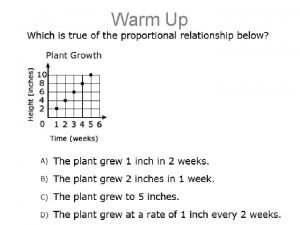
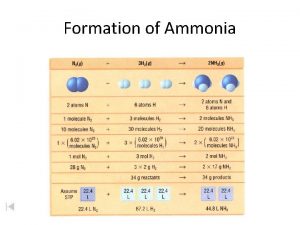
Stoichiometry Stoichiometric Calculations A Proportional Relationships 2 14











- Slides: 23

Stoichiometry Stoichiometric Calculations

A. Proportional Relationships 2 1/4 c. flour 1 tsp. baking soda 1 tsp. salt 1 c. butter 3/4 c. sugar 3/4 c. brown sugar 1 tsp vanilla extract 2 eggs 2 c. chocolate chips Makes 5 dozen cookies. b. I have 5 eggs. How many cookies can I make? 5 eggs 5 doz. 2 eggs Ratio of eggs to cookies = 12. 5 dozen cookies

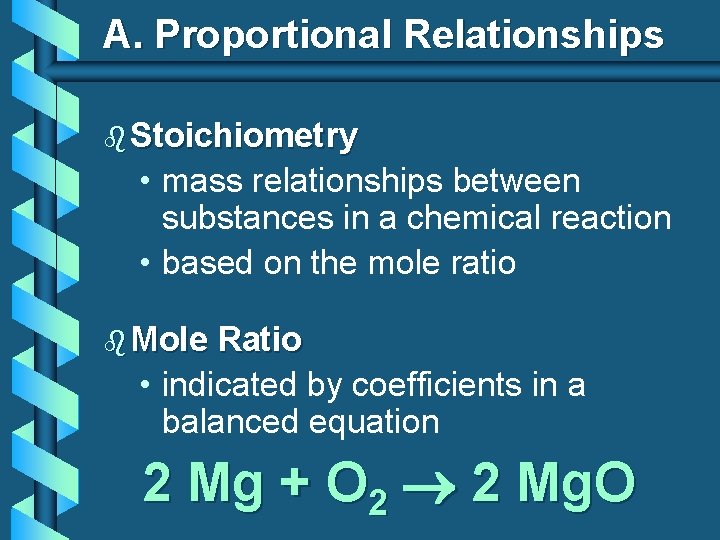
A. Proportional Relationships b Stoichiometry • mass relationships between substances in a chemical reaction • based on the mole ratio b Mole Ratio • indicated by coefficients in a balanced equation 2 Mg + O 2 2 Mg. O

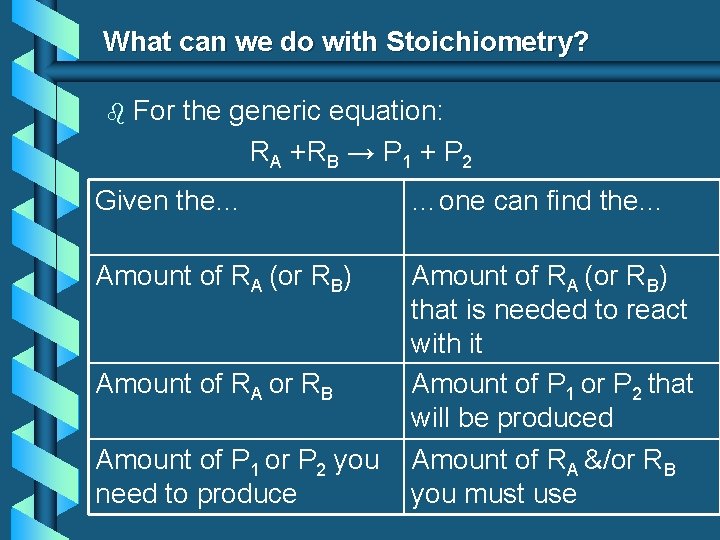
What can we do with Stoichiometry? b For the generic equation: RA +RB → P 1 + P 2 Given the… …one can find the… Amount of RA (or RB) that is needed to react with it Amount of P 1 or P 2 that will be produced Amount of RA or RB Amount of P 1 or P 2 you need to produce Amount of RA &/or RB you must use

