecture Presentation Chapter 3 Stoichiometry Calculations with Chemical













- Slides: 96

Lecture Presentation Chapter 3 Stoichiometry: Calculations with Chemical Formulas and Equations © 2012 Pearson Education, Inc. John D. Bookstaver St. Charles Community College Cottleville, MO

Law of Conservation of Mass “We may lay it down as an incontestable axiom that, in all the operations of art and nature, nothing is created; an equal amount of matter exists both before and after the experiment. Upon this principle, the whole art of performing chemical experiments depends. ” --Antoine Lavoisier, 1789 © 2012 Pearson Education, Inc. Stoichiometry

Chemical Equations Chemical equations are concise representations of chemical reactions. Stoichiometry © 2012 Pearson Education, Inc.

Chemical Equations CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(g) • CH 4 and O 2 are the reactants, and CO 2 and H 2 O are the products • the (g) after the formulas tells us the state of the chemical • the number in front of each substance tells us the numbers of those molecules in the reaction coefficients ücalled the ______ 4

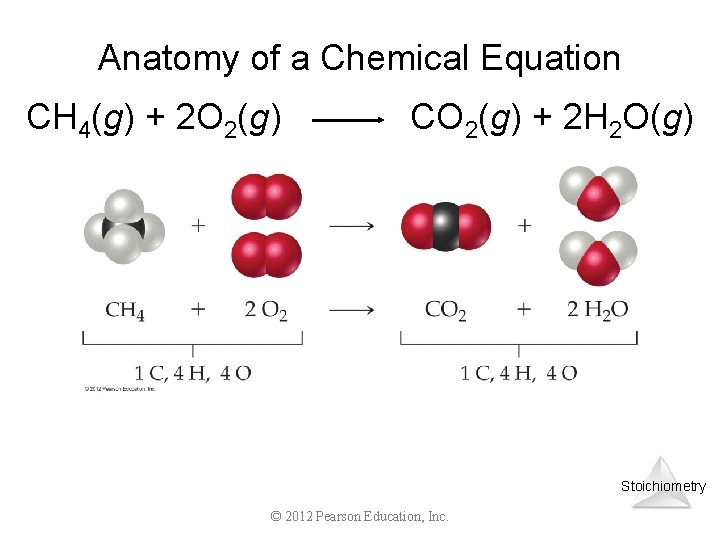
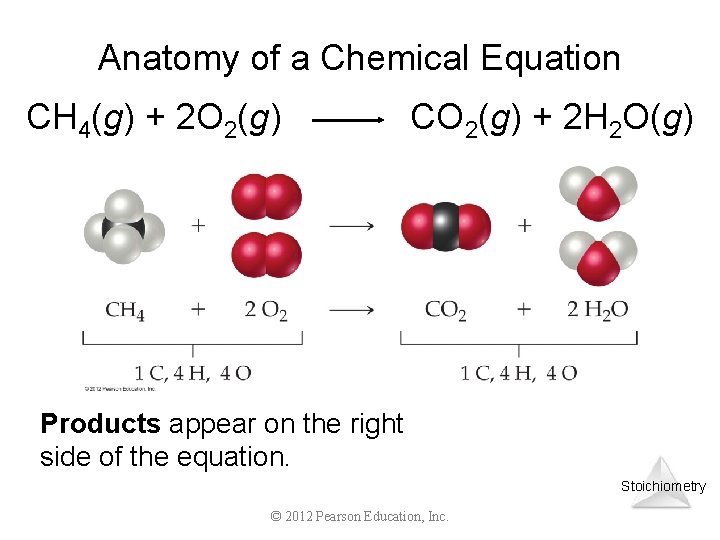
Anatomy of a Chemical Equation CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(g) Stoichiometry © 2012 Pearson Education, Inc.

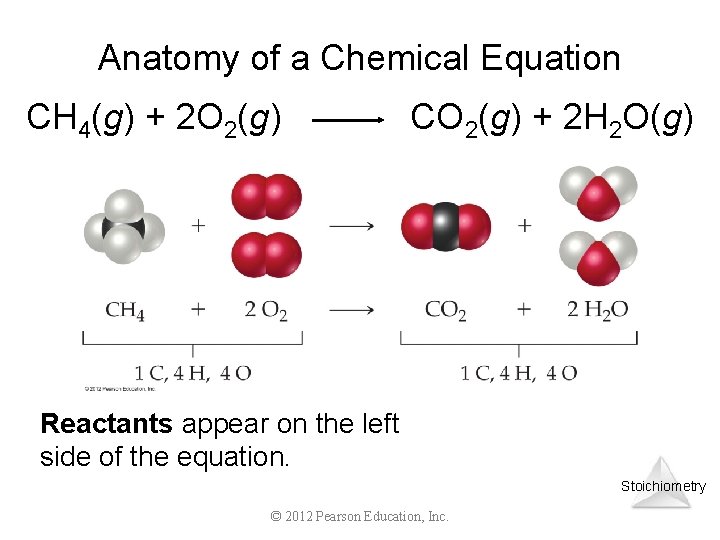
Anatomy of a Chemical Equation CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(g) Reactants appear on the left side of the equation. Stoichiometry © 2012 Pearson Education, Inc.

Anatomy of a Chemical Equation CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(g) Products appear on the right side of the equation. Stoichiometry © 2012 Pearson Education, Inc.

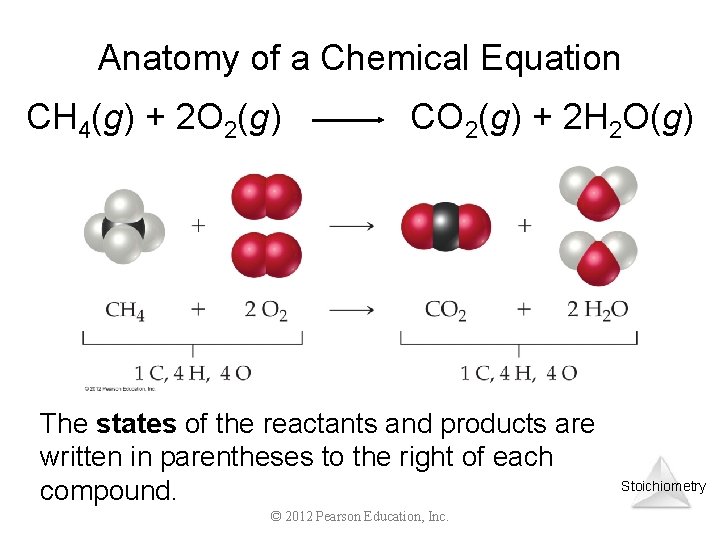
Anatomy of a Chemical Equation CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(g) The states of the reactants and products are written in parentheses to the right of each compound. © 2012 Pearson Education, Inc. Stoichiometry

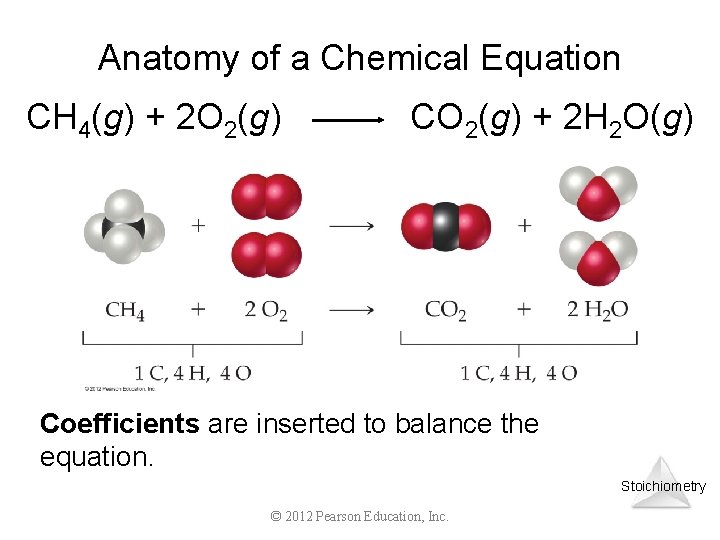
Anatomy of a Chemical Equation CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(g) Coefficients are inserted to balance the equation. Stoichiometry © 2012 Pearson Education, Inc.

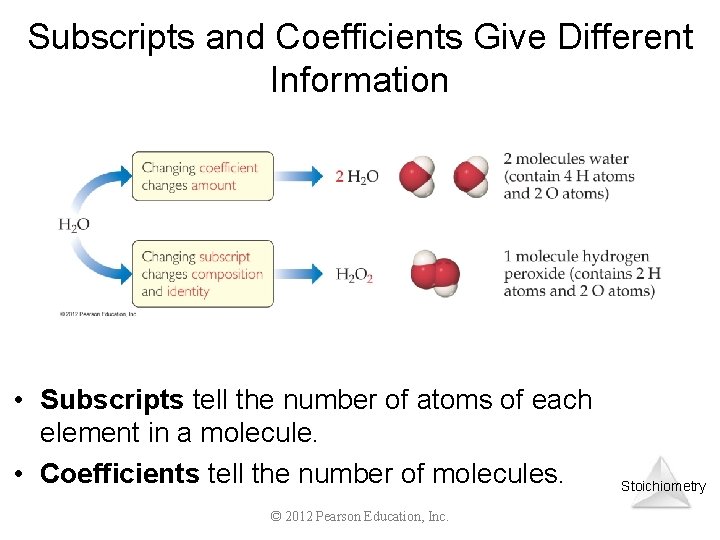
Subscripts and Coefficients Give Different Information • Subscripts tell the number of atoms of each element in a molecule. • Coefficients tell the number of molecules. © 2012 Pearson Education, Inc. Stoichiometry

Reaction Types Stoichiometry © 2012 Pearson Education, Inc.

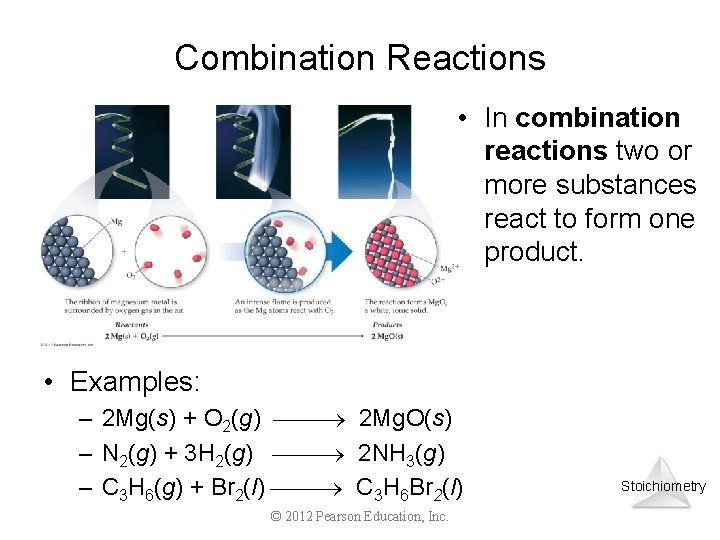
Combination Reactions • In combination reactions two or more substances react to form one product. • Examples: – 2 Mg(s) + O 2(g) 2 Mg. O(s) – N 2(g) + 3 H 2(g) 2 NH 3(g) – C 3 H 6(g) + Br 2(l) C 3 H 6 Br 2(l) © 2012 Pearson Education, Inc. Stoichiometry

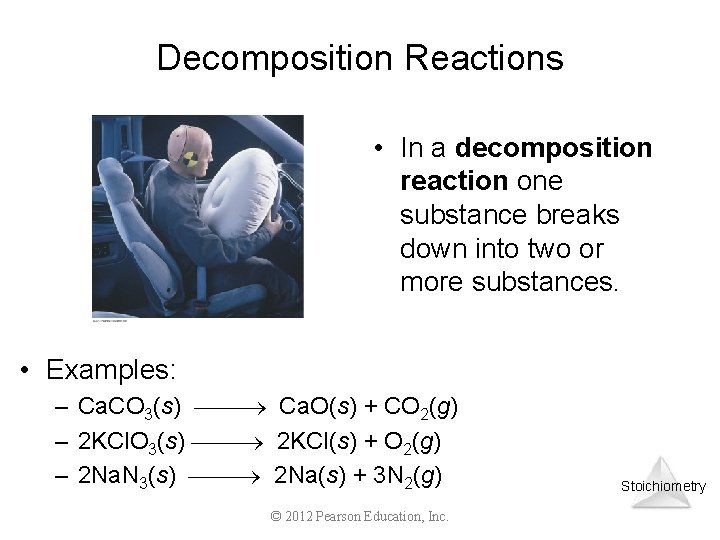
Decomposition Reactions • In a decomposition reaction one substance breaks down into two or more substances. • Examples: – Ca. CO 3(s) Ca. O(s) + CO 2(g) – 2 KCl. O 3(s) 2 KCl(s) + O 2(g) – 2 Na. N 3(s) 2 Na(s) + 3 N 2(g) © 2012 Pearson Education, Inc. Stoichiometry

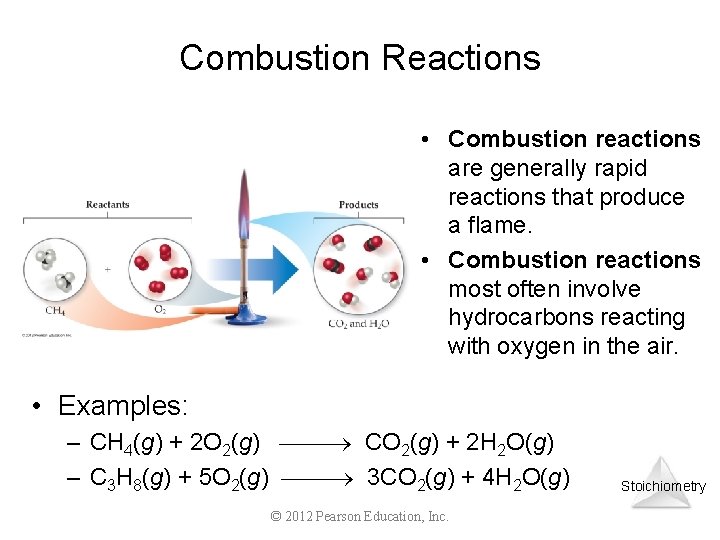
Combustion Reactions • Combustion reactions are generally rapid reactions that produce a flame. • Combustion reactions most often involve hydrocarbons reacting with oxygen in the air. • Examples: – CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(g) – C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O(g) © 2012 Pearson Education, Inc. Stoichiometry

Formula Weights Stoichiometry © 2012 Pearson Education, Inc.

Formula Weight (FW) • A formula weight is the sum of the atomic weights for the atoms in a chemical formula. • So, the formula weight of calcium chloride, Ca. Cl 2, would be Ca: 1(40. 08 amu) + Cl: 2(35. 453 amu) 110. 99 amu • Formula weights are generally reported for ionic compounds. Stoichiometry © 2012 Pearson Education, Inc.

