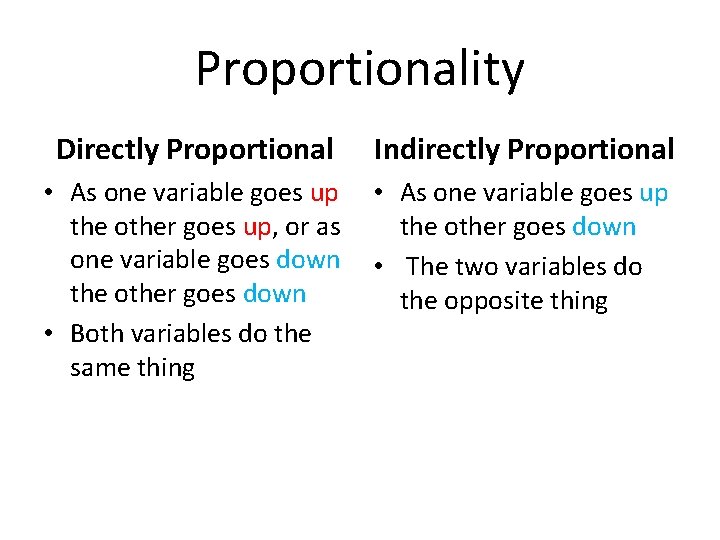
The Gas Laws Proportionality Directly Proportional Indirectly Proportional








- Slides: 17

The Gas Laws

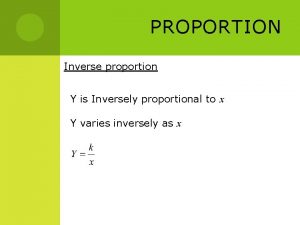
Proportionality Directly Proportional Indirectly Proportional • As one variable goes up the other goes up, or as one variable goes down the other goes down • Both variables do the same thing • As one variable goes up the other goes down • The two variables do the opposite thing

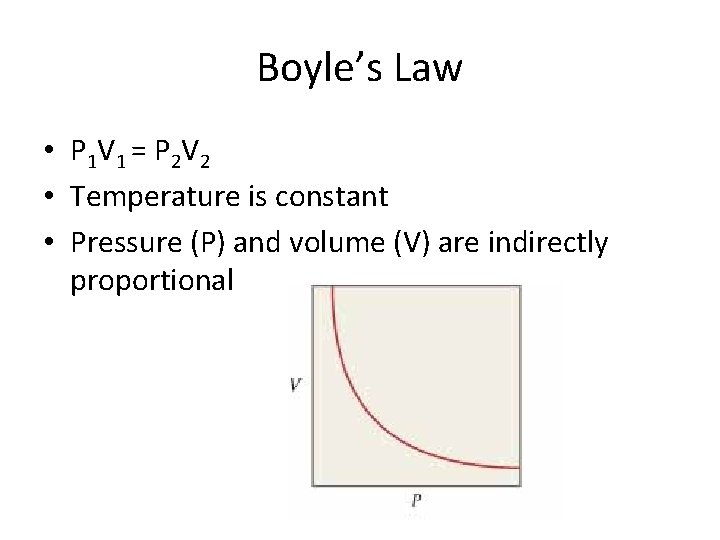
Boyle’s Law • P 1 V 1 = P 2 V 2 • Temperature is constant • Pressure (P) and volume (V) are indirectly proportional

Demo Cartesian Diver 1. Draw or describe the initial observation 2. Claim: If the pressure inside the bottle is increased then… 3. What are the variables? (list in table) 4. What is the Gas Law relationship? (write the gas law and proportionality)

Demo Marshmallow Madness 1. Draw or describe the initial observation 2. Claim: If the pressure inside the syringe is decreased then… 3. What are the variables? (list in table) 4. What is the Gas Law relationship? (write the gas law and proportionality)

Example 1 A helium balloon was compressed from 4. 0 L to 2. 5 L at a constant temperature. If the pressure of the gas in the 4. 0 L balloon is 210 k. PA, what will the pressure be at 2. 5 L? V 1 = 4. 0 L P 1= 210 k. Pa Given: V 2 = 2. 5 L P 2 = ? P 1 V 1 = P 2 V 2 (210 k. Pa) × (4. 0 L) = P 2 × (2. 5 L) P 2 = 336 k. Pa ≈ 340 k. Pa