Given the equation… 2 Ti. O 2 + 4 Cl 2 + 3 C → 2 Ti. Cl 4 + CO 2 + 2 CO b How many mol chlorine will react with 4. 55 mol carbon? b X mol Cl 2 = 4. 55 mol C (4 mol Cl 2/ 3 mol C) b 6. 07 mol Cl 2 will react with 4. 55 mol carbon

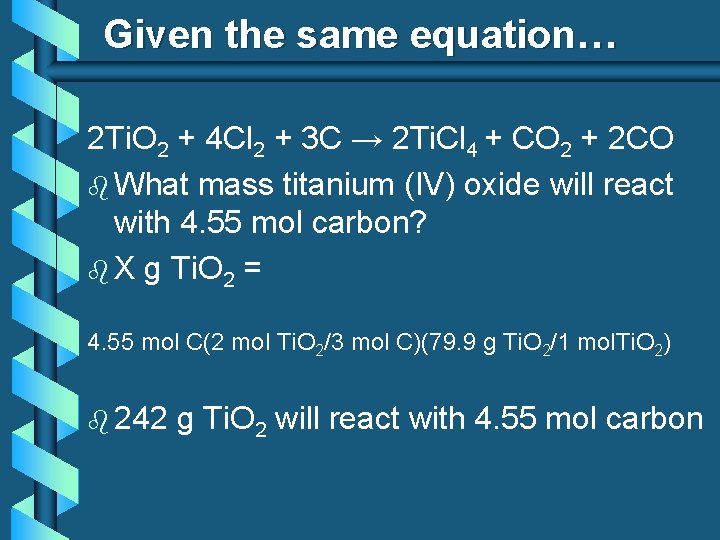
Given the same equation… 2 Ti. O 2 + 4 Cl 2 + 3 C → 2 Ti. Cl 4 + CO 2 + 2 CO b What mass titanium (IV) oxide will react with 4. 55 mol carbon? b X g Ti. O 2 = 4. 55 mol C(2 mol Ti. O 2/3 mol C)(79. 9 g Ti. O 2/1 mol. Ti. O 2) b 242 g Ti. O 2 will react with 4. 55 mol carbon

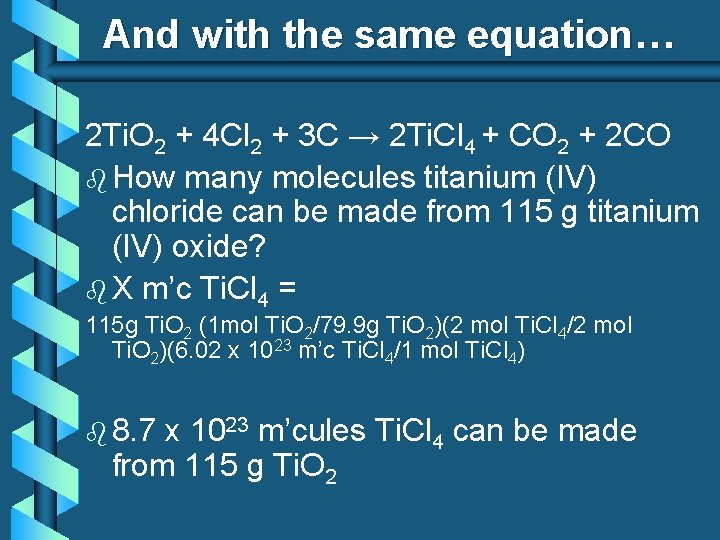
And with the same equation… 2 Ti. O 2 + 4 Cl 2 + 3 C → 2 Ti. Cl 4 + CO 2 + 2 CO b How many molecules titanium (IV) chloride can be made from 115 g titanium (IV) oxide? b X m’c Ti. Cl 4 = 115 g Ti. O 2 (1 mol Ti. O 2/79. 9 g Ti. O 2)(2 mol Ti. Cl 4/2 mol Ti. O 2)(6. 02 x 1023 m’c Ti. Cl 4/1 mol Ti. Cl 4) b 8. 7 x 1023 m’cules Ti. Cl 4 can be made from 115 g Ti. O 2