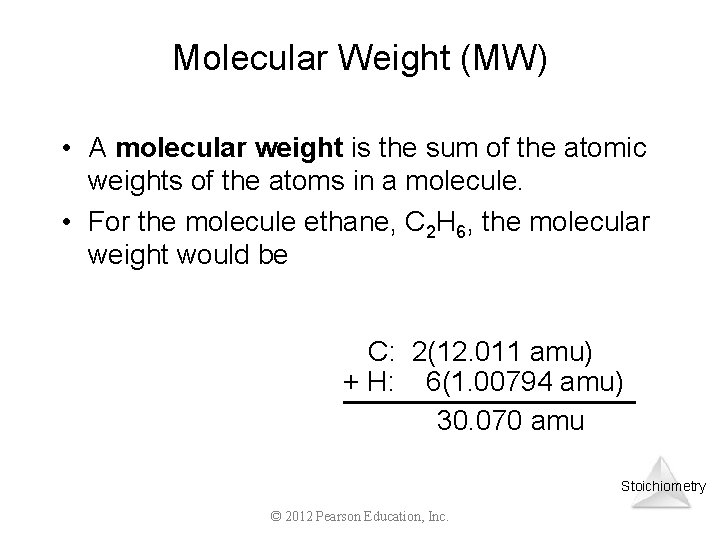
Molecular Weight (MW) • A molecular weight is the sum of the atomic weights of the atoms in a molecule. • For the molecule ethane, C 2 H 6, the molecular weight would be C: 2(12. 011 amu) + H: 6(1. 00794 amu) 30. 070 amu Stoichiometry © 2012 Pearson Education, Inc.

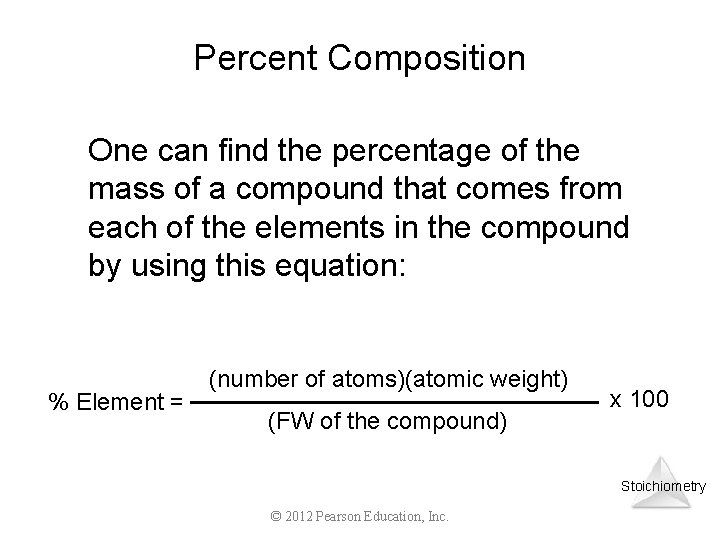
Percent Composition One can find the percentage of the mass of a compound that comes from each of the elements in the compound by using this equation: % Element = (number of atoms)(atomic weight) (FW of the compound) x 100 Stoichiometry © 2012 Pearson Education, Inc.

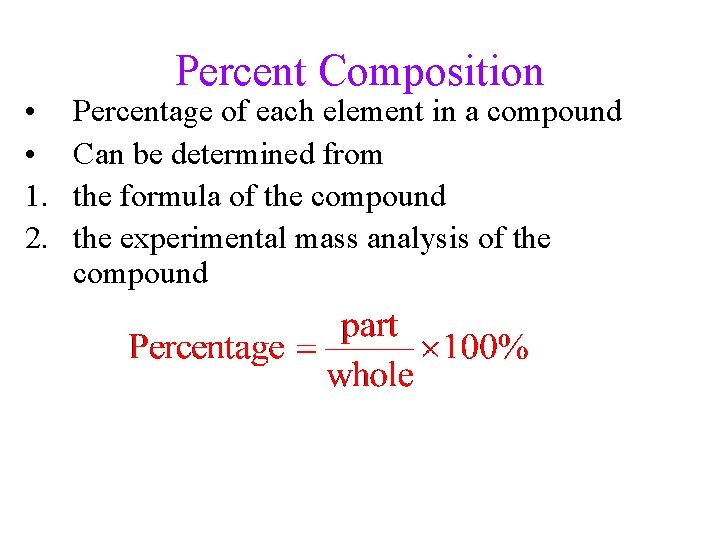
• • 1. 2. Percent Composition Percentage of each element in a compound Can be determined from the formula of the compound the experimental mass analysis of the compound

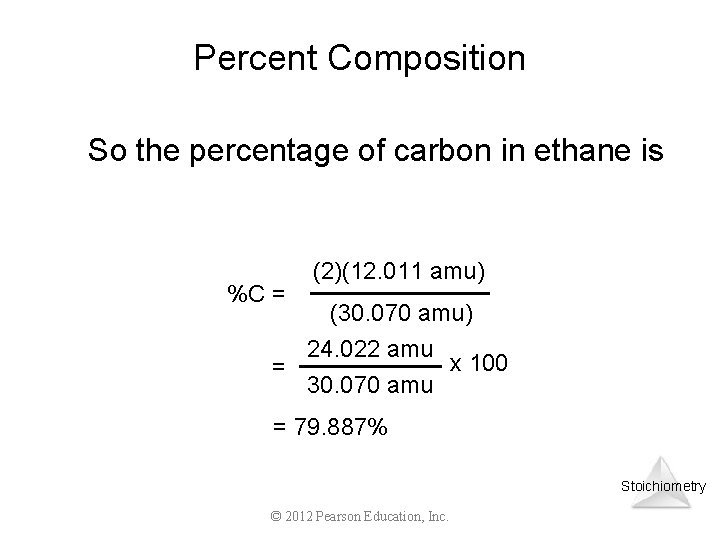
Percent Composition So the percentage of carbon in ethane is %C = (2)(12. 011 amu) (30. 070 amu) 24. 022 amu x 100 = 30. 070 amu = 79. 887% Stoichiometry © 2012 Pearson Education, Inc.

Moles Stoichiometry © 2012 Pearson Education, Inc.

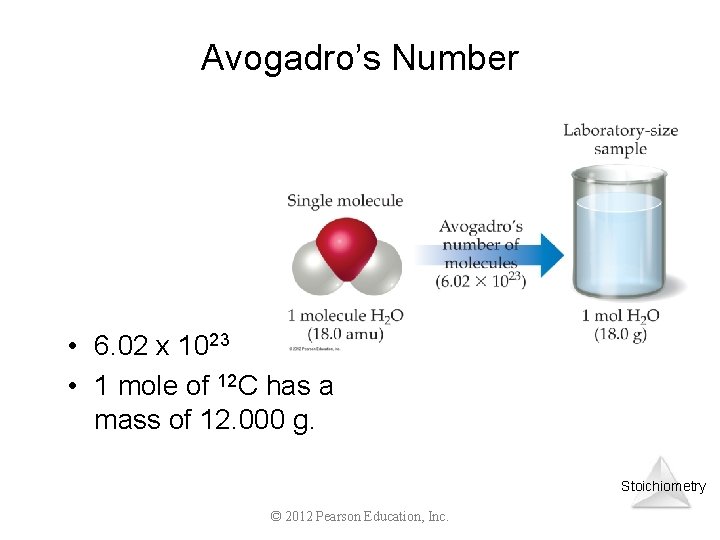
The Mole • mole (mol) = number of particles equal to the number of atoms in 12 g of C-12 üone mole of anything is 6. 022 x 1023 units of that thing üwhere 6. 022 x 1023 is known as NA Avogadro’s Number(often denoted ___) _________ ü 1 mol of marbles = 6. 022 x 1023 marbles x 1023 He atoms ü 1 mol of He = 6. 022 _____ ü 1 mol of CO 2 = _____ 6. 022 x 1023 CO 2 molecules

Avogadro’s Number • 6. 02 x 1023 • 1 mole of 12 C has a mass of 12. 000 g. Stoichiometry © 2012 Pearson Education, Inc.

Molar Mass • By definition, a molar mass is the mass of 1 mol of a substance (i. e. , g/mol). – The molar mass of an element is the mass number for the element that we find on the periodic table. – The formula weight (in amu’s) will be the same number as the molar mass (in g/mol). Stoichiometry © 2012 Pearson Education, Inc.

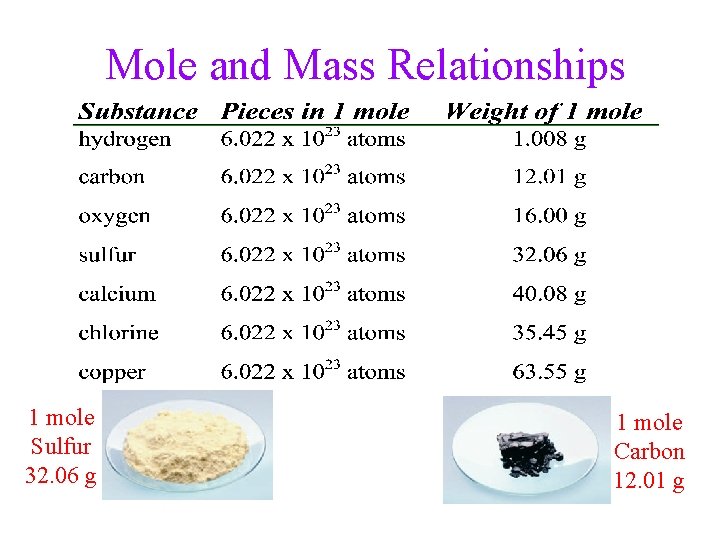
Relationship Between Moles and Mass • The mass of one mole of atoms is called the molar mass _____ • The molar mass of an element, in grams, is numerically equal to the element’s atomic mass, in amu ü atomic mass of Cu = 63. 55 _____ g/mol ü molar mass of Cu = 63. 55 ______

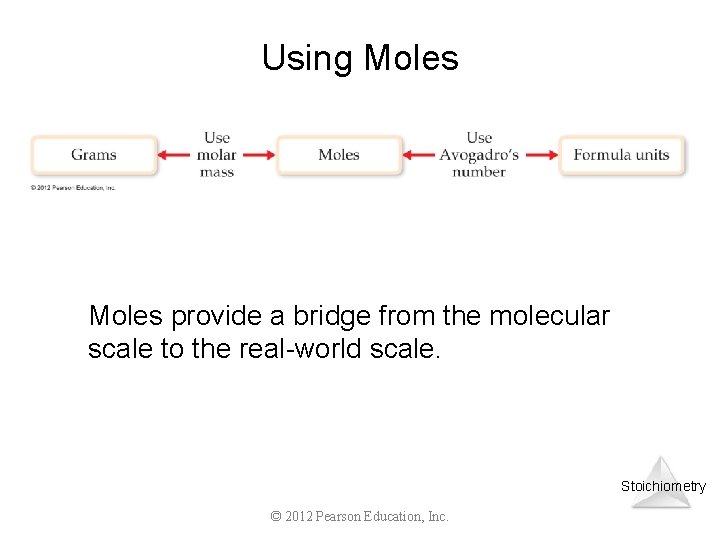
Using Moles provide a bridge from the molecular scale to the real-world scale. Stoichiometry © 2012 Pearson Education, Inc.

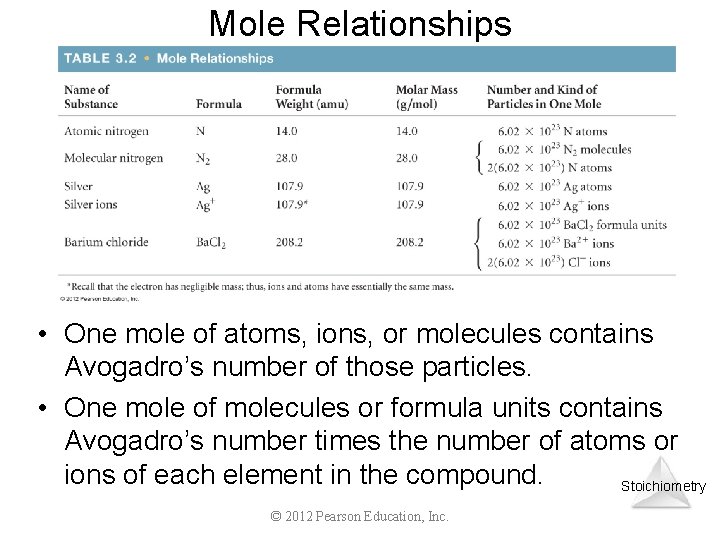
Mole Relationships • One mole of atoms, ions, or molecules contains Avogadro’s number of those particles. • One mole of molecules or formula units contains Avogadro’s number times the number of atoms or ions of each element in the compound. Stoichiometry © 2012 Pearson Education, Inc.

Mole and Mass Relationships 1 mole Sulfur 32. 06 g 1 mole Carbon 12. 01 g

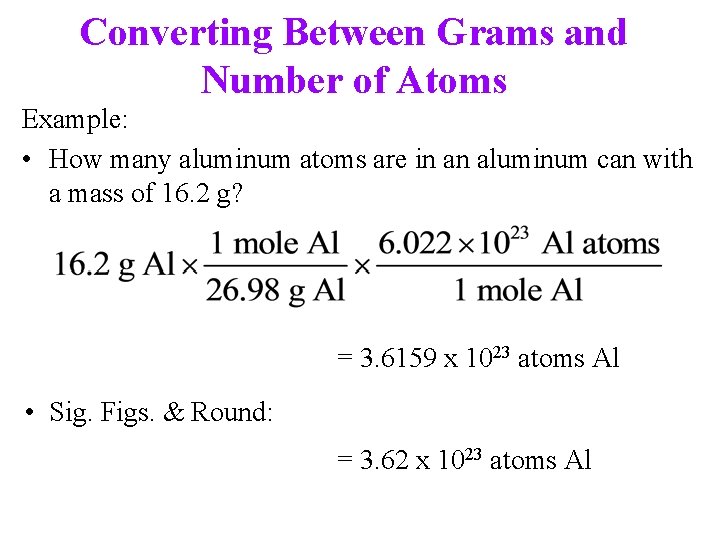
Converting Between Grams and Number of Atoms Example: • How many aluminum atoms are in an aluminum can with a mass of 16. 2 g? = 3. 6159 x 1023 atoms Al • Sig. Figs. & Round: = 3. 62 x 1023 atoms Al

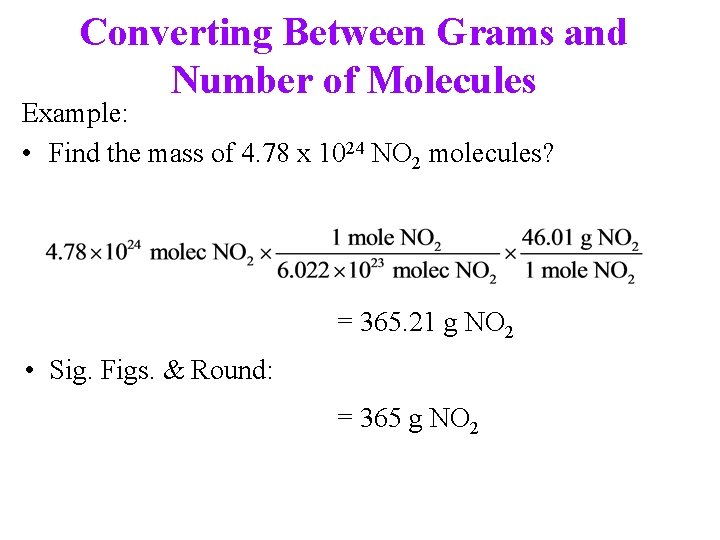
Converting Between Grams and Number of Molecules Example: • Find the mass of 4. 78 x 1024 NO 2 molecules? = 365. 21 g NO 2 • Sig. Figs. & Round: = 365 g NO 2