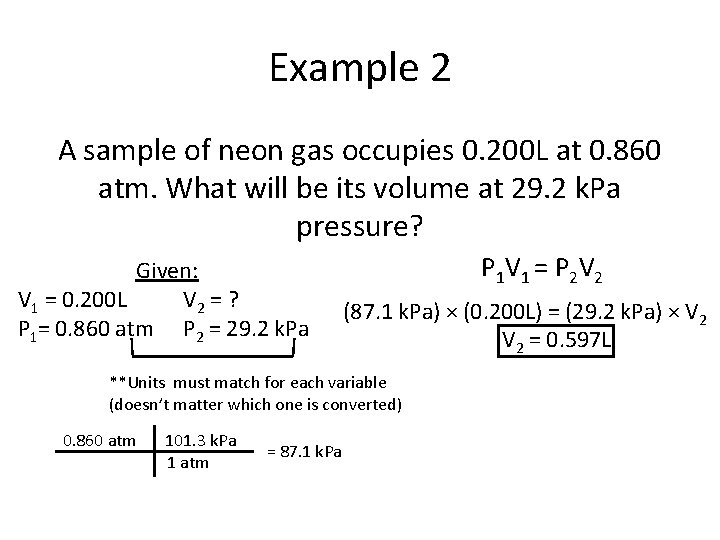
Example 2 A sample of neon gas occupies 0. 200 L at 0. 860 atm. What will be its volume at 29. 2 k. Pa pressure? Given: V 1 = 0. 200 L V 2 = ? P 1= 0. 860 atm P 2 = 29. 2 k. Pa P 1 V 1 = P 2 V 2 (87. 1 k. Pa) × (0. 200 L) = (29. 2 k. Pa) × V 2 = 0. 597 L **Units must match for each variable (doesn’t matter which one is converted) 0. 860 atm 101. 3 k. Pa 1 atm = 87. 1 k. Pa

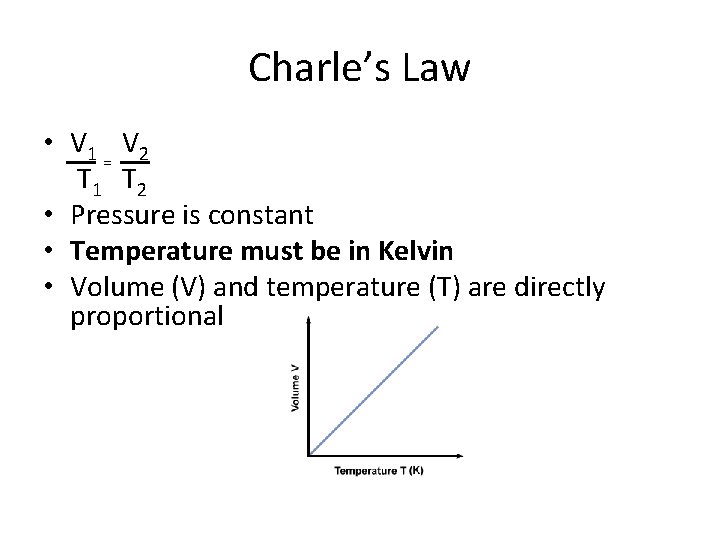
Charle’s Law • V 1 V 2 = T 1 T 2 • Pressure is constant • Temperature must be in Kelvin • Volume (V) and temperature (T) are directly proportional

Demo Balloon in a Bottle 1. Draw or describe the initial observation 2. Claim: If the temperature inside the flask is decreased then… 3. What are the variables? (list in table) 4. What is the Gas Law relationship? (write the gas law and proportionality)

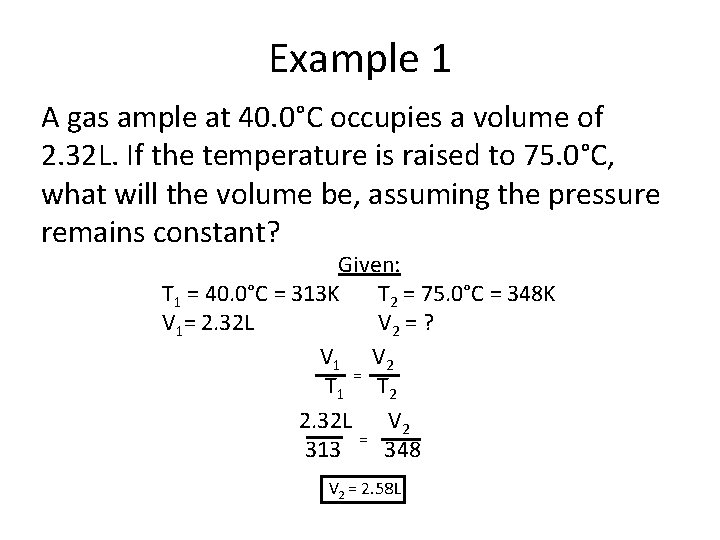
Example 1 A gas ample at 40. 0°C occupies a volume of 2. 32 L. If the temperature is raised to 75. 0°C, what will the volume be, assuming the pressure remains constant? Given: T 1 = 40. 0°C = 313 K T 2 = 75. 0°C = 348 K V 1= 2. 32 L V 2 = ? V 1 V 2 T 1 = T 2 2. 32 L V 2 313 = 348 V 2 = 2. 58 L