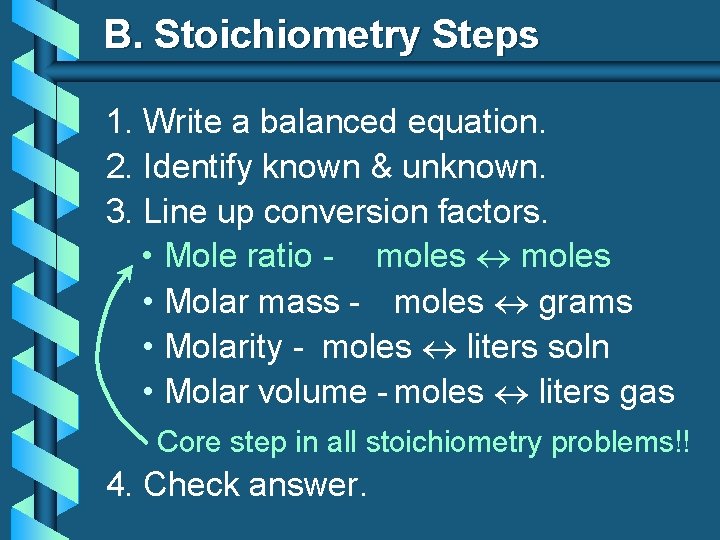
B. Stoichiometry Steps 1. Write a balanced equation. 2. Identify known & unknown. 3. Line up conversion factors. • Mole ratio - moles • Molar mass - moles grams • Molarity - moles liters soln • Molar volume - moles liters gas Core step in all stoichiometry problems!! 4. Check answer.

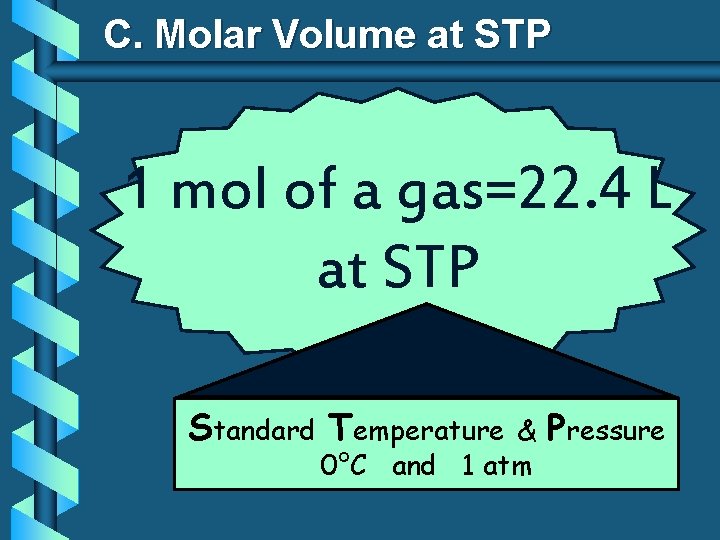
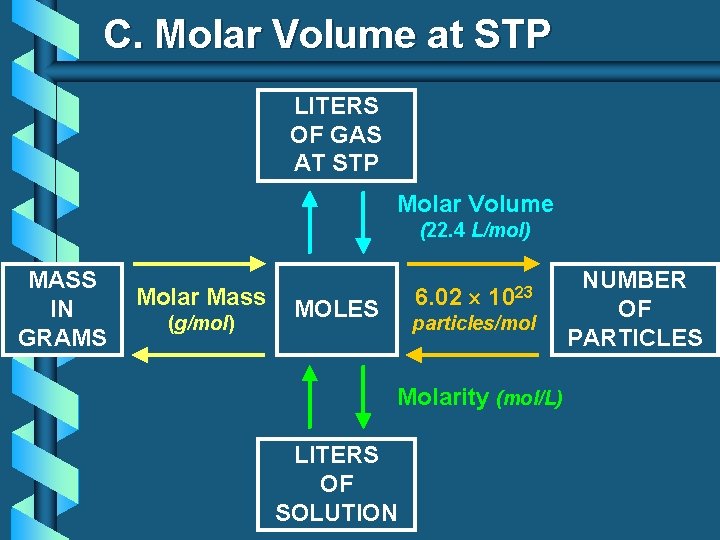
C. Molar Volume at STP 1 mol of a gas=22. 4 L at STP Standard Temperature & 0°C and 1 atm Pressure