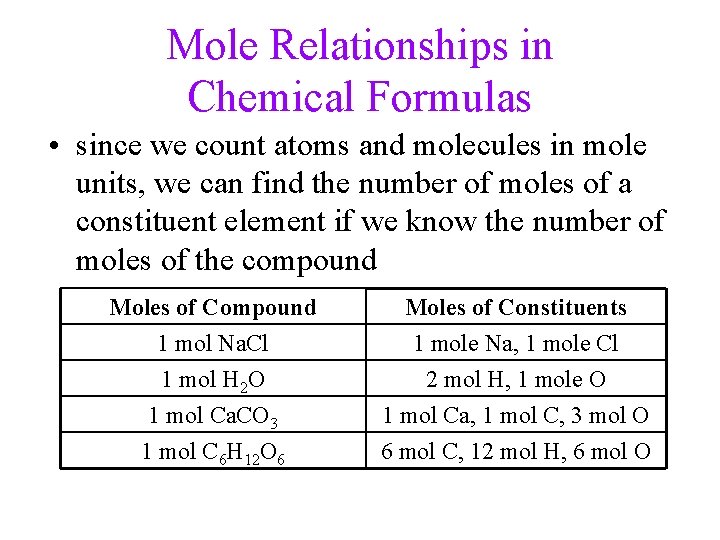
Mole Relationships in Chemical Formulas • since we count atoms and molecules in mole units, we can find the number of moles of a constituent element if we know the number of moles of the compound Moles of Compound 1 mol Na. Cl 1 mol H 2 O 1 mol Ca. CO 3 Moles of Constituents 1 mole Na, 1 mole Cl 2 mol H, 1 mole O 1 mol Ca, 1 mol C, 3 mol O 1 mol C 6 H 12 O 6 6 mol C, 12 mol H, 6 mol O

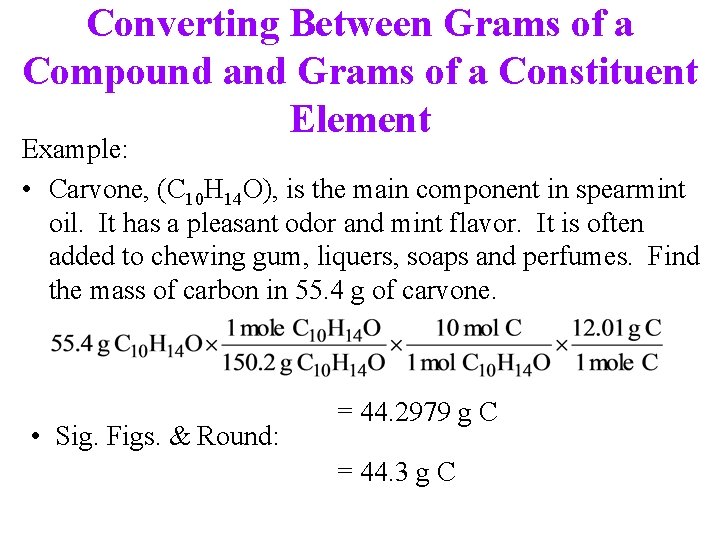
Converting Between Grams of a Compound and Grams of a Constituent Element Example: • Carvone, (C 10 H 14 O), is the main component in spearmint oil. It has a pleasant odor and mint flavor. It is often added to chewing gum, liquers, soaps and perfumes. Find the mass of carbon in 55. 4 g of carvone. • Sig. Figs. & Round: = 44. 2979 g C = 44. 3 g C

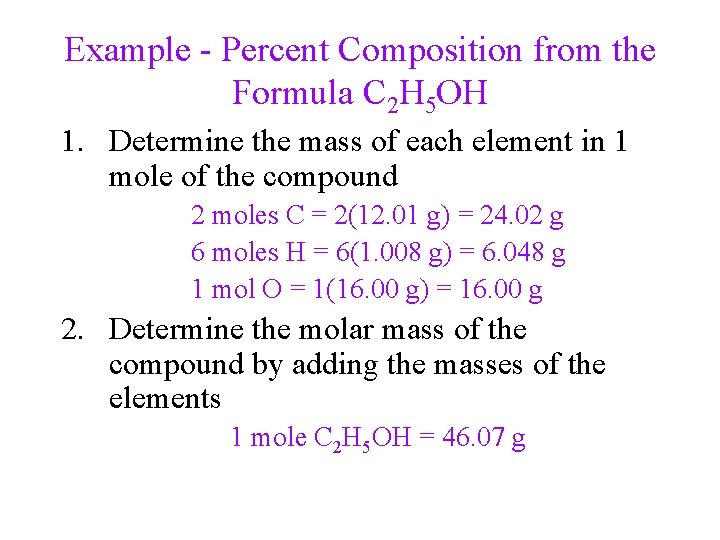
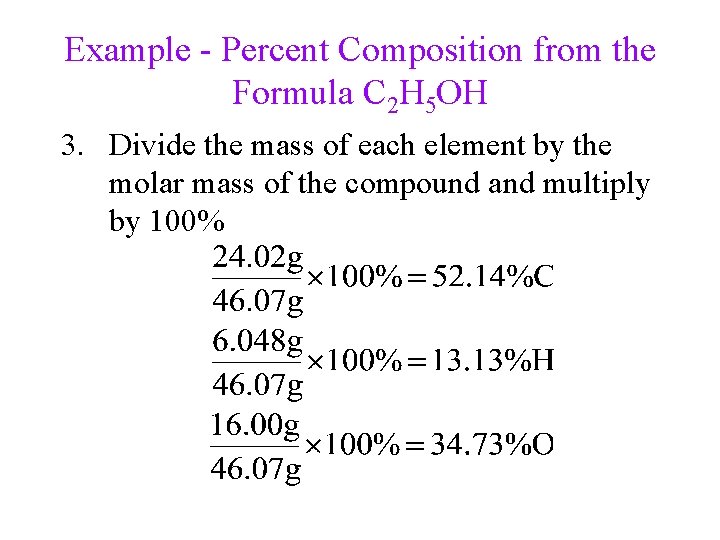
Example - Percent Composition from the Formula C 2 H 5 OH 1. Determine the mass of each element in 1 mole of the compound 2 moles C = 2(12. 01 g) = 24. 02 g 6 moles H = 6(1. 008 g) = 6. 048 g 1 mol O = 1(16. 00 g) = 16. 00 g 2. Determine the molar mass of the compound by adding the masses of the elements 1 mole C 2 H 5 OH = 46. 07 g

Example - Percent Composition from the Formula C 2 H 5 OH 3. Divide the mass of each element by the molar mass of the compound and multiply by 100%

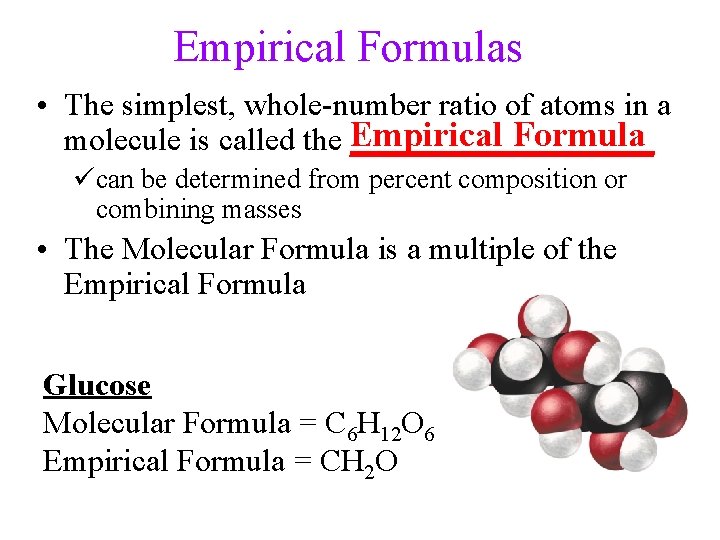
Empirical Formulas • The simplest, whole-number ratio of atoms in a Formula molecule is called the Empirical __________ ücan be determined from percent composition or combining masses • The Molecular Formula is a multiple of the Empirical Formula Glucose Molecular Formula = C 6 H 12 O 6 Empirical Formula = CH 2 O

Finding Empirical Formulas Stoichiometry © 2012 Pearson Education, Inc.

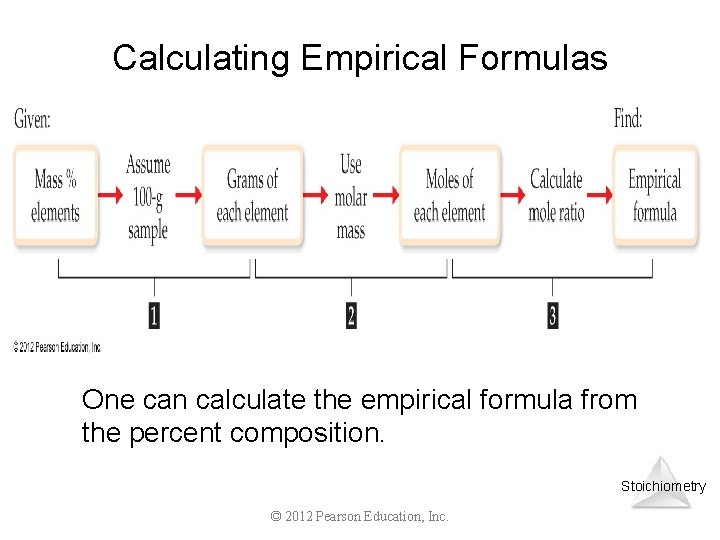
Calculating Empirical Formulas One can calculate the empirical formula from the percent composition. Stoichiometry © 2012 Pearson Education, Inc.

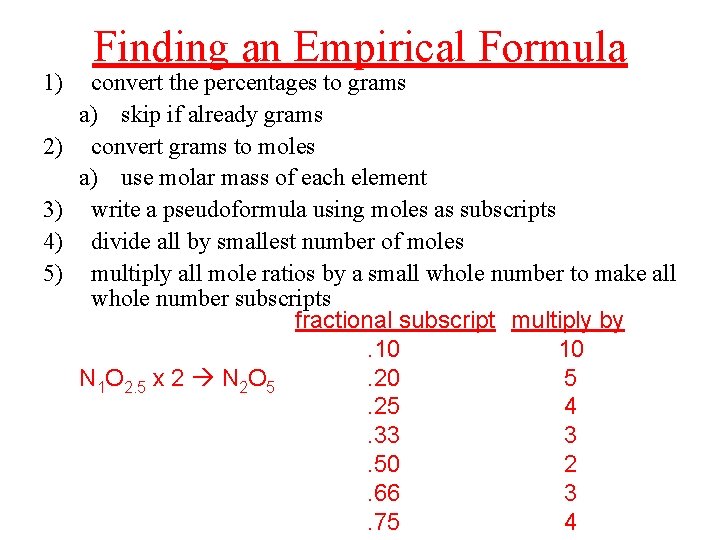
1) 2) 3) 4) 5) Finding an Empirical Formula convert the percentages to grams a) skip if already grams convert grams to moles a) use molar mass of each element write a pseudoformula using moles as subscripts divide all by smallest number of moles multiply all mole ratios by a small whole number to make all whole number subscripts fractional subscript multiply by. 10 10 N 1 O 2. 5 x 2 N 2 O 5. 20 5. 25 4. 33 3. 50 2. 66 3. 75 4

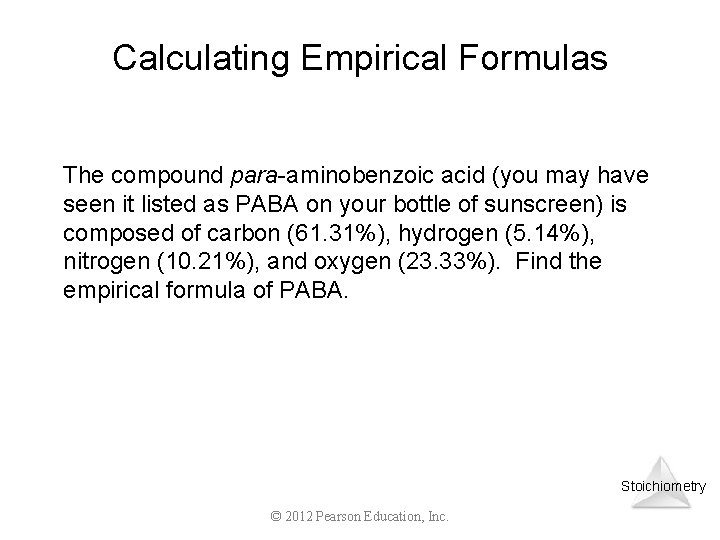
Calculating Empirical Formulas The compound para-aminobenzoic acid (you may have seen it listed as PABA on your bottle of sunscreen) is composed of carbon (61. 31%), hydrogen (5. 14%), nitrogen (10. 21%), and oxygen (23. 33%). Find the empirical formula of PABA. Stoichiometry © 2012 Pearson Education, Inc.

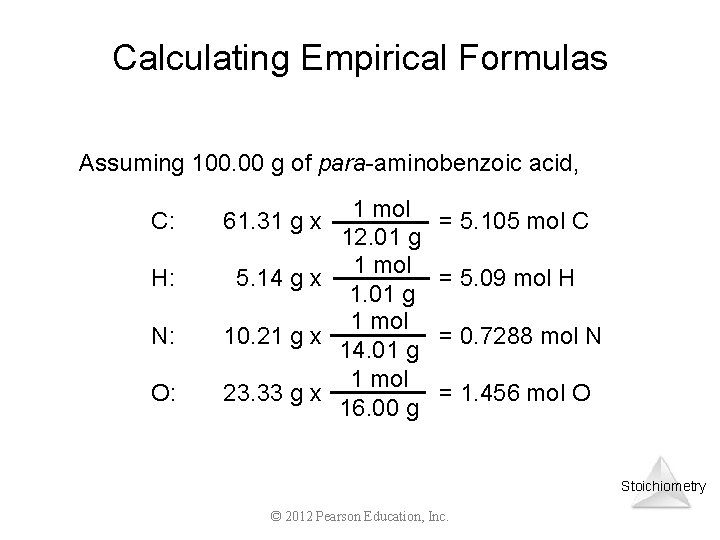
Calculating Empirical Formulas Assuming 100. 00 g of para-aminobenzoic acid, C: H: N: O: 1 mol 12. 01 g 1 mol 5. 14 g x 1. 01 g 1 mol 10. 21 g x 14. 01 g 1 mol 23. 33 g x 16. 00 g 61. 31 g x = 5. 105 mol C = 5. 09 mol H = 0. 7288 mol N = 1. 456 mol O Stoichiometry © 2012 Pearson Education, Inc.

