Stoichiometry I Stoichiometric Calculations A Proportional Relationships 2











- Slides: 18

Stoichiometry I. Stoichiometric Calculations

A. Proportional Relationships 2 1/4 c. flour 1 tsp. baking soda 1 tsp. salt 1 c. butter 3/4 c. sugar 3/4 c. brown sugar 1 tsp vanilla extract 2 eggs 2 c. chocolate chips Makes 5 dozen cookies. b. I have 5 eggs. How many cookies can I make? 5 eggs 5 doz. 2 eggs Ratio of eggs to cookies = 12. 5 dozen cookies

A. Proportional Relationships b Stoichiometry • mass relationships between substances in a chemical reaction • based on the mole ratio b Mole Ratio • indicated by coefficients in a balanced equation 2 Mg + O 2 2 Mg. O

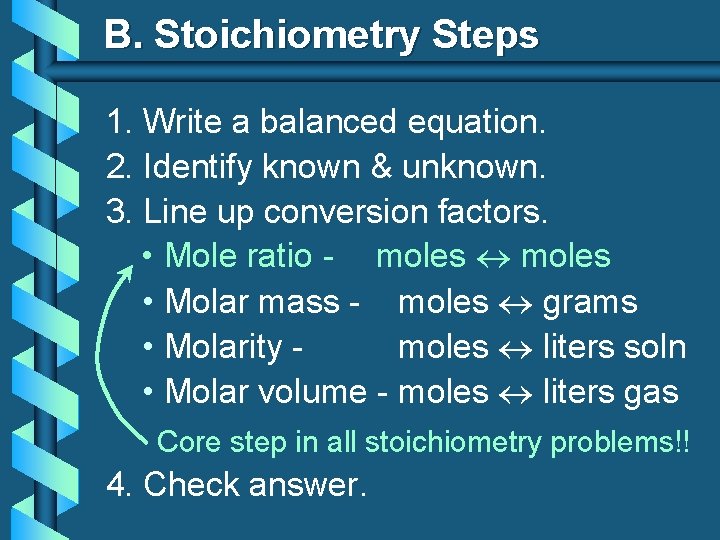
B. Stoichiometry Steps 1. Write a balanced equation. 2. Identify known & unknown. 3. Line up conversion factors. • Mole ratio - moles • Molar mass - moles grams • Molarity moles liters soln • Molar volume - moles liters gas Core step in all stoichiometry problems!! 4. Check answer.

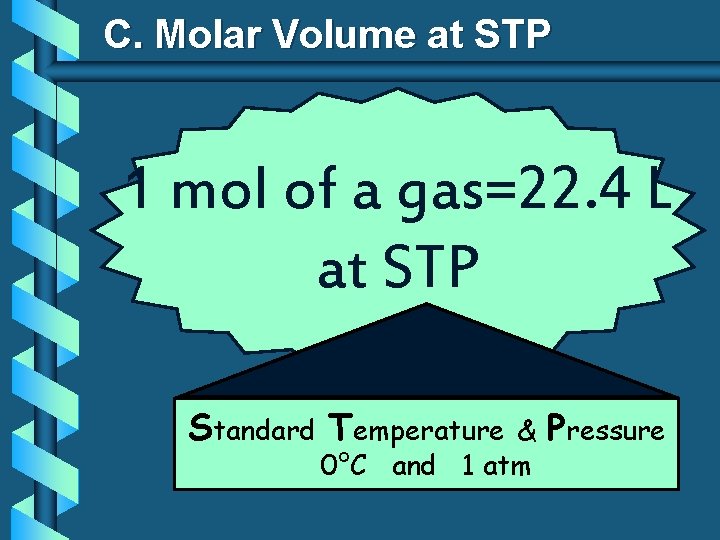
C. Molar Volume at STP 1 mol of a gas=22. 4 L at STP Standard Temperature & 0°C and 1 atm Pressure

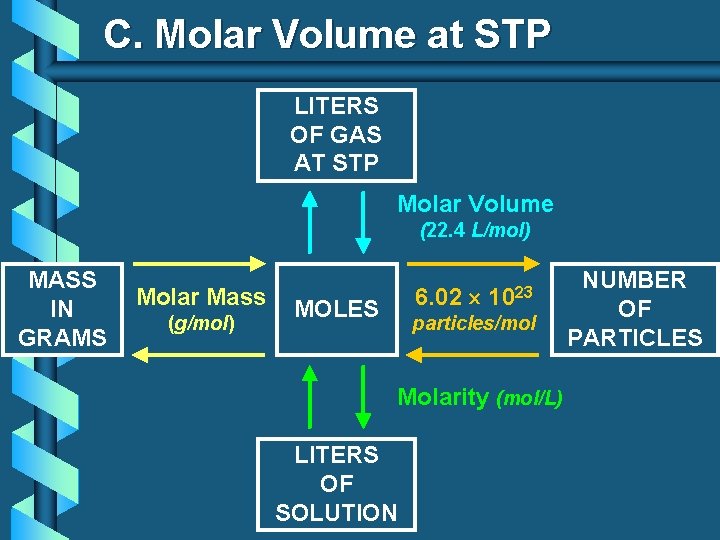
C. Molar Volume at STP LITERS OF GAS AT STP Molar Volume (22. 4 L/mol) MASS IN GRAMS Molar Mass (g/mol) 6. 02 MOLES 1023 particles/mol Molarity (mol/L) LITERS OF SOLUTION NUMBER OF PARTICLES