C. Molar Volume at STP LITERS OF GAS AT STP Molar Volume (22. 4 L/mol) MASS IN GRAMS Molar Mass (g/mol) 6. 02 MOLES 1023 particles/mol Molarity (mol/L) LITERS OF SOLUTION NUMBER OF PARTICLES

Stoichiometry “Pop” Quiz b b b Iron (III) reacts with water… • Write the complete balanced equation • How many liters of hydrogen (@ STP) will be produced from 8 moles of iron? • How many grams of water are needed to produce 68 grams of Iron (III) oxide? • 5. 2 x 1025 particles of iron will produce how many moles of hydrogen? For the complete combustion of butane (C 4 H 10)… • Write the complete balanced equation • In a room at 0°C and 1 atm pressure, how many m’cules oxygen react with 632 L butane? • If 316 L liters of carbon dioxide gas are produced, how much butane (in moles) was combusted? Silver nitrate reacts with sodium chloride… • Write the complete balanced equation (including states of matter) • How much precipitate (in g) is formed when 1. 5 L of 0. 10 M silver nitrate is used? • How many molecules of sodium chloride do you need to react with that amount of silver nitrate?

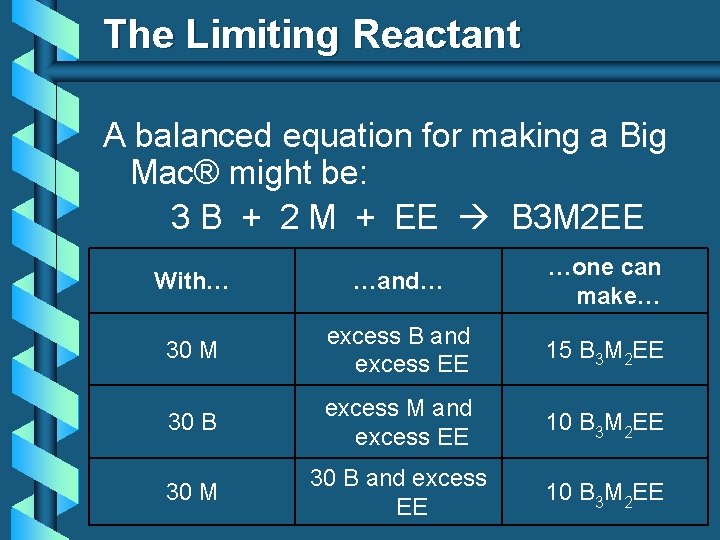
The Limiting Reactant A balanced equation for making a Big Mac® might be: 3 B + 2 M + EE B 3 M 2 EE With… …and… …one can make… 30 M excess B and excess EE 15 B 3 M 2 EE 30 B excess M and excess EE 10 B 3 M 2 EE 30 M 30 B and excess EE 10 B 3 M 2 EE

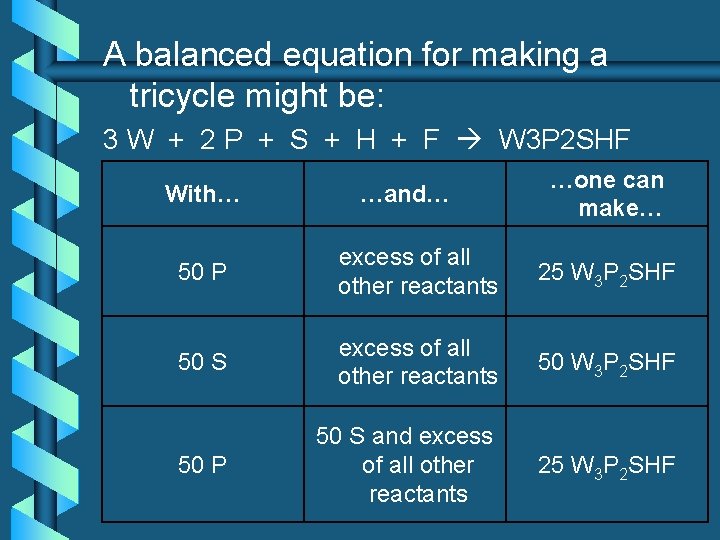
A balanced equation for making a tricycle might be: 3 W + 2 P + S + H + F W 3 P 2 SHF With… …and… …one can make… 50 P excess of all other reactants 25 W 3 P 2 SHF 50 S excess of all other reactants 50 W 3 P 2 SHF 50 P 50 S and excess of all other reactants 25 W 3 P 2 SHF