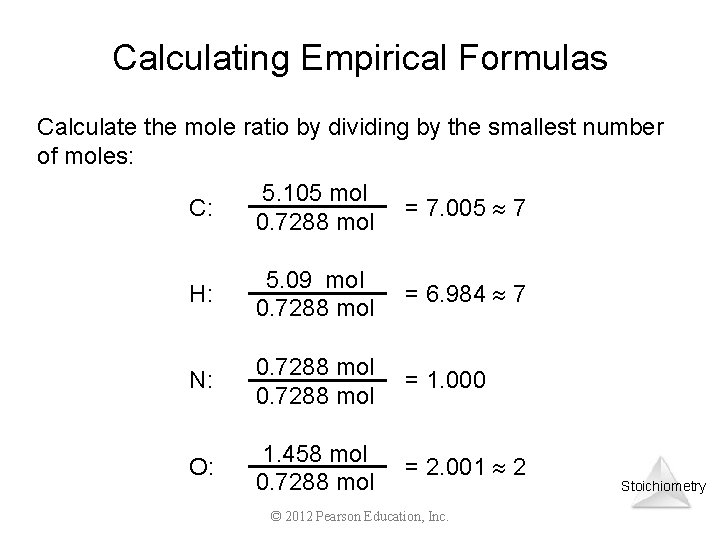
Calculating Empirical Formulas Calculate the mole ratio by dividing by the smallest number of moles: C: 5. 105 mol 0. 7288 mol = 7. 005 7 H: 5. 09 mol 0. 7288 mol = 6. 984 7 N: 0. 7288 mol = 1. 000 O: 1. 458 mol 0. 7288 mol = 2. 001 2 © 2012 Pearson Education, Inc. Stoichiometry

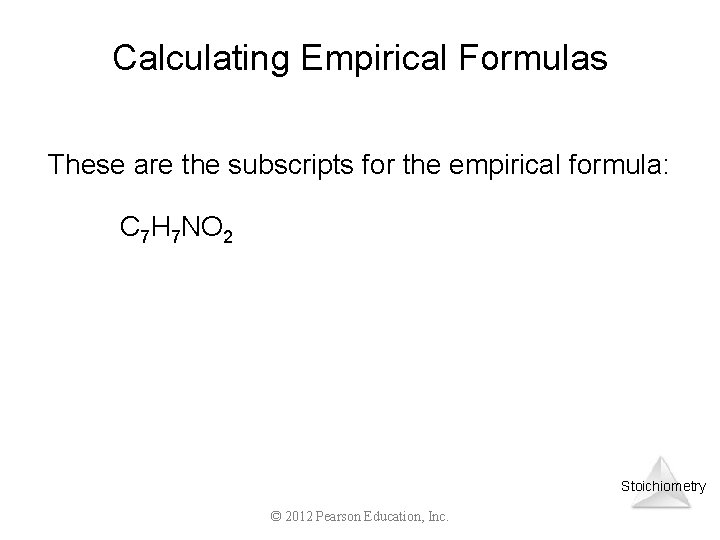
Calculating Empirical Formulas These are the subscripts for the empirical formula: C 7 H 7 NO 2 Stoichiometry © 2012 Pearson Education, Inc.

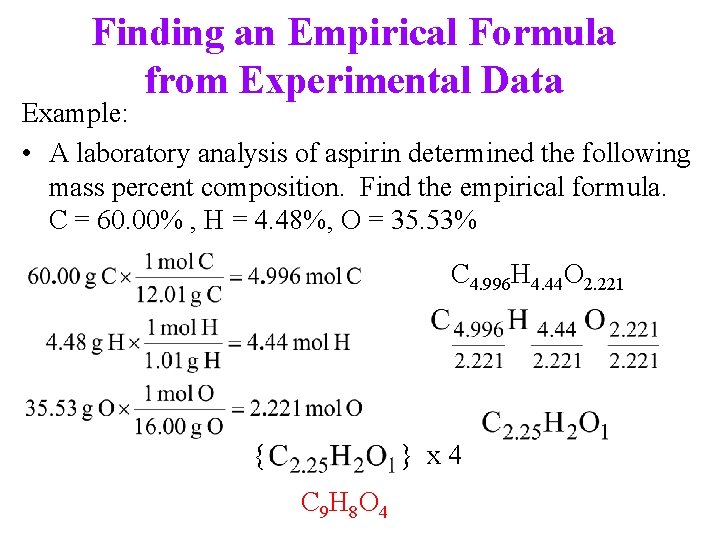
Finding an Empirical Formula from Experimental Data Example: • A laboratory analysis of aspirin determined the following mass percent composition. Find the empirical formula. C = 60. 00% , H = 4. 48%, O = 35. 53% C 4. 996 H 4. 44 O 2. 221 { } x 4 C 9 H 8 O 4

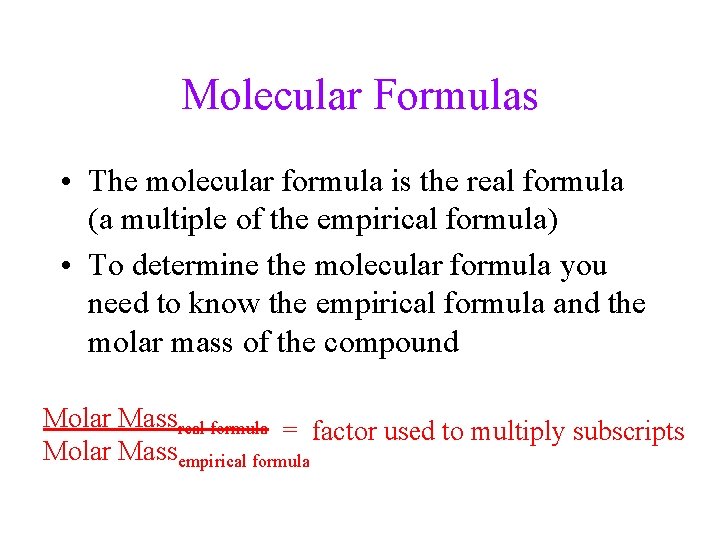
Molecular Formulas • The molecular formula is the real formula (a multiple of the empirical formula) • To determine the molecular formula you need to know the empirical formula and the molar mass of the compound Molar Massreal formula = factor used to multiply subscripts Molar Massempirical formula

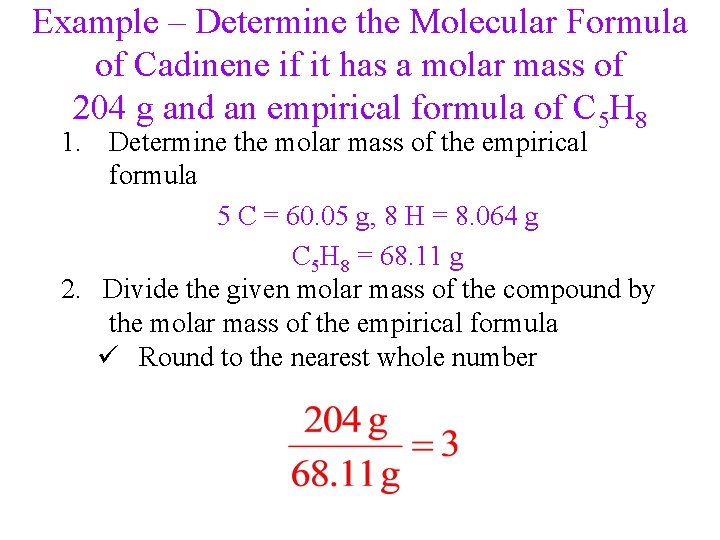
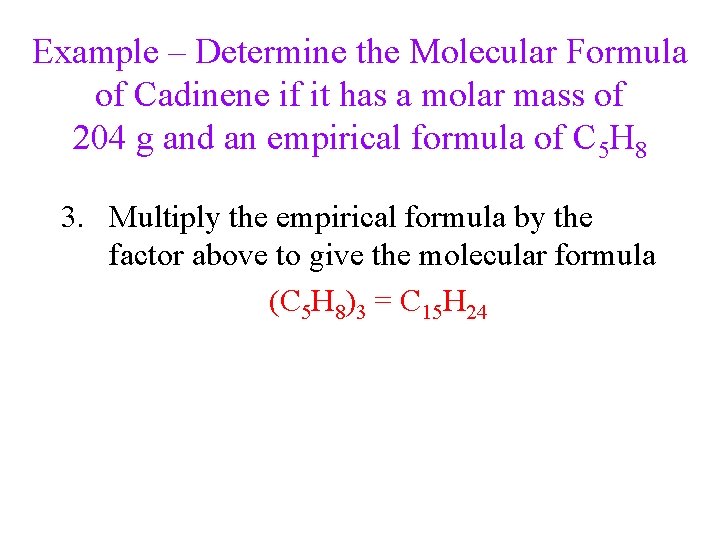
Example – Determine the Molecular Formula of Cadinene if it has a molar mass of 204 g and an empirical formula of C 5 H 8 1. Determine the molar mass of the empirical formula 5 C = 60. 05 g, 8 H = 8. 064 g C 5 H 8 = 68. 11 g 2. Divide the given molar mass of the compound by the molar mass of the empirical formula ü Round to the nearest whole number

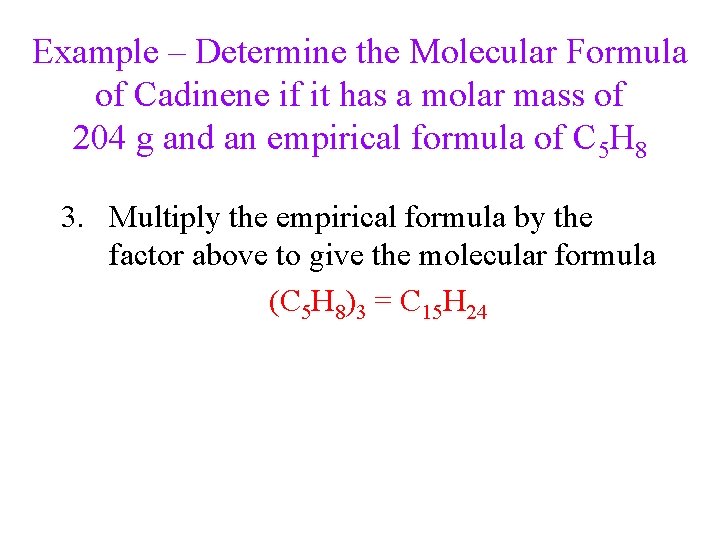
Example – Determine the Molecular Formula of Cadinene if it has a molar mass of 204 g and an empirical formula of C 5 H 8 3. Multiply the empirical formula by the factor above to give the molecular formula (C 5 H 8)3 = C 15 H 24

Example – Determine the Molecular Formula of Cadinene if it has a molar mass of 204 g and an empirical formula of C 5 H 8 3. Multiply the empirical formula by the factor above to give the molecular formula (C 5 H 8)3 = C 15 H 24

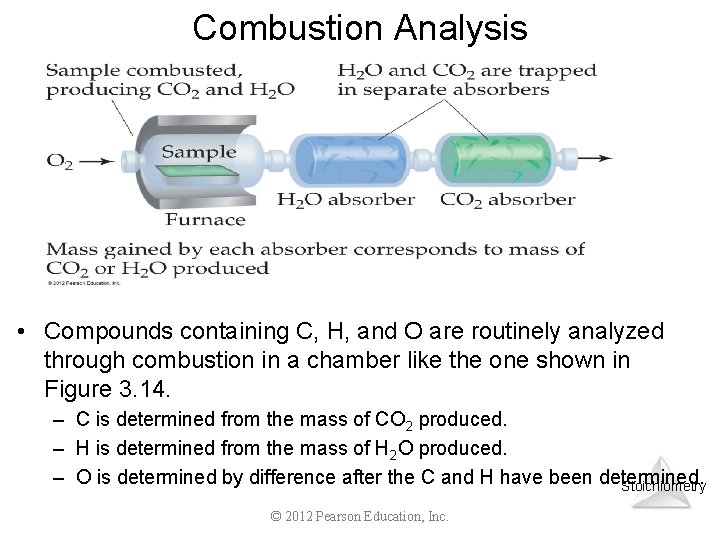
Combustion Analysis • Compounds containing C, H, and O are routinely analyzed through combustion in a chamber like the one shown in Figure 3. 14. – C is determined from the mass of CO 2 produced. – H is determined from the mass of H 2 O produced. – O is determined by difference after the C and H have been determined. Stoichiometry © 2012 Pearson Education, Inc.

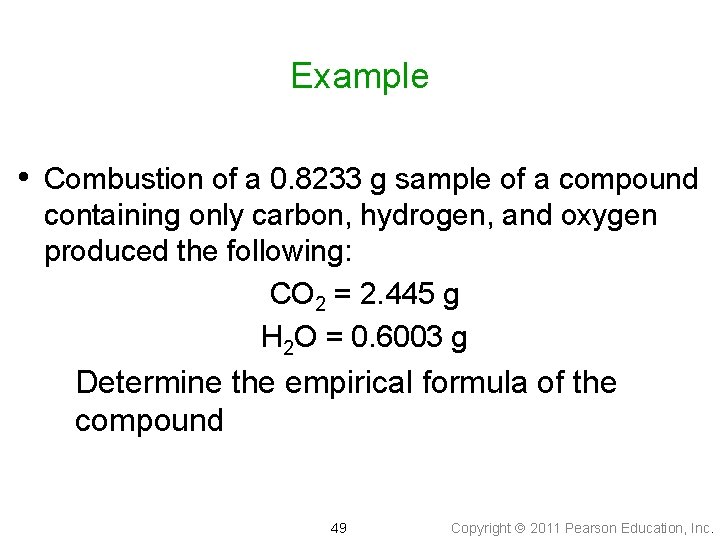
Example • Combustion of a 0. 8233 g sample of a compound containing only carbon, hydrogen, and oxygen produced the following: CO 2 = 2. 445 g H 2 O = 0. 6003 g Determine the empirical formula of the compound 49 Copyright 2011 Pearson Education, Inc.

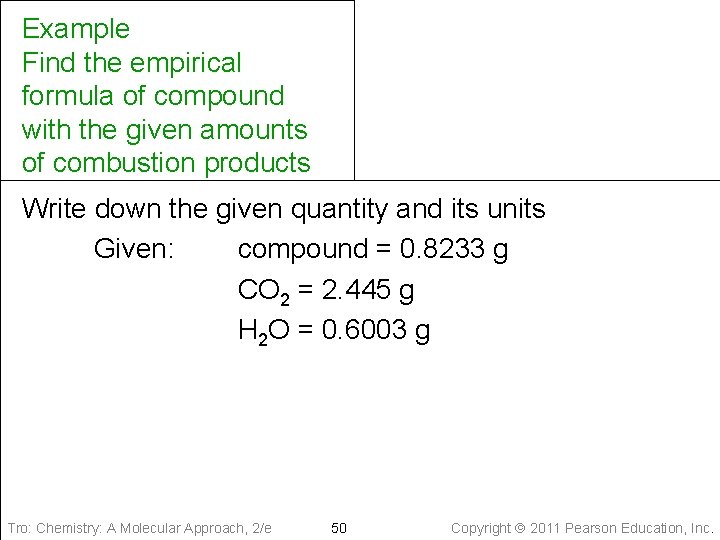
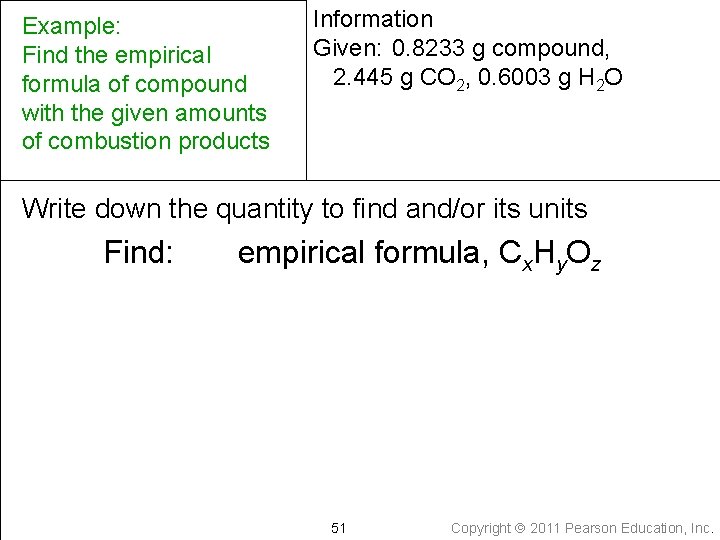
Example Find the empirical formula of compound with the given amounts of combustion products Write down the given quantity and its units Given: compound = 0. 8233 g CO 2 = 2. 445 g H 2 O = 0. 6003 g Tro: Chemistry: A Molecular Approach, 2/e 50 Copyright 2011 Pearson Education, Inc.