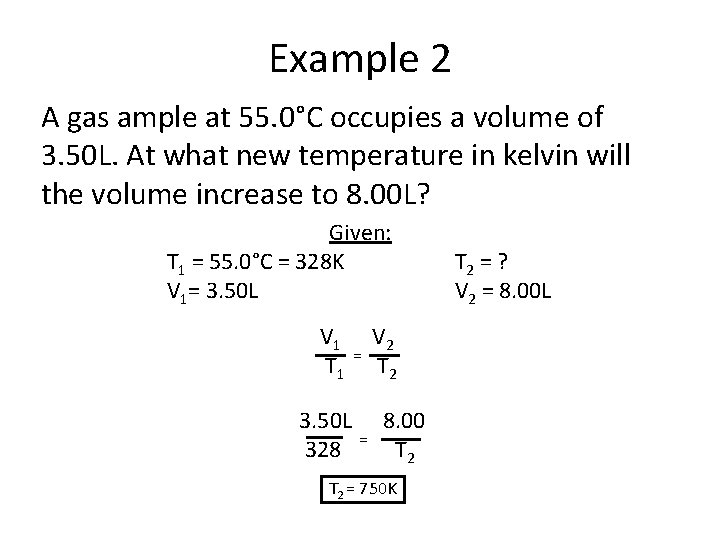
Example 2 A gas ample at 55. 0°C occupies a volume of 3. 50 L. At what new temperature in kelvin will the volume increase to 8. 00 L? Given: T 1 = 55. 0°C = 328 K V 1= 3. 50 L V 1 T 1 3. 50 L 328 = = V 2 T 2 8. 00 T 2 = 750 K T 2 = ? V 2 = 8. 00 L

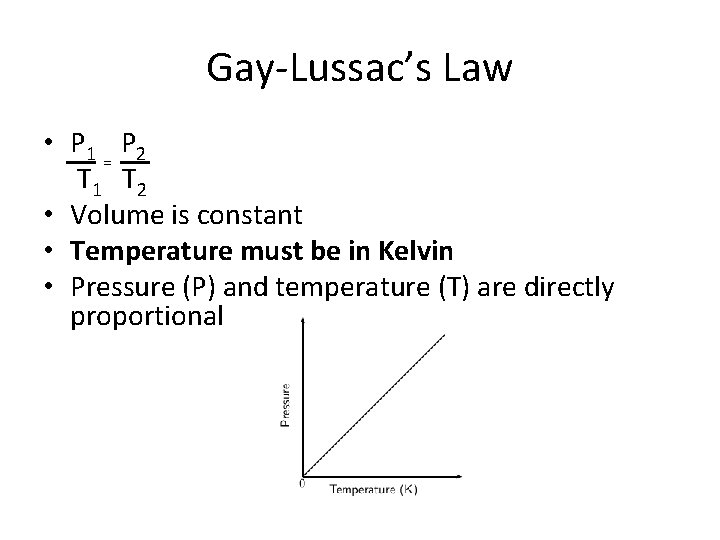
Gay-Lussac’s Law • P 1 P 2 = T 1 T 2 • Volume is constant • Temperature must be in Kelvin • Pressure (P) and temperature (T) are directly proportional

Demo Can Crusher 1. Draw or describe the initial observation 2. Claim: If the temperature inside the bottle is increased and suddenly decreased then… 3. What are the variables? (list in table) 4. What is the Gas Law relationship? (write the gas law and proportionality)

Demo Boiling Water 1. Draw or describe the initial observation 2. Claim: If the temperature inside the flask is decreased then… 3. What are the variables? (list in table) 4. What is the Gas Law relationship? (write the gas law and proportionality)

Demo Egg in a Bottle 1. Draw or describe the initial observation 2. Claim: If the temperature inside the bottle is increased then… 3. What are the variables? (list in table) 4. What is the Gas Law relationship? (write the gas law and proportionality)