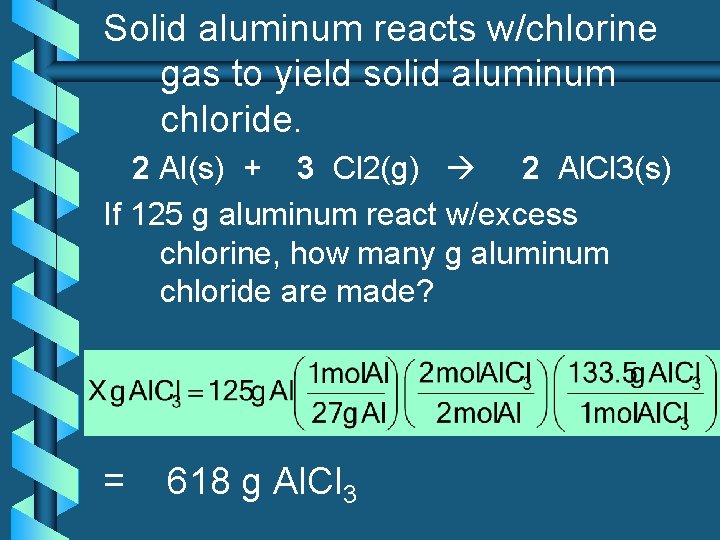
Solid aluminum reacts w/chlorine gas to yield solid aluminum chloride. 2 Al(s) + 3 Cl 2(g) 2 Al. Cl 3(s) If 125 g aluminum react w/excess chlorine, how many g aluminum chloride are made? = 618 g Al. Cl 3

If 125 g chlorine react w/excess aluminum, how many g aluminum chloride are made? = 157 g Al. Cl 3 b If 125 g aluminum react w/125 g chlorine, how many g aluminum chloride are made? 157 g Al. Cl 3 b We’re out of Cl 2.

blimiting reactant (LR): the reactant that runs out first • Amount of product is based on LR. b. Any reactant you don’t run out of is an excess reactant (ER).

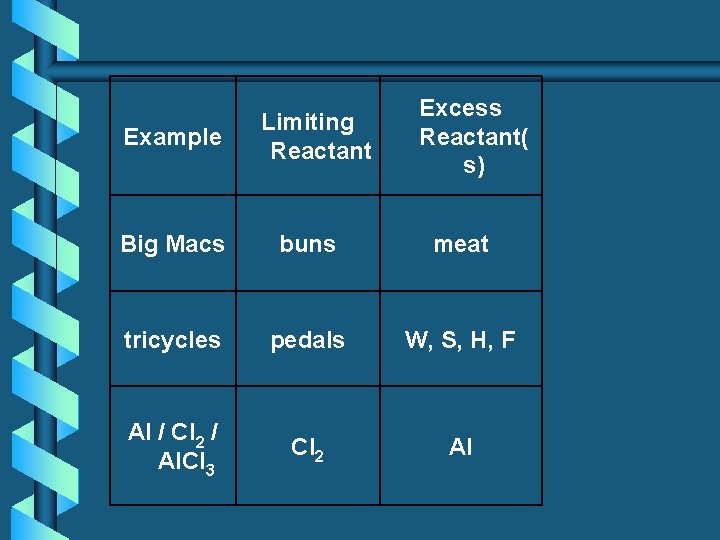
Example Limiting Reactant Excess Reactant( s) Big Macs buns meat tricycles pedals W, S, H, F Al / Cl 2 / Al. Cl 3 Cl 2 Al

