Stoichiometry Stoichiometric Calculations Proportional Relationships 2 14 c




- Slides: 10

Stoichiometry Stoichiometric Calculations

Proportional Relationships 2 1/4 c. flour 1 tsp. baking soda 1 tsp. salt 1 c. butter 3/4 c. sugar 3/4 c. brown sugar 1 tsp vanilla extract 2 eggs 2 c. chocolate chips Makes 5 dozen cookies. b. I have 5 eggs. How many cookies can I make? 5 eggs 5 doz. 2 eggs Ratio of eggs to cookies = 12. 5 dozen cookies

Proportional Relationships b Stoichiometry • mass relationships between substances in a chemical reaction • based on the mole ratio b Mole Ratio • indicated by coefficients in a balanced equation 2 Mg + O 2 2 Mg. O

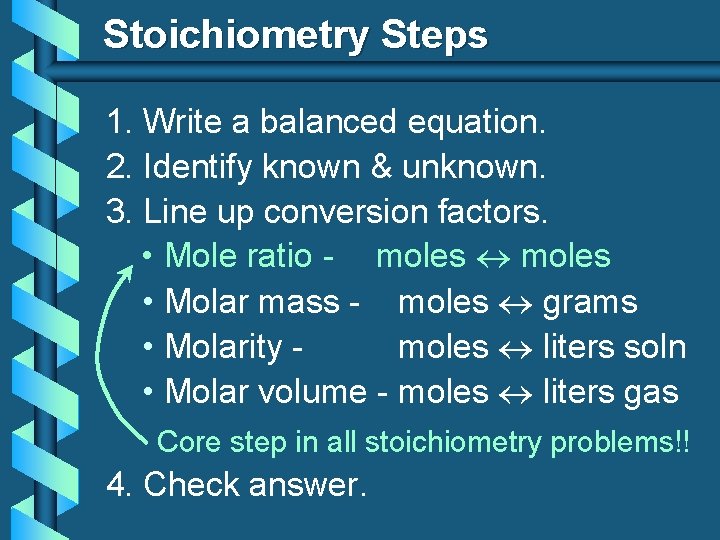
Stoichiometry Steps 1. Write a balanced equation. 2. Identify known & unknown. 3. Line up conversion factors. • Mole ratio - moles • Molar mass - moles grams • Molarity moles liters soln • Molar volume - moles liters gas Core step in all stoichiometry problems!! 4. Check answer.

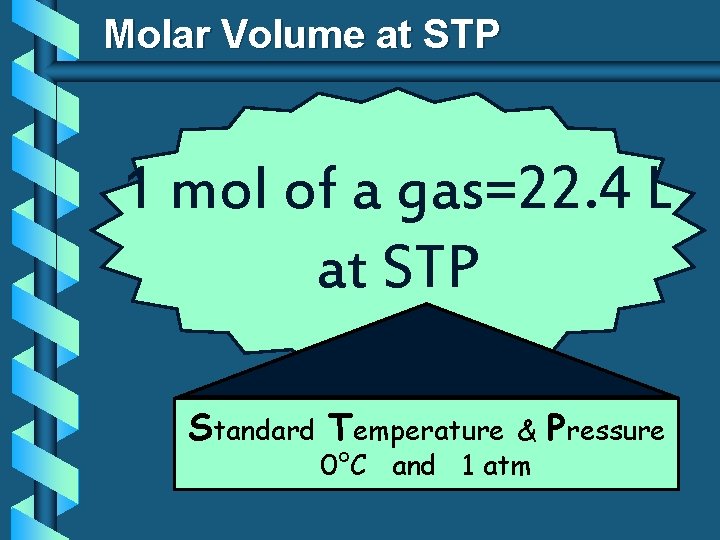
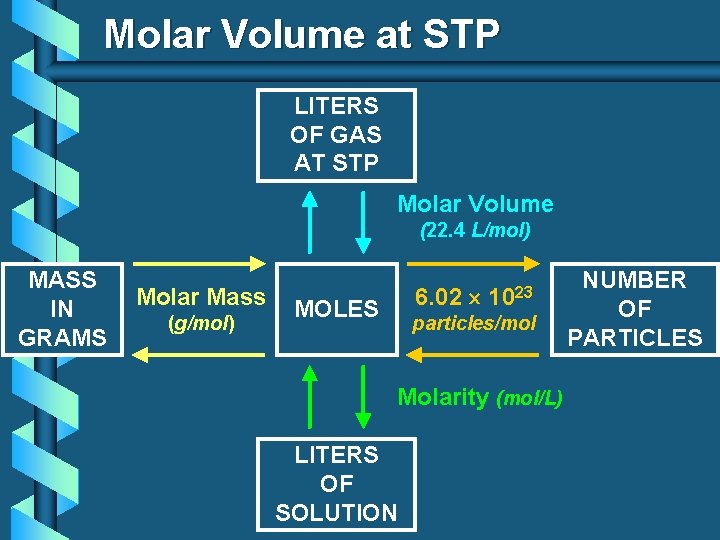
Molar Volume at STP 1 mol of a gas=22. 4 L at STP Standard Temperature & 0°C and 1 atm Pressure

Molar Volume at STP LITERS OF GAS AT STP Molar Volume (22. 4 L/mol) MASS IN GRAMS Molar Mass (g/mol) 6. 02 MOLES 1023 particles/mol Molarity (mol/L) LITERS OF SOLUTION NUMBER OF PARTICLES

Stoichiometry Problems b How many moles of KCl. O 3 must decompose in order to produce 9 moles of oxygen gas? 2 KCl. O 3 2 KCl + 3 O 2 ? mol 9 mol O 2 2 mol KCl. O 3 3 mol O 2 9 mol = 6 mol KCl. O 3