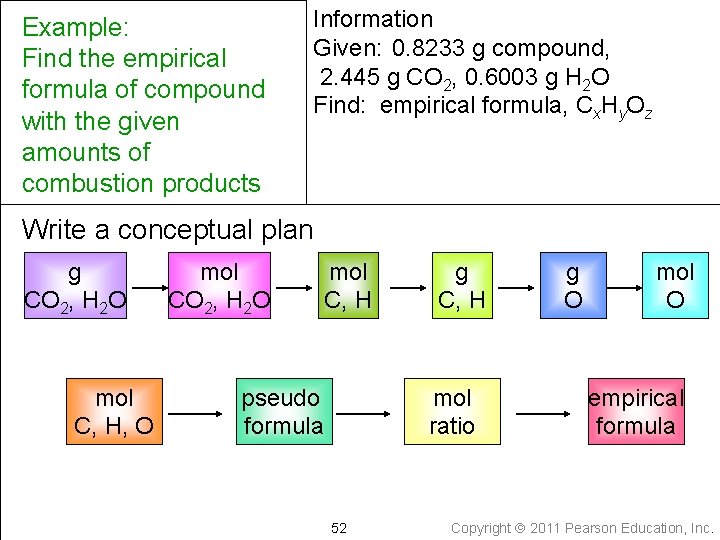
Example: Find the empirical formula of compound with the given amounts of combustion products Information Given: 0. 8233 g compound, 2. 445 g CO 2, 0. 6003 g H 2 O Write down the quantity to find and/or its units Find: empirical formula, Cx. Hy. Oz 51 Copyright 2011 Pearson Education, Inc.

Example: Find the empirical formula of compound with the given amounts of combustion products Information Given: 0. 8233 g compound, 2. 445 g CO 2, 0. 6003 g H 2 O Find: empirical formula, Cx. Hy. Oz Write a conceptual plan g CO 2, H 2 O mol C, H, O mol CO 2, H 2 O mol C, H pseudo formula g C, H mol ratio 52 g O mol O empirical formula Copyright 2011 Pearson Education, Inc.

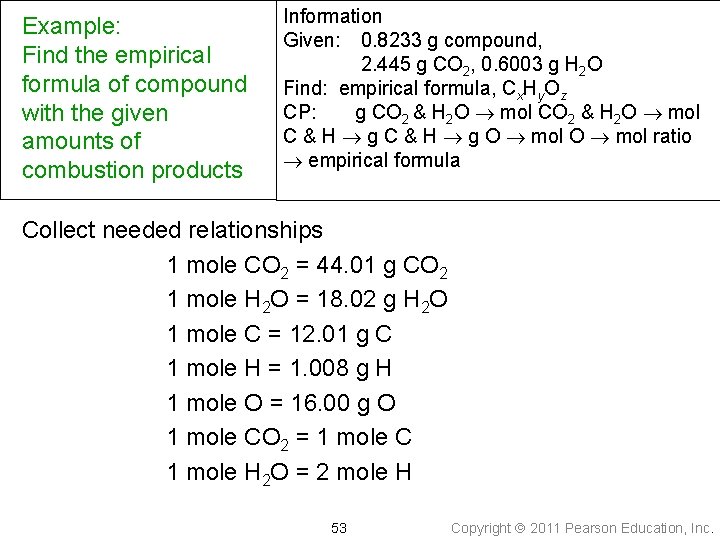
Example: Find the empirical formula of compound with the given amounts of combustion products Information Given: 0. 8233 g compound, 2. 445 g CO 2, 0. 6003 g H 2 O Find: empirical formula, Cx. Hy. Oz CP: g CO 2 & H 2 O mol C & H g O mol ratio empirical formula Collect needed relationships 1 mole CO 2 = 44. 01 g CO 2 1 mole H 2 O = 18. 02 g H 2 O 1 mole C = 12. 01 g C 1 mole H = 1. 008 g H 1 mole O = 16. 00 g O 1 mole CO 2 = 1 mole C 1 mole H 2 O = 2 mole H 53 Copyright 2011 Pearson Education, Inc.

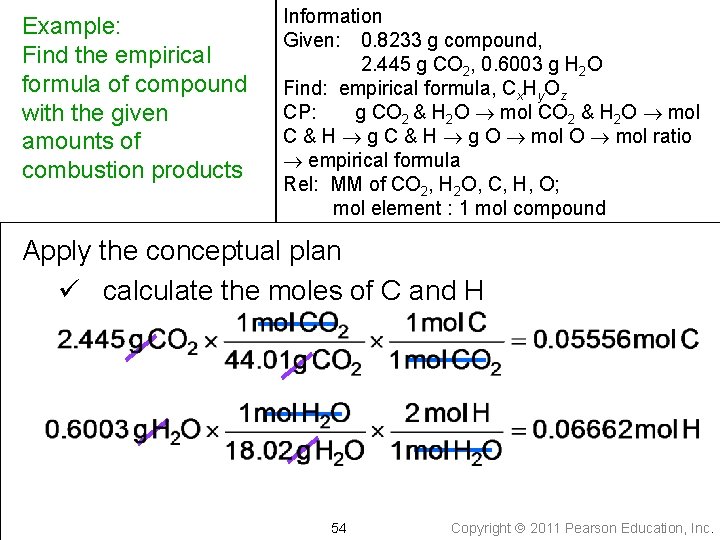
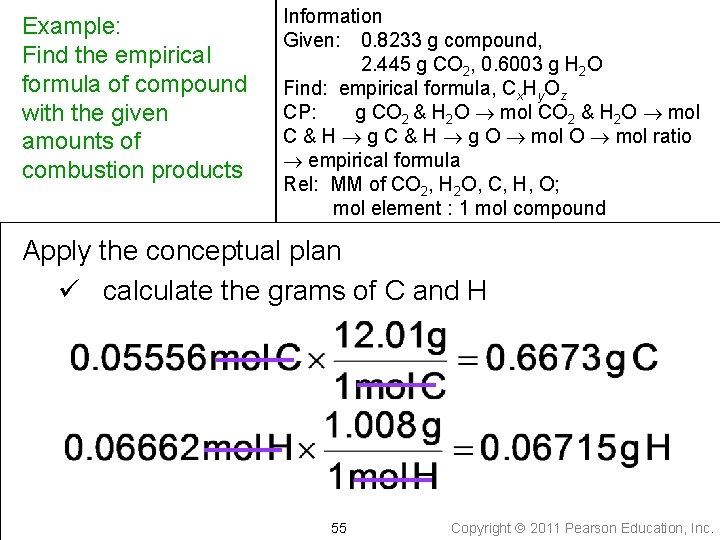
Example: Find the empirical formula of compound with the given amounts of combustion products Information Given: 0. 8233 g compound, 2. 445 g CO 2, 0. 6003 g H 2 O Find: empirical formula, Cx. Hy. Oz CP: g CO 2 & H 2 O mol C & H g O mol ratio empirical formula Rel: MM of CO 2, H 2 O, C, H, O; mol element : 1 mol compound Apply the conceptual plan ü calculate the moles of C and H 54 Copyright 2011 Pearson Education, Inc.

Example: Find the empirical formula of compound with the given amounts of combustion products Information Given: 0. 8233 g compound, 2. 445 g CO 2, 0. 6003 g H 2 O Find: empirical formula, Cx. Hy. Oz CP: g CO 2 & H 2 O mol C & H g O mol ratio empirical formula Rel: MM of CO 2, H 2 O, C, H, O; mol element : 1 mol compound Apply the conceptual plan ü calculate the grams of C and H 55 Copyright 2011 Pearson Education, Inc.

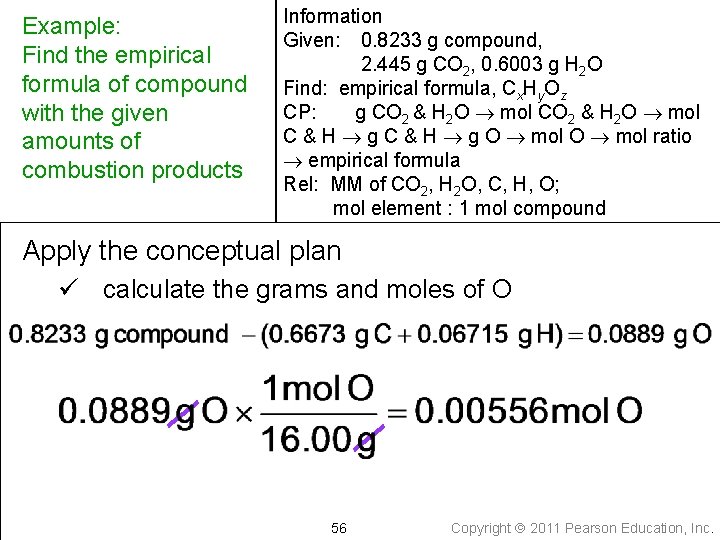
Example: Find the empirical formula of compound with the given amounts of combustion products Information Given: 0. 8233 g compound, 2. 445 g CO 2, 0. 6003 g H 2 O Find: empirical formula, Cx. Hy. Oz CP: g CO 2 & H 2 O mol C & H g O mol ratio empirical formula Rel: MM of CO 2, H 2 O, C, H, O; mol element : 1 mol compound Apply the conceptual plan ü calculate the grams and moles of O 56 Copyright 2011 Pearson Education, Inc.

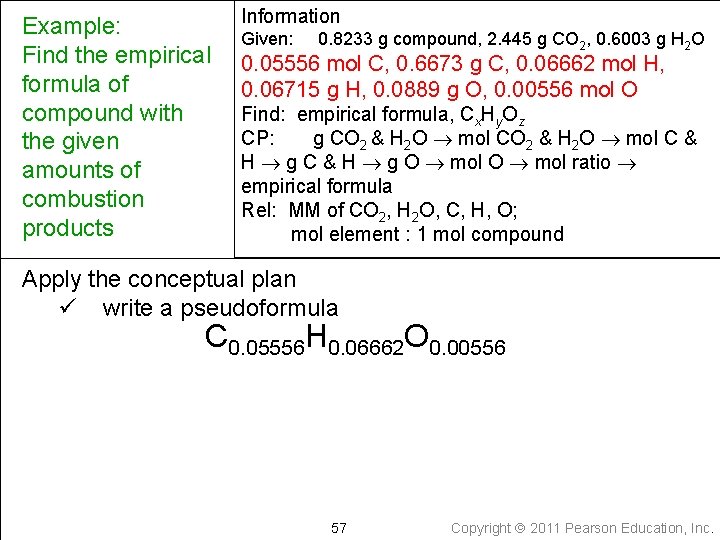
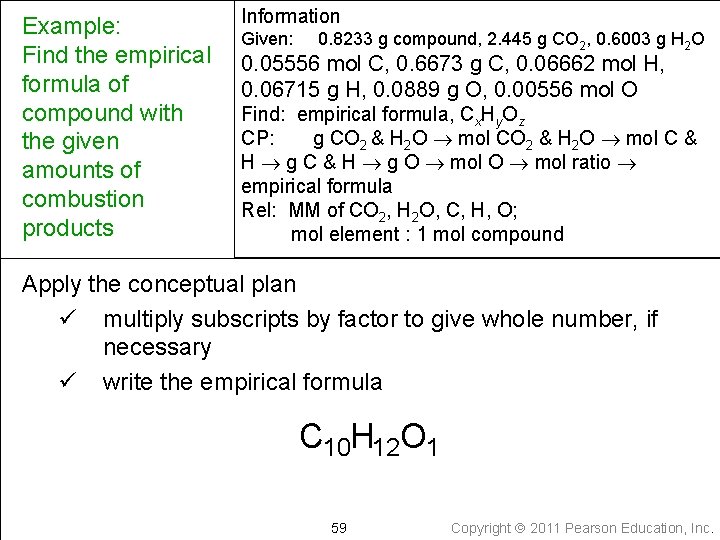
Example: Find the empirical formula of compound with the given amounts of combustion products Information Given: 0. 8233 g compound, 2. 445 g CO 2, 0. 6003 g H 2 O 0. 05556 mol C, 0. 6673 g C, 0. 06662 mol H, 0. 06715 g H, 0. 0889 g O, 0. 00556 mol O Find: empirical formula, Cx. Hy. Oz CP: g CO 2 & H 2 O mol C & H g O mol ratio empirical formula Rel: MM of CO 2, H 2 O, C, H, O; mol element : 1 mol compound Apply the conceptual plan ü write a pseudoformula C 0. 05556 H 0. 06662 O 0. 00556 57 Copyright 2011 Pearson Education, Inc.

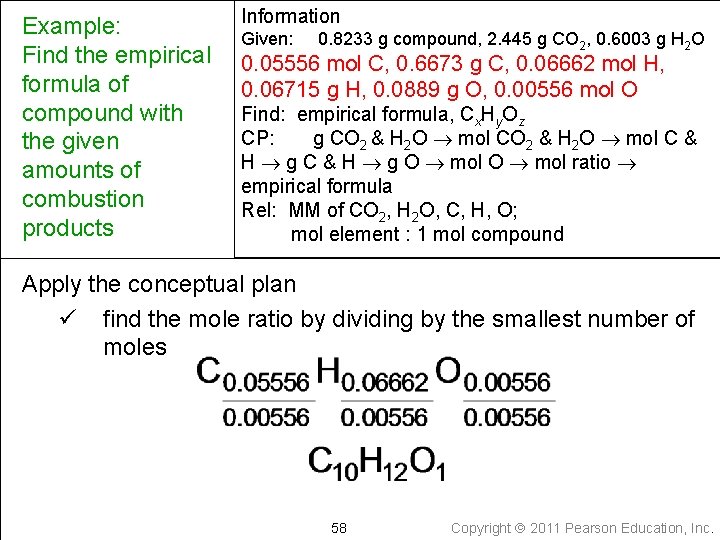
Example: Find the empirical formula of compound with the given amounts of combustion products Information Given: 0. 8233 g compound, 2. 445 g CO 2, 0. 6003 g H 2 O 0. 05556 mol C, 0. 6673 g C, 0. 06662 mol H, 0. 06715 g H, 0. 0889 g O, 0. 00556 mol O Find: empirical formula, Cx. Hy. Oz CP: g CO 2 & H 2 O mol C & H g O mol ratio empirical formula Rel: MM of CO 2, H 2 O, C, H, O; mol element : 1 mol compound Apply the conceptual plan ü find the mole ratio by dividing by the smallest number of moles 58 Copyright 2011 Pearson Education, Inc.

Example: Find the empirical formula of compound with the given amounts of combustion products Information Given: 0. 8233 g compound, 2. 445 g CO 2, 0. 6003 g H 2 O 0. 05556 mol C, 0. 6673 g C, 0. 06662 mol H, 0. 06715 g H, 0. 0889 g O, 0. 00556 mol O Find: empirical formula, Cx. Hy. Oz CP: g CO 2 & H 2 O mol C & H g O mol ratio empirical formula Rel: MM of CO 2, H 2 O, C, H, O; mol element : 1 mol compound Apply the conceptual plan ü multiply subscripts by factor to give whole number, if necessary ü write the empirical formula C 10 H 12 O 1 59 Copyright 2011 Pearson Education, Inc.

Quantities in Chemical Reactions • The amount of every substance used and made in a chemical reaction is related to the amounts of all the other substances in the reaction ü Law of Conservation of Mass ü Balancing equations by balancing atoms • The study of the numerical relationship between chemical quantities in a chemical reaction is called stoichiometry 60 Copyright © 2011 Pearson Education, Inc.