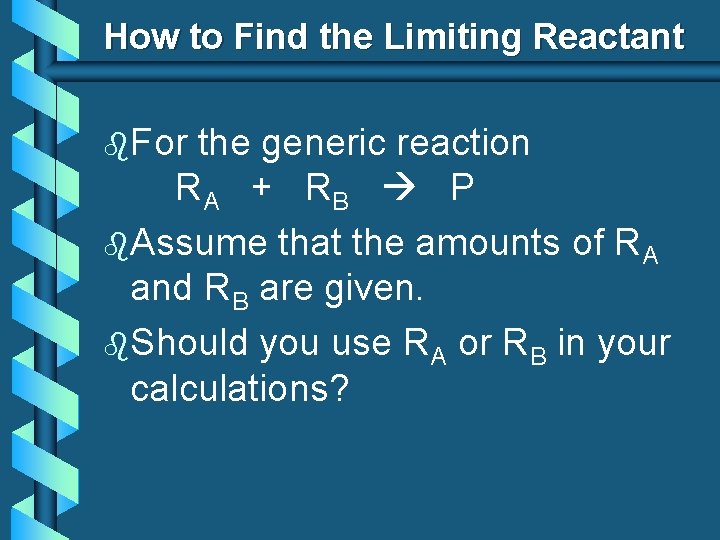
How to Find the Limiting Reactant b. For the generic reaction RA + RB P b. Assume that the amounts of RA and RB are given. b. Should you use RA or RB in your calculations?

How to find LR steps… 1. Calc. # of mol of RA and RB you have. 2. Use the mole ratio from the balanced equation to calculate the required amount of the other reactant. 3. If resulting amount is less than what is given, that reactant is the LR.

% Yield molten + solid sodium aluminum sodium oxide Al 3+ O 2– Na 1+ O 2– ___Na(l) + ___Al 2 O 3(s) ___Al(l) +___Na 2 O(s) 6 Na(l)+ 1 Al 2 O 3(s) 2 Al(l) + 3 Na 2 O(s) Find mass of aluminum produced if you start w/575 g sodium and 357 g aluminum oxide. = 189 g Al

% Yield b This amount of product is theoretical yield. • amt. we get if reaction is perfect • found by calculation b Now suppose that we perform this reaction in the lab and get only 172 grams of aluminum. Why? • couldn’t collect all Al • not all Na and Al 2 O 3 reacted • some reactant or product spilled and was lost

% Yield Calculation: % yield can never be > 100%

Find % Yield for the Previous Problem
 Stoichiometric
Stoichiometric Nonproportional table
Nonproportional table Stoichiometry example
Stoichiometry example Defining stoichiometry
Defining stoichiometry Structural steel connection calculations calculations
Structural steel connection calculations calculations Directly vs indirectly proportional
Directly vs indirectly proportional Proportional and non proportional
Proportional and non proportional What is proportional relationship
What is proportional relationship Inveresly
Inveresly Proportional vs non proportional graphs worksheet
Proportional vs non proportional graphs worksheet Math antics proportional relationships
Math antics proportional relationships Lesson 4-4 proportional and nonproportional situations
Lesson 4-4 proportional and nonproportional situations Graph proportional
Graph proportional Proportional function graph
Proportional function graph Proportional relationships jeopardy
Proportional relationships jeopardy Proportional web
Proportional web Proportional relationship
Proportional relationship 7-5 using proportional relationships
7-5 using proportional relationships Lesson 4 proportional and nonproportional relationships
Lesson 4 proportional and nonproportional relationships Lesson 3-1 representing proportional relationships
Lesson 3-1 representing proportional relationships Using proportional relationships
Using proportional relationships To find the height h of a dinosaur in a museum
To find the height h of a dinosaur in a museum Proportional and nonproportional relationships answer key
Proportional and nonproportional relationships answer key Interpreting graphs of proportional relationships
Interpreting graphs of proportional relationships