Stoichiometry II. Stoichiometry in the Real World

A. Limiting Reactants b. Available Ingredients • 4 slices of bread • 1 jar of peanut butter • 1/2 jar of jelly b. Limiting Reactant • bread b. Excess Reactants • peanut butter and jelly

A. Limiting Reactants b. Limiting Reactant • used up in a reaction • determines the amount of product b. Excess Reactant • added to ensure that the other reactant is completely used up • cheaper & easier to recycle

A. Limiting Reactants 1. Write a balanced equation. 2. For each reactant, calculate the amount of product formed. 3. Smaller answer indicates: • limiting reactant • amount of product

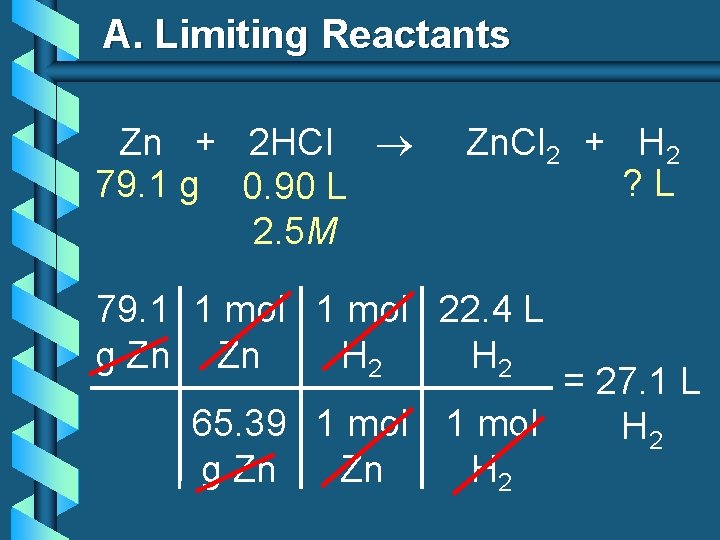
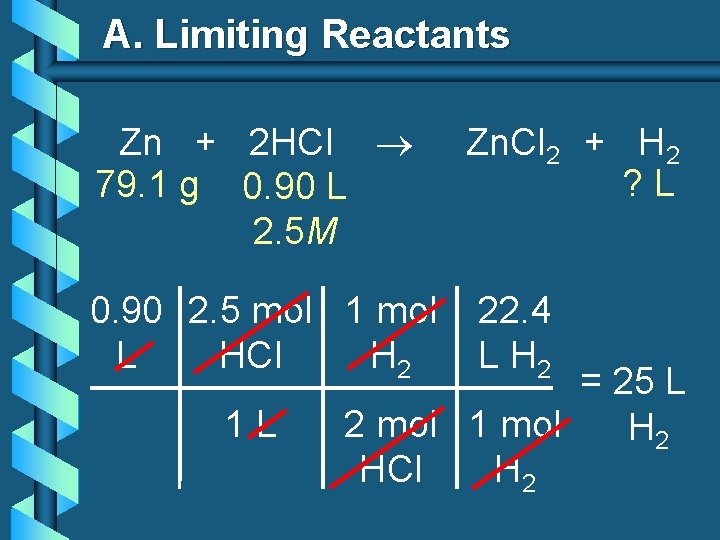
A. Limiting Reactants b 79. 1 g of zinc react with 0. 90 L of 2. 5 M HCl. Identify the limiting and excess reactants. How many liters of hydrogen are formed at STP? Zn + 2 HCl 79. 1 g 0. 90 L 2. 5 M Zn. Cl 2 + H 2 ? L

A. Limiting Reactants Zn + 2 HCl 79. 1 g 0. 90 L 2. 5 M Zn. Cl 2 + H 2 ? L 79. 1 1 mol 22. 4 L g Zn Zn H 2 = 27. 1 L 65. 39 1 mol H 2 g Zn Zn H 2

A. Limiting Reactants Zn + 2 HCl 79. 1 g 0. 90 L 2. 5 M 0. 90 2. 5 mol 1 mol L HCl H 2 1 L Zn. Cl 2 + H 2 ? L 22. 4 L H 2 = 25 L 2 mol 1 mol H 2 HCl H 2

A. Limiting Reactants Zn: 27. 1 L H 2 HCl: 25 L H 2 Limiting reactant: HCl Excess reactant: Zn Product Formed: 25 L H 2 left over zinc