Reaction Stoichiometry • The coefficients in a balanced chemical equation specify the relative amounts in moles of each of the substances involved in the reaction 2 C 8 H 18(l) + 25 O 2(g) 16 CO 2(g) + 18 H 2 O(g) 2 molecules of C 8 H 18 react with 25 molecules of O 2 to form 16 molecules of CO 2 and 18 molecules of H 2 O 2 moles of C 8 H 18 react with 25 moles of O 2 to form 16 moles of CO 2 and 18 moles of H 2 O 2 mol C 8 H 18 : 25 mol O 2 : 16 mol CO 2 : 18 mol H 2 O 61 Copyright © 2011 Pearson Education, Inc.

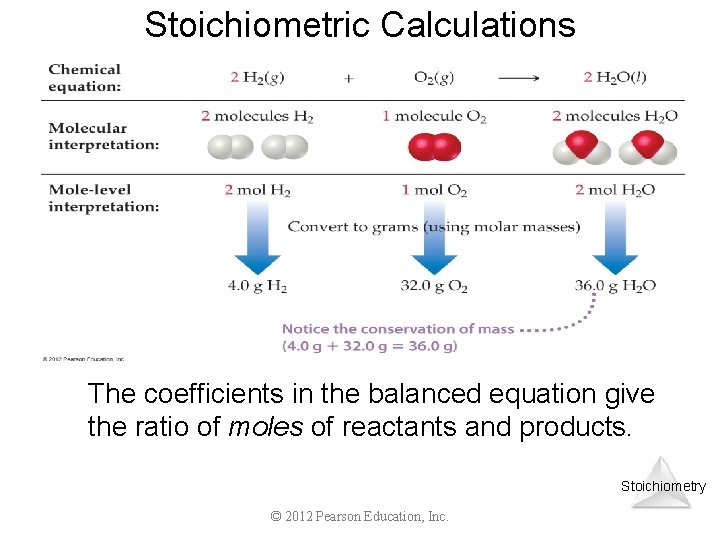
Stoichiometric Calculations The coefficients in the balanced equation give the ratio of moles of reactants and products. Stoichiometry © 2012 Pearson Education, Inc.

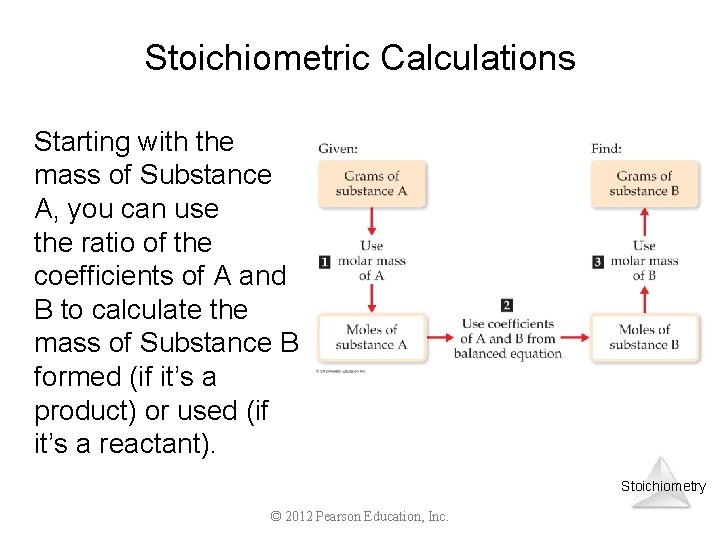
Stoichiometric Calculations Starting with the mass of Substance A, you can use the ratio of the coefficients of A and B to calculate the mass of Substance B formed (if it’s a product) or used (if it’s a reactant). Stoichiometry © 2012 Pearson Education, Inc.

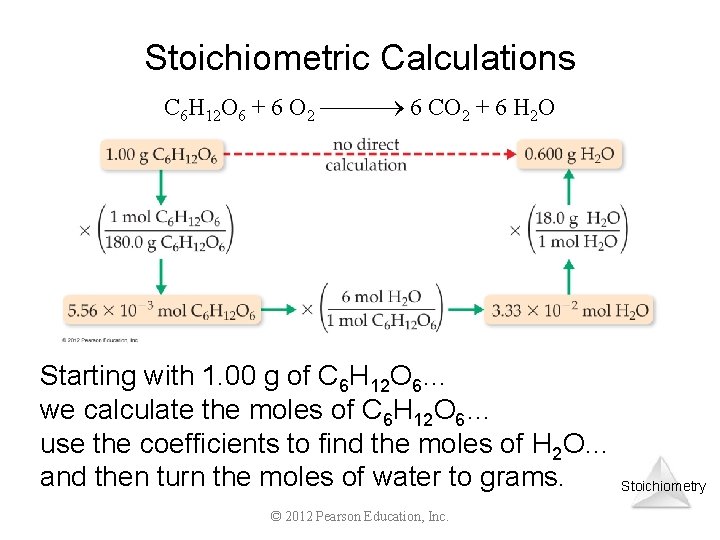
Stoichiometric Calculations C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O Starting with 1. 00 g of C 6 H 12 O 6… we calculate the moles of C 6 H 12 O 6… use the coefficients to find the moles of H 2 O… and then turn the moles of water to grams. © 2012 Pearson Education, Inc. Stoichiometry

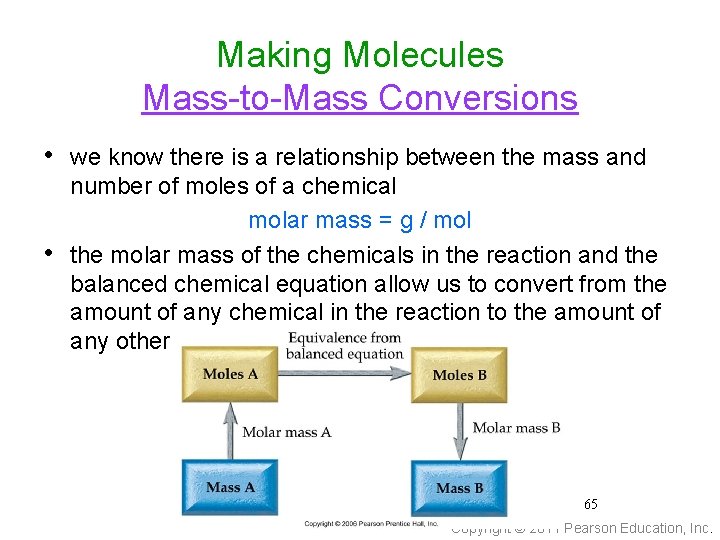
Making Molecules Mass-to-Mass Conversions • we know there is a relationship between the mass and • number of moles of a chemical molar mass = g / mol the molar mass of the chemicals in the reaction and the balanced chemical equation allow us to convert from the amount of any chemical in the reaction to the amount of any other 65 Copyright © 2011 Pearson Education, Inc.

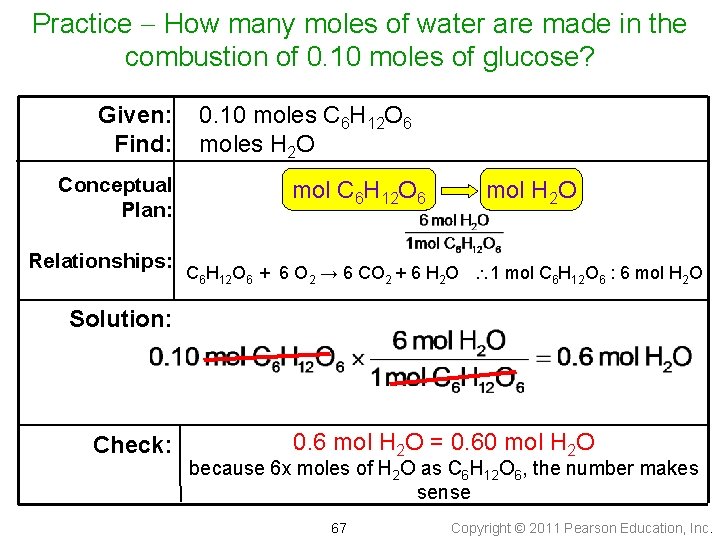
Practice • According to the following equation, how many moles of water are made in the combustion of 0. 10 moles of glucose? C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O 66 Copyright © 2011 Pearson Education, Inc.

Practice How many moles of water are made in the combustion of 0. 10 moles of glucose? Given: Find: Conceptual Plan: Relationships: 0. 10 moles C 6 H 12 O 6 moles H 2 O mol C 6 H 12 O 6 mol H 2 O C 6 H 12 O 6 + 6 O 2 → 6 CO 2 + 6 H 2 O 1 mol C 6 H 12 O 6 : 6 mol H 2 O Solution: Check: 0. 6 mol H 2 O = 0. 60 mol H 2 O because 6 x moles of H 2 O as C 6 H 12 O 6, the number makes sense 67 Copyright © 2011 Pearson Education, Inc.

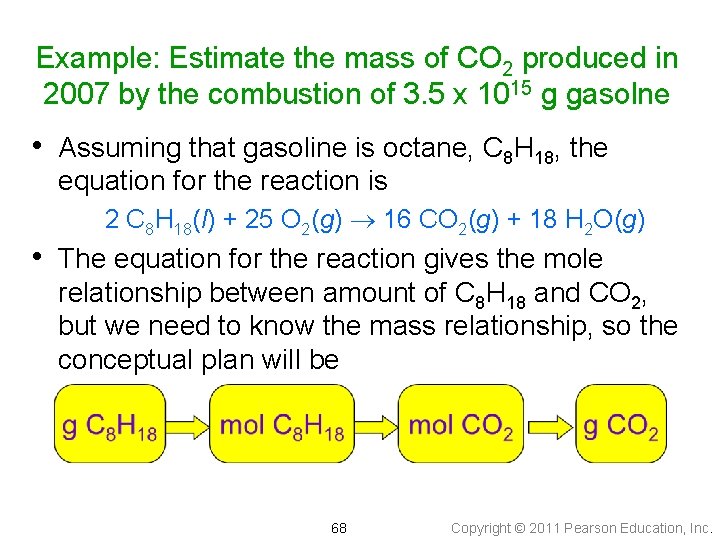
Example: Estimate the mass of CO 2 produced in 2007 by the combustion of 3. 5 x 1015 g gasolne • Assuming that gasoline is octane, C 8 H 18, the equation for the reaction is 2 C 8 H 18(l) + 25 O 2(g) 16 CO 2(g) + 18 H 2 O(g) • The equation for the reaction gives the mole relationship between amount of C 8 H 18 and CO 2, but we need to know the mass relationship, so the conceptual plan will be 68 Copyright © 2011 Pearson Education, Inc.

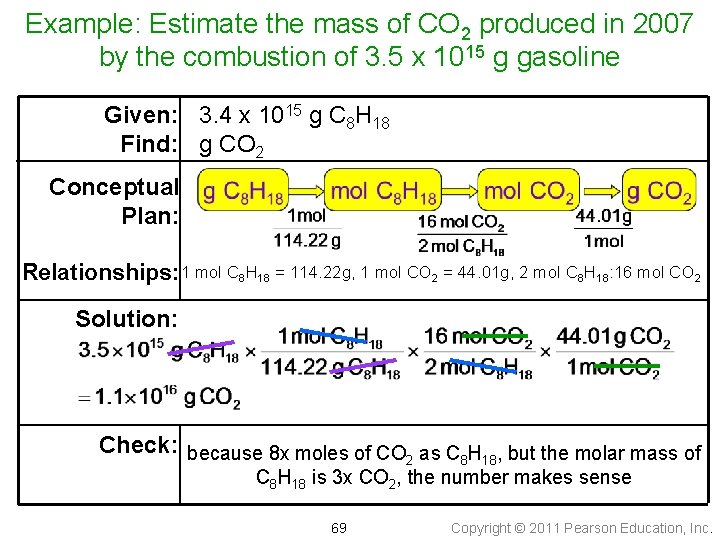
Example: Estimate the mass of CO 2 produced in 2007 by the combustion of 3. 5 x 1015 g gasoline Given: 3. 4 x 1015 g C 8 H 18 Find: g CO 2 Conceptual Plan: Relationships: 1 mol C 8 H 18 = 114. 22 g, 1 mol CO 2 = 44. 01 g, 2 mol C 8 H 18: 16 mol CO 2 Solution: Check: because 8 x moles of CO as C H , but the molar mass of 2 8 18 C 8 H 18 is 3 x CO 2, the number makes sense 69 Copyright © 2011 Pearson Education, Inc.

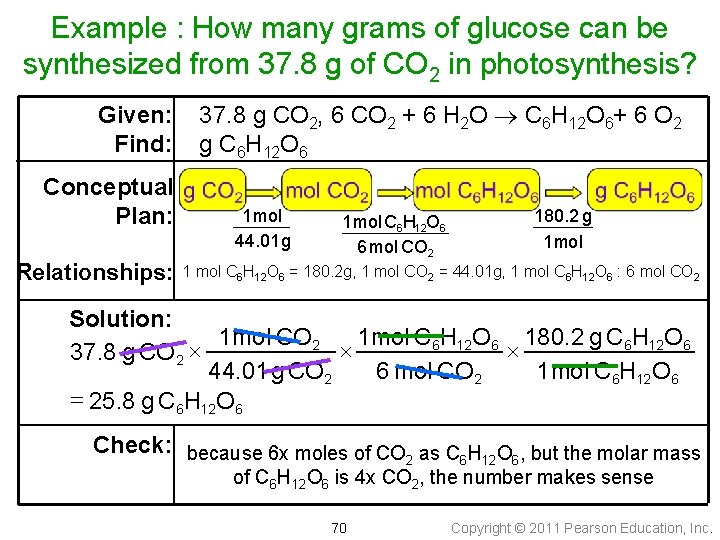
Example : How many grams of glucose can be synthesized from 37. 8 g of CO 2 in photosynthesis? Given: Find: Conceptual Plan: Relationships: 37. 8 g CO 2, 6 CO 2 + 6 H 2 O C 6 H 12 O 6+ 6 O 2 g C 6 H 12 O 6 1 mol 44. 01 g 1 mol C 6 H 12 O 6 6 mol CO 2 180. 2 g 1 mol C 6 H 12 O 6 = 180. 2 g, 1 mol CO 2 = 44. 01 g, 1 mol C 6 H 12 O 6 : 6 mol CO 2 Solution: 1 mol C 6 H 12 O 6 180. 2 g C 6 H 12 O 6 1 mol CO 2 37. 8 g CO 2 44. 01 g CO 2 6 mol CO 2 1 mol C 6 H 12 O 6 = 25. 8 g C 6 H 12 O 6 Check: because 6 x moles of CO as C H O , but the molar mass 2 6 12 6 of C 6 H 12 O 6 is 4 x CO 2, the number makes sense 70 Copyright © 2011 Pearson Education, Inc.