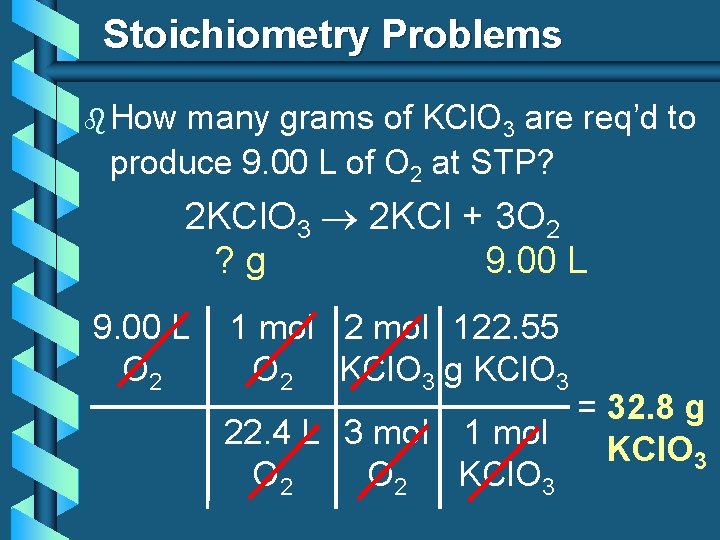
Stoichiometry Problems b How many grams of KCl. O 3 are req’d to produce 9. 00 L of O 2 at STP? 2 KCl. O 3 2 KCl + 3 O 2 ? g 9. 00 L O 2 1 mol 2 mol 122. 55 O 2 KCl. O 3 g KCl. O 3 = 32. 8 g 22. 4 L 3 mol 1 mol KCl. O 3 O 2 KCl. O 3

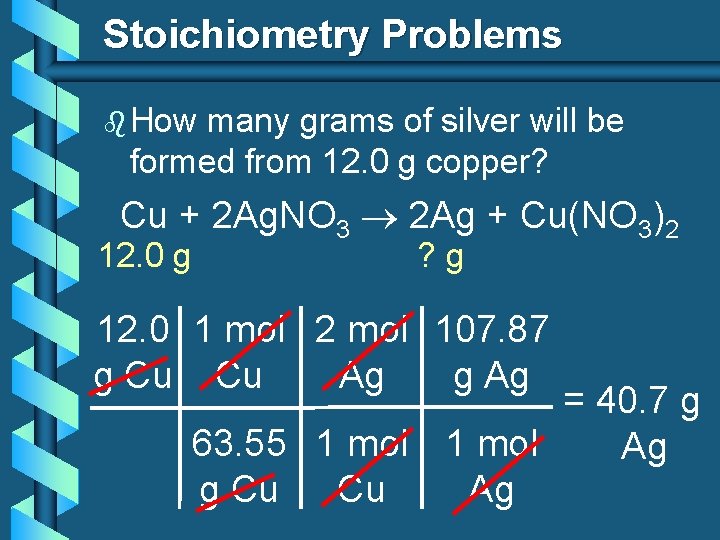
Stoichiometry Problems b How many grams of silver will be formed from 12. 0 g copper? Cu + 2 Ag. NO 3 2 Ag + Cu(NO 3)2 12. 0 g ? g 12. 0 1 mol 2 mol 107. 87 g Cu Cu Ag g Ag = 40. 7 g 63. 55 1 mol Ag g Cu Cu Ag

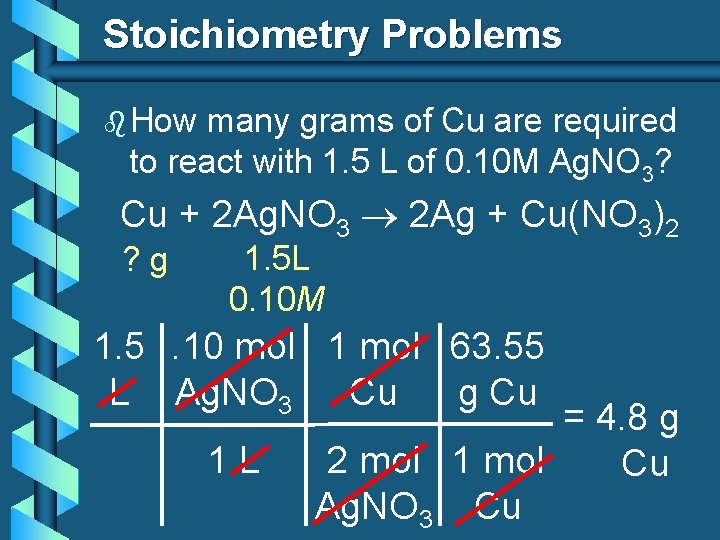
Stoichiometry Problems b How many grams of Cu are required to react with 1. 5 L of 0. 10 M Ag. NO 3? Cu + 2 Ag. NO 3 2 Ag + Cu(NO 3)2 ? g 1. 5 L 0. 10 M 1. 5. 10 mol 1 mol 63. 55 L Ag. NO 3 Cu g Cu 1 L = 4. 8 g 2 mol 1 mol Cu Ag. NO 3 Cu
 Stoichiometric
Stoichiometric Proportional vs nonproportional
Proportional vs nonproportional Stoichiometry example
Stoichiometry example Defining stoichiometry
Defining stoichiometry Structural steel connection calculations calculations
Structural steel connection calculations calculations Directly vs indirectly proportional
Directly vs indirectly proportional Non proportional graph
Non proportional graph Proportional and nonproportional relationships
Proportional and nonproportional relationships Inversely proportional
Inversely proportional Identifying graphs worksheet
Identifying graphs worksheet Stoichiometry mole island diagram
Stoichiometry mole island diagram