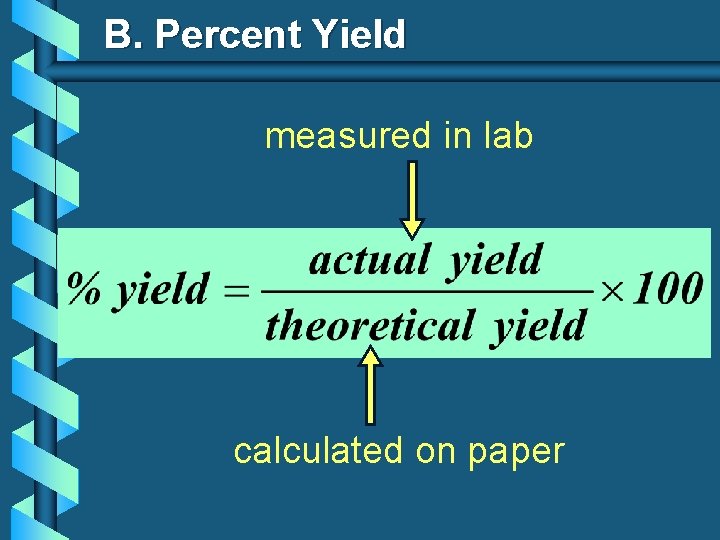
B. Percent Yield measured in lab calculated on paper

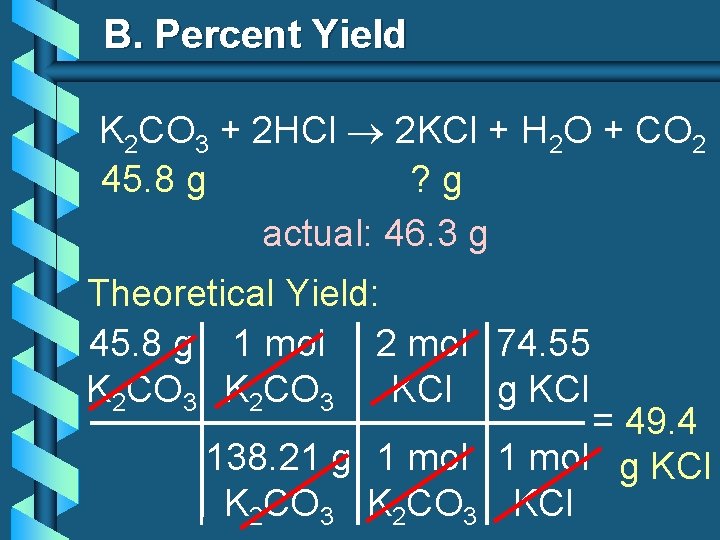
B. Percent Yield b. When 45. 8 g of K 2 CO 3 react with excess HCl, 46. 3 g of KCl are formed. Calculate theoretical and % yields of KCl. K 2 CO 3 + 2 HCl 2 KCl + H 2 O + CO 2 45. 8 g ? g actual: 46. 3 g

B. Percent Yield K 2 CO 3 + 2 HCl 2 KCl + H 2 O + CO 2 45. 8 g ? g actual: 46. 3 g Theoretical Yield: 45. 8 g 1 mol 2 mol 74. 55 K 2 CO 3 KCl g KCl = 49. 4 138. 21 g 1 mol g KCl K 2 CO 3 KCl

B. Percent Yield K 2 CO 3 + 2 HCl 2 KCl + H 2 O + CO 2 45. 8 g 49. 4 g actual: 46. 3 g Theoretical Yield = 49. 4 g KCl % Yield = 46. 3 g 49. 4 g 100 = 93. 7%
 Stoichiometric
Stoichiometric Non proportional relationship
Non proportional relationship Limiting reactant definition
Limiting reactant definition All stoichiometric calculations begin with a
All stoichiometric calculations begin with a Structural steel connection calculations calculations
Structural steel connection calculations calculations Non proportional relationship
Non proportional relationship Proportional and non-proportional
Proportional and non-proportional Inversely proportional and directly proportional
Inversely proportional and directly proportional Proportional graphs worksheet
Proportional graphs worksheet Directly proportional vs indirectly proportional
Directly proportional vs indirectly proportional How to find proportional relationship on a graph
How to find proportional relationship on a graph Graphing proportional relationships quiz
Graphing proportional relationships quiz Unit rate triangle
Unit rate triangle Proportional web
Proportional web Identifying proportional relationships in graphs worksheet
Identifying proportional relationships in graphs worksheet 7.5 using proportional relationships
7.5 using proportional relationships Lesson 4-4 proportional and nonproportional situations
Lesson 4-4 proportional and nonproportional situations Lesson 3 representing proportional relationships
Lesson 3 representing proportional relationships Using proportional relationships
Using proportional relationships