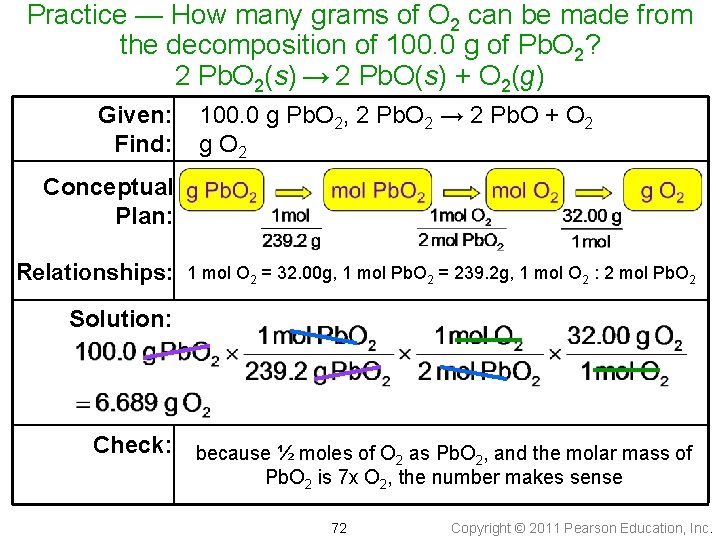
Practice — How many grams of O 2 can be made from the decomposition of 100. 0 g of Pb. O 2? 2 Pb. O 2(s) → 2 Pb. O(s) + O 2(g) (Pb. O 2 = 239. 2, O 2 = 32. 00) 71 Copyright © 2011 Pearson Education, Inc.

Practice — How many grams of O 2 can be made from the decomposition of 100. 0 g of Pb. O 2? 2 Pb. O 2(s) → 2 Pb. O(s) + O 2(g) Given: Find: 100. 0 g Pb. O 2, 2 Pb. O 2 → 2 Pb. O + O 2 g O 2 Conceptual Plan: Relationships: 1 mol O 2 = 32. 00 g, 1 mol Pb. O 2 = 239. 2 g, 1 mol O 2 : 2 mol Pb. O 2 Solution: Check: because ½ moles of O as Pb. O , and the molar mass of 2 2 Pb. O 2 is 7 x O 2, the number makes sense 72 Copyright © 2011 Pearson Education, Inc.

Limiting Reactants Stoichiometry © 2012 Pearson Education, Inc.

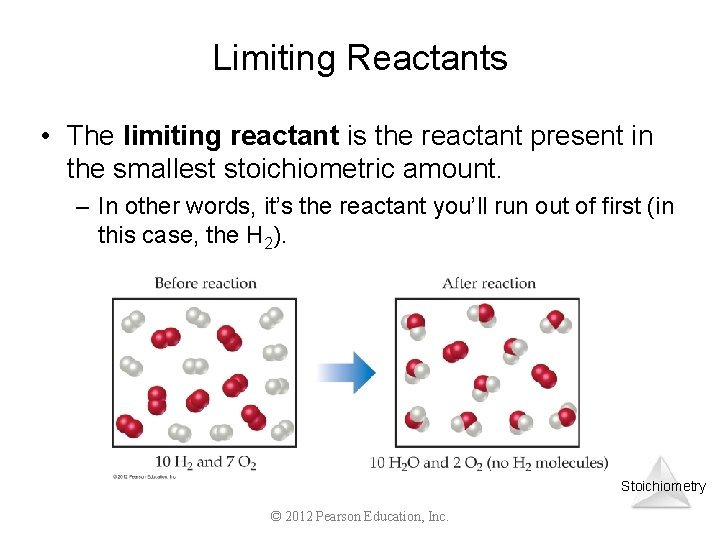
Limiting Reactants • The limiting reactant is the reactant present in the smallest stoichiometric amount. – In other words, it’s the reactant you’ll run out of first (in this case, the H 2). Stoichiometry © 2012 Pearson Education, Inc.

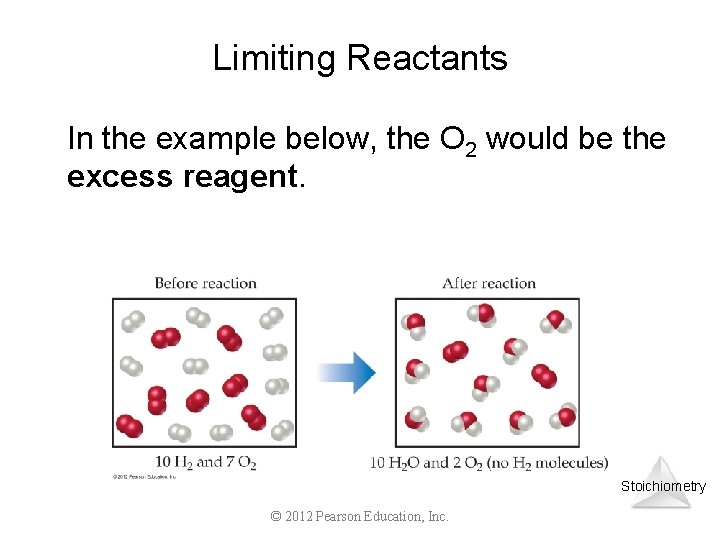
Limiting Reactants In the example below, the O 2 would be the excess reagent. Stoichiometry © 2012 Pearson Education, Inc.

The Limiting Reactant • For reactions with multiple reactants, it is likely • • that one of the reactants will be completely used before the others When this reactant is used up, the reaction stops and no more product is made The reactant that limits the amount of product is called the limiting reactant ü sometimes called the limiting reagent ü the limiting reactant gets completely consumed • Reactants not completely consumed are called • excess reactants The amount of product that can be made from the limiting reactant is called theoretical yield 76 Copyright © 2011 Pearson Education, Inc.

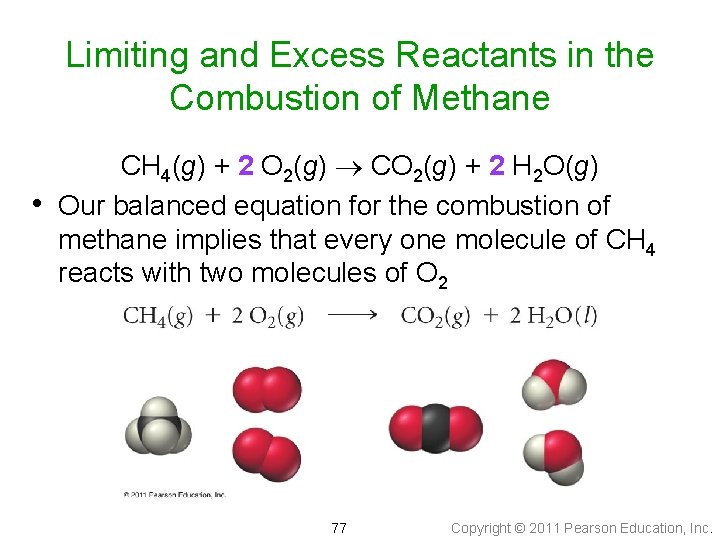
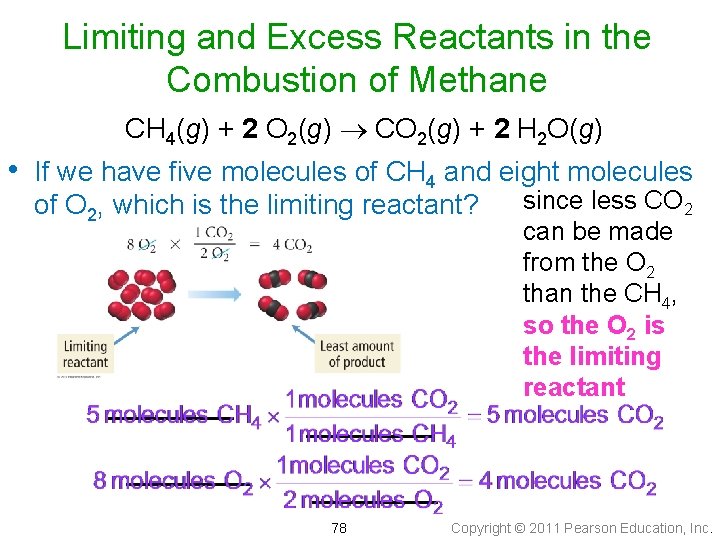
Limiting and Excess Reactants in the Combustion of Methane • CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(g) Our balanced equation for the combustion of methane implies that every one molecule of CH 4 reacts with two molecules of O 2 77 Copyright © 2011 Pearson Education, Inc.

Limiting and Excess Reactants in the Combustion of Methane CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(g) • If we have five molecules of CH 4 and eight molecules of O 2, which is the limiting reactant? 78 since less CO 2 can be made from the O 2 than the CH 4, so the O 2 is the limiting reactant Copyright © 2011 Pearson Education, Inc.

Theoretical Yield • The theoretical yield is the maximum amount of product that can be made. – In other words, it’s the amount of product possible as calculated through the stoichiometry problem. • This is different from the actual yield, which is the amount one actually produces and measures. Stoichiometry © 2012 Pearson Education, Inc.

Theoretical and Actual Yield • As we did with the pizzas, in order to determine • theoretical yield, we should use reaction stoichiometry to determine the amount of product each of our reactants could make The theoretical yield will always be the least possible amount of product ü theoretical yield will always come from the limiting reactant • Because of both controllable and uncontrollable factors, the actual yield of product will always be less than theoretical yield 80 Copyright © 2011 Pearson Education, Inc.

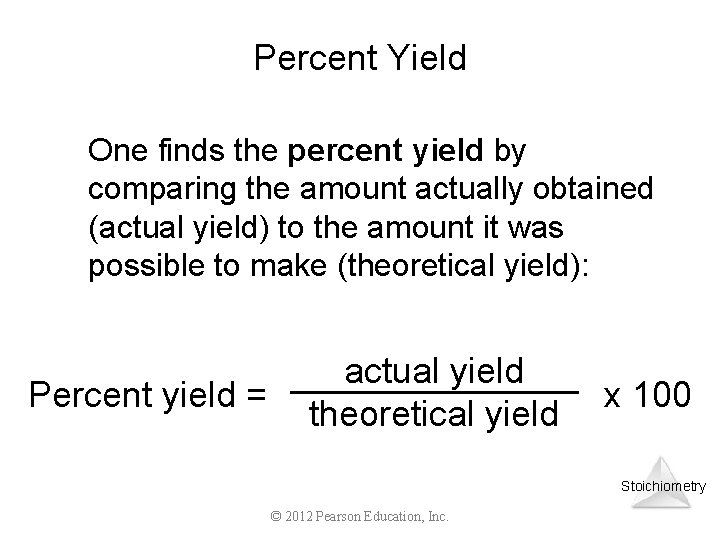
Percent Yield One finds the percent yield by comparing the amount actually obtained (actual yield) to the amount it was possible to make (theoretical yield): Percent yield = actual yield theoretical yield x 100 Stoichiometry © 2012 Pearson Education, Inc.

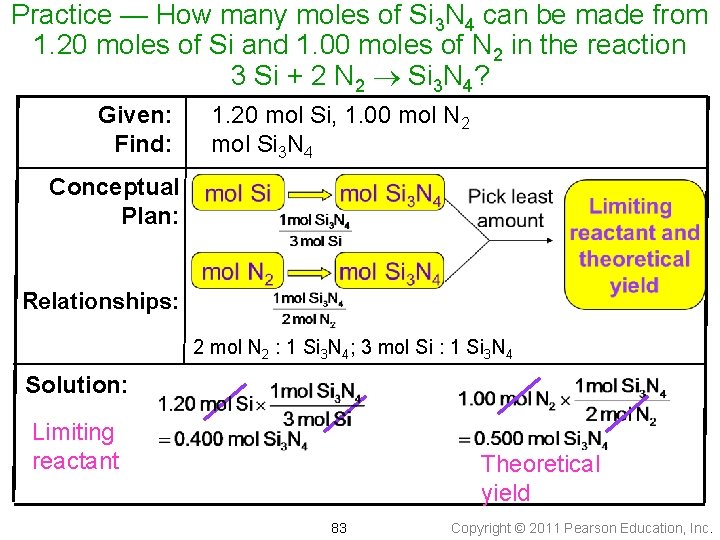
Practice — How many moles of Si 3 N 4 can be made from 1. 20 moles of Si and 1. 00 moles of N 2 in the reaction 3 Si + 2 N 2 Si 3 N 4? 82 Copyright © 2011 Pearson Education, Inc.

Practice — How many moles of Si 3 N 4 can be made from 1. 20 moles of Si and 1. 00 moles of N 2 in the reaction 3 Si + 2 N 2 Si 3 N 4? Given: Find: 1. 20 mol Si, 1. 00 mol N 2 mol Si 3 N 4 Conceptual Plan: Relationships: 2 mol N 2 : 1 Si 3 N 4; 3 mol Si : 1 Si 3 N 4 Solution: Limiting reactant Theoretical yield 83 Copyright © 2011 Pearson Education, Inc.

Example Finding limiting reactant, theoretical yield, and percent yield Copyright © 2011 Pearson Education, Inc.

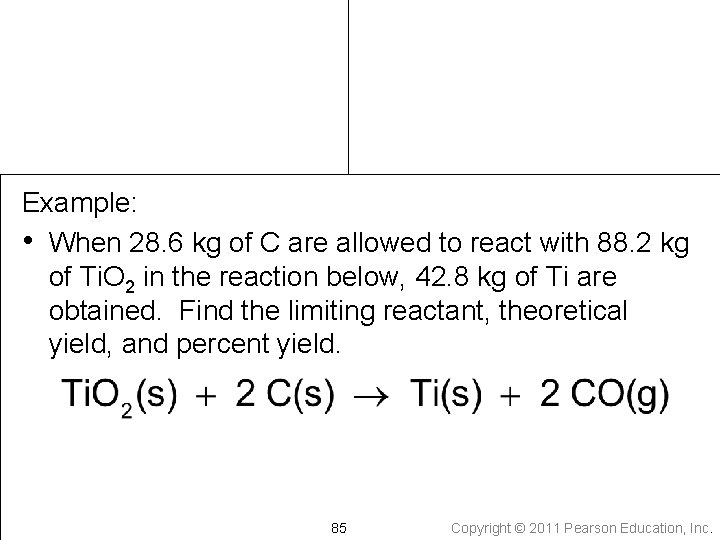
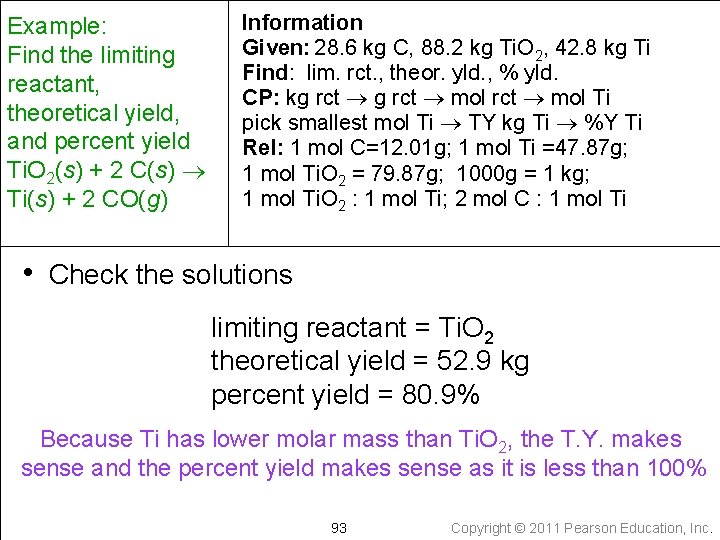
Example: • When 28. 6 kg of C are allowed to react with 88. 2 kg of Ti. O 2 in the reaction below, 42. 8 kg of Ti are obtained. Find the limiting reactant, theoretical yield, and percent yield. 85 Copyright © 2011 Pearson Education, Inc.