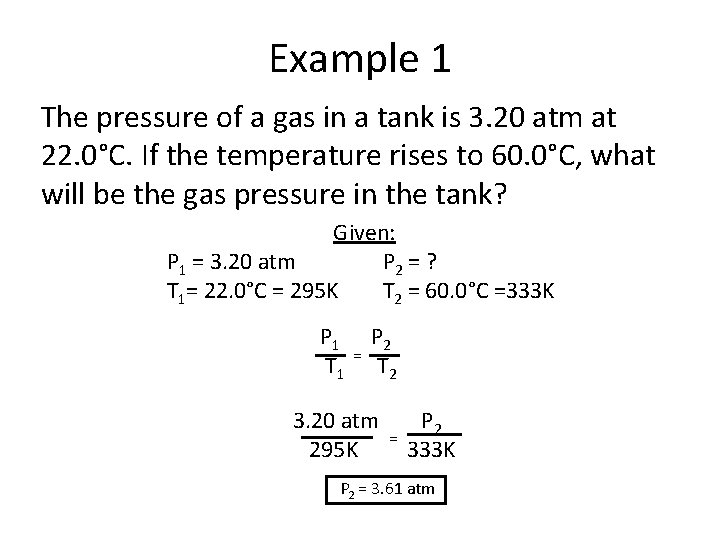
Example 1 The pressure of a gas in a tank is 3. 20 atm at 22. 0°C. If the temperature rises to 60. 0°C, what will be the gas pressure in the tank? Given: P 1 = 3. 20 atm P 2 = ? T 1= 22. 0°C = 295 K T 2 = 60. 0°C =333 K P 1 T 1 = P 2 T 2 3. 20 atm 295 K = P 2 333 K P 2 = 3. 61 atm

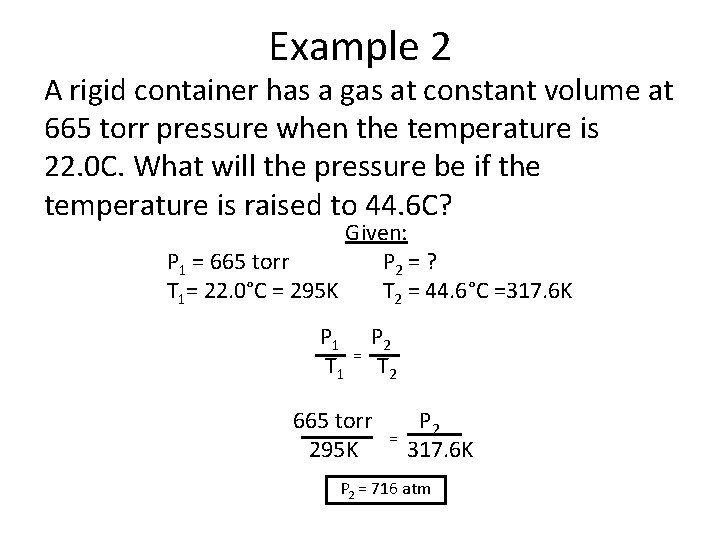
Example 2 A rigid container has a gas at constant volume at 665 torr pressure when the temperature is 22. 0 C. What will the pressure be if the temperature is raised to 44. 6 C? Given: P 1 = 665 torr P 2 = ? T 1= 22. 0°C = 295 K T 2 = 44. 6°C =317. 6 K P 1 T 1 = P 2 T 2 665 torr 295 K = P 2 317. 6 K P 2 = 716 atm
 Boyle's law direct or indirect
Boyle's law direct or indirect Inversely proportional
Inversely proportional Never directly or indirectly view an electric arc without
Never directly or indirectly view an electric arc without Who gets affected directly or indirectly due to accident
Who gets affected directly or indirectly due to accident Y is inversely proportional to d^2
Y is inversely proportional to d^2 Boyle's law graph
Boyle's law graph Boyle's law practice problems
Boyle's law practice problems Directly proportional equation
Directly proportional equation Directly proportional graph
Directly proportional graph Direct proportion worksheet
Direct proportion worksheet What is dispersive power of plane transmission grating?
What is dispersive power of plane transmission grating? Directly proportional formula
Directly proportional formula Directly proportional equation
Directly proportional equation Directly proportional formula
Directly proportional formula Non proportional relationships
Non proportional relationships Non proportional equation
Non proportional equation Proportional graphs worksheet
Proportional graphs worksheet What is not a proportional relationship
What is not a proportional relationship