Example: When 28. 6 kg of C reacts with 88. 2 kg of Ti. O 2, 42. 8 kg of Ti are obtained. Find the limiting reactant, theoretical yield, and percent yield Ti. O 2(s) + 2 C(s) Ti(s) + 2 CO(g) • Write down the given quantity and its units Given: 28. 6 kg C 88. 2 kg Ti. O 2 42. 8 kg Ti produced 86 Copyright © 2011 Pearson Education, Inc.

Information Example: Given: 28. 6 kg C, 88. 2 kg Ti. O 2, 42. 8 kg Ti Find the limiting reactant, theoretical yield, and percent yield Ti. O 2(s) + 2 C(s) Ti(s) + 2 CO(g) • Write down the quantity to find and/or its units Find: limiting reactant theoretical yield percent yield 87 Copyright © 2011 Pearson Education, Inc.

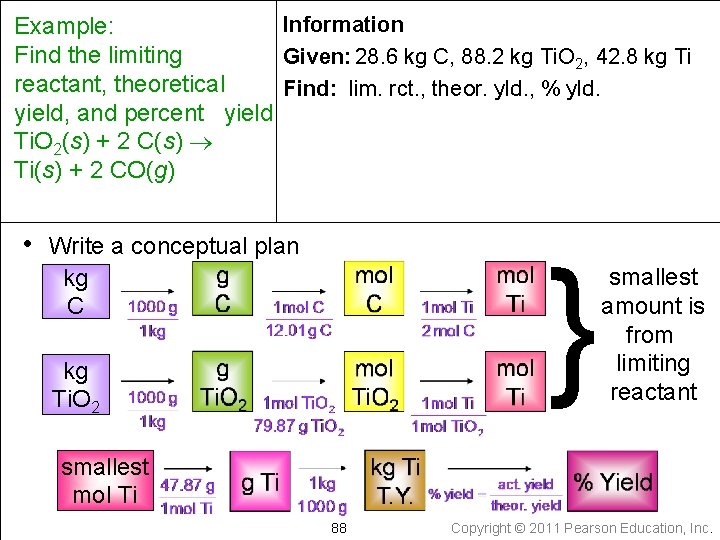
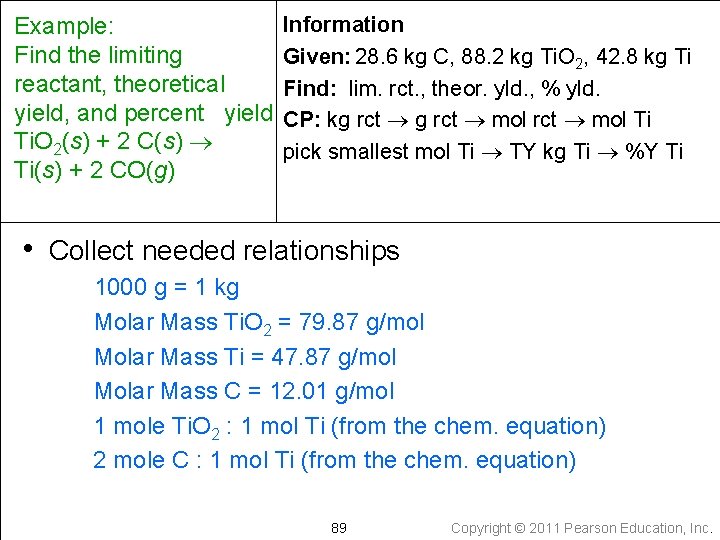
Information Example: Find the limiting Given: 28. 6 kg C, 88. 2 kg Ti. O 2, 42. 8 kg Ti reactant, theoretical Find: lim. rct. , theor. yld. , % yld. yield, and percent yield Ti. O 2(s) + 2 C(s) Ti(s) + 2 CO(g) • Write a conceptual plan } kg C kg Ti. O 2 smallest amount is from limiting reactant smallest mol Ti 88 Copyright © 2011 Pearson Education, Inc.

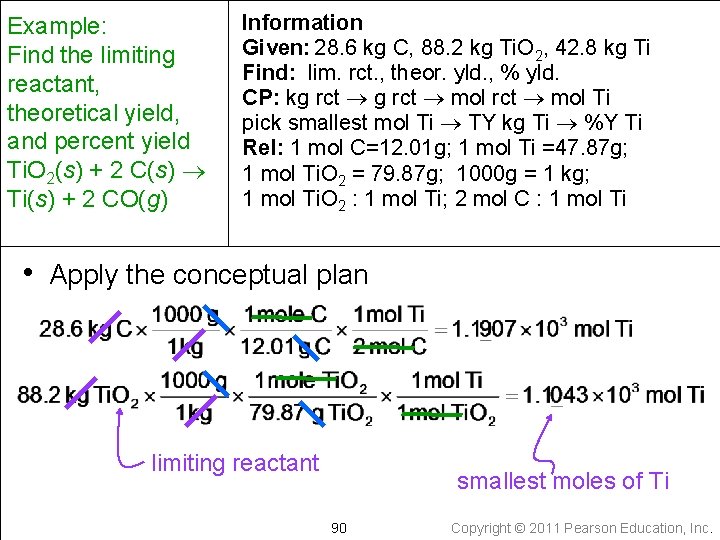
Example: Find the limiting reactant, theoretical yield, and percent yield Ti. O 2(s) + 2 C(s) Ti(s) + 2 CO(g) Information Given: 28. 6 kg C, 88. 2 kg Ti. O 2, 42. 8 kg Ti Find: lim. rct. , theor. yld. , % yld. CP: kg rct mol Ti pick smallest mol Ti TY kg Ti %Y Ti • Collect needed relationships 1000 g = 1 kg Molar Mass Ti. O 2 = 79. 87 g/mol Molar Mass Ti = 47. 87 g/mol Molar Mass C = 12. 01 g/mol 1 mole Ti. O 2 : 1 mol Ti (from the chem. equation) 2 mole C : 1 mol Ti (from the chem. equation) 89 Copyright © 2011 Pearson Education, Inc.

Example: Find the limiting reactant, theoretical yield, and percent yield Ti. O 2(s) + 2 C(s) Ti(s) + 2 CO(g) Information Given: 28. 6 kg C, 88. 2 kg Ti. O 2, 42. 8 kg Ti Find: lim. rct. , theor. yld. , % yld. CP: kg rct mol Ti pick smallest mol Ti TY kg Ti %Y Ti Rel: 1 mol C=12. 01 g; 1 mol Ti =47. 87 g; 1 mol Ti. O 2 = 79. 87 g; 1000 g = 1 kg; 1 mol Ti. O 2 : 1 mol Ti; 2 mol C : 1 mol Ti • Apply the conceptual plan limiting reactant smallest moles of Ti 90 Copyright © 2011 Pearson Education, Inc.

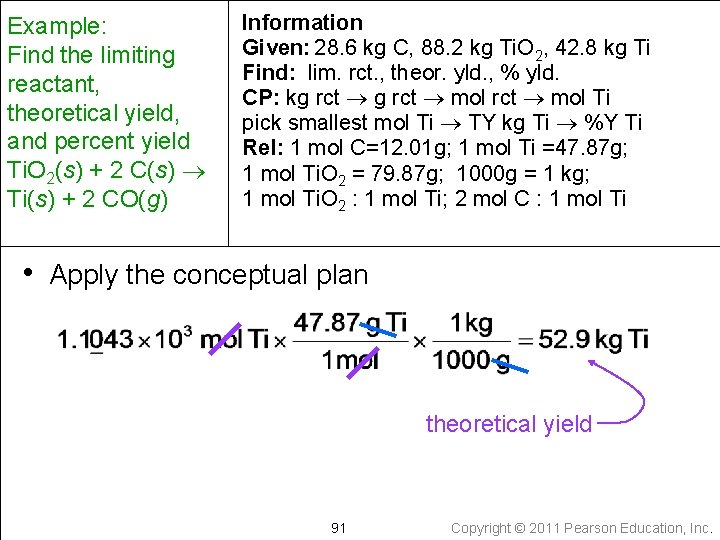
Example: Find the limiting reactant, theoretical yield, and percent yield Ti. O 2(s) + 2 C(s) Ti(s) + 2 CO(g) Information Given: 28. 6 kg C, 88. 2 kg Ti. O 2, 42. 8 kg Ti Find: lim. rct. , theor. yld. , % yld. CP: kg rct mol Ti pick smallest mol Ti TY kg Ti %Y Ti Rel: 1 mol C=12. 01 g; 1 mol Ti =47. 87 g; 1 mol Ti. O 2 = 79. 87 g; 1000 g = 1 kg; 1 mol Ti. O 2 : 1 mol Ti; 2 mol C : 1 mol Ti • Apply the conceptual plan theoretical yield 91 Copyright © 2011 Pearson Education, Inc.

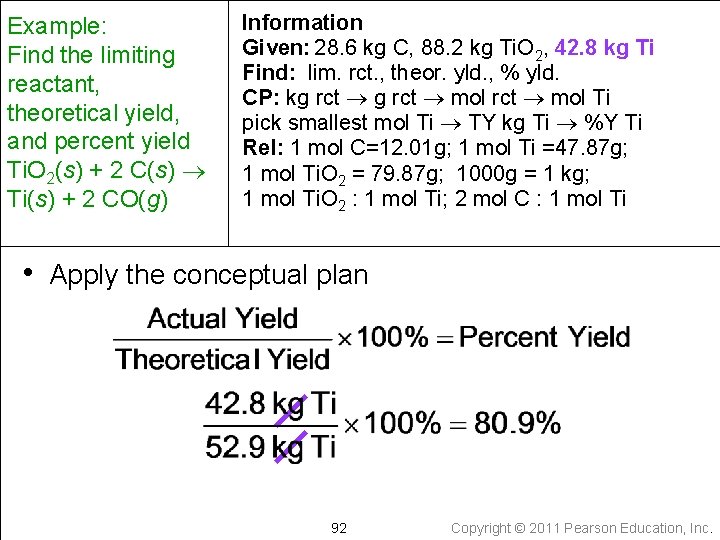
Example: Find the limiting reactant, theoretical yield, and percent yield Ti. O 2(s) + 2 C(s) Ti(s) + 2 CO(g) Information Given: 28. 6 kg C, 88. 2 kg Ti. O 2, 42. 8 kg Ti Find: lim. rct. , theor. yld. , % yld. CP: kg rct mol Ti pick smallest mol Ti TY kg Ti %Y Ti Rel: 1 mol C=12. 01 g; 1 mol Ti =47. 87 g; 1 mol Ti. O 2 = 79. 87 g; 1000 g = 1 kg; 1 mol Ti. O 2 : 1 mol Ti; 2 mol C : 1 mol Ti • Apply the conceptual plan 92 Copyright © 2011 Pearson Education, Inc.

Example: Find the limiting reactant, theoretical yield, and percent yield Ti. O 2(s) + 2 C(s) Ti(s) + 2 CO(g) Information Given: 28. 6 kg C, 88. 2 kg Ti. O 2, 42. 8 kg Ti Find: lim. rct. , theor. yld. , % yld. CP: kg rct mol Ti pick smallest mol Ti TY kg Ti %Y Ti Rel: 1 mol C=12. 01 g; 1 mol Ti =47. 87 g; 1 mol Ti. O 2 = 79. 87 g; 1000 g = 1 kg; 1 mol Ti. O 2 : 1 mol Ti; 2 mol C : 1 mol Ti • Check the solutions limiting reactant = Ti. O 2 theoretical yield = 52. 9 kg percent yield = 80. 9% Because Ti has lower molar mass than Ti. O 2, the T. Y. makes sense and the percent yield makes sense as it is less than 100% 93 Copyright © 2011 Pearson Education, Inc.

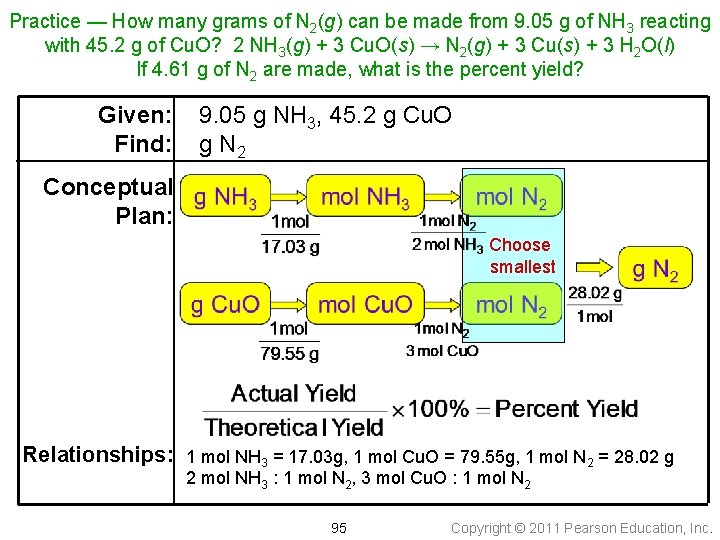
Practice — How many grams of N 2(g) can be made from 9. 05 g of NH 3 reacting with 45. 2 g of Cu. O? 2 NH 3(g) + 3 Cu. O(s) → N 2(g) + 3 Cu(s) + 3 H 2 O(l) If 4. 61 g of N 2 are made, what is the percent yield? 94 Copyright © 2011 Pearson Education, Inc.

Practice — How many grams of N 2(g) can be made from 9. 05 g of NH 3 reacting with 45. 2 g of Cu. O? 2 NH 3(g) + 3 Cu. O(s) → N 2(g) + 3 Cu(s) + 3 H 2 O(l) If 4. 61 g of N 2 are made, what is the percent yield? Given: Find: 9. 05 g NH 3, 45. 2 g Cu. O g N 2 Conceptual Plan: Choose smallest Relationships: 1 mol NH 3 = 17. 03 g, 1 mol Cu. O = 79. 55 g, 1 mol N 2 = 28. 02 g 2 mol NH 3 : 1 mol N 2, 3 mol Cu. O : 1 mol N 2 95 Copyright © 2011 Pearson Education, Inc.

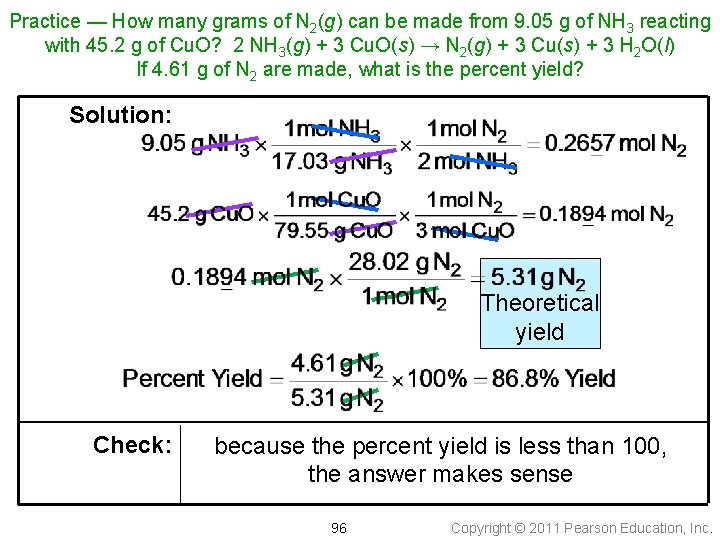
Practice — How many grams of N 2(g) can be made from 9. 05 g of NH 3 reacting with 45. 2 g of Cu. O? 2 NH 3(g) + 3 Cu. O(s) → N 2(g) + 3 Cu(s) + 3 H 2 O(l) If 4. 61 g of N 2 are made, what is the percent yield? Solution: Theoretical yield Check: because the percent yield is less than 100, the answer makes sense 96 Copyright © 2011 Pearson Education, Inc.