Ch 9 Stoichiometry 9 19 2 STOICHIOMETRIC CALCULATIONS


- Slides: 11

Ch. 9 Stoichiometry 9. 1/9. 2 STOICHIOMETRIC CALCULATIONS

Stoichiometry �Deals with mass relationships between reactants and products in a chemical reaction �Can be a conversion between two reactants, reactants and products or two products �Review Conversion Basics: Should you write numbers or units first? What do you use to convert between g and moles of a compound? In this conversion, what number goes next to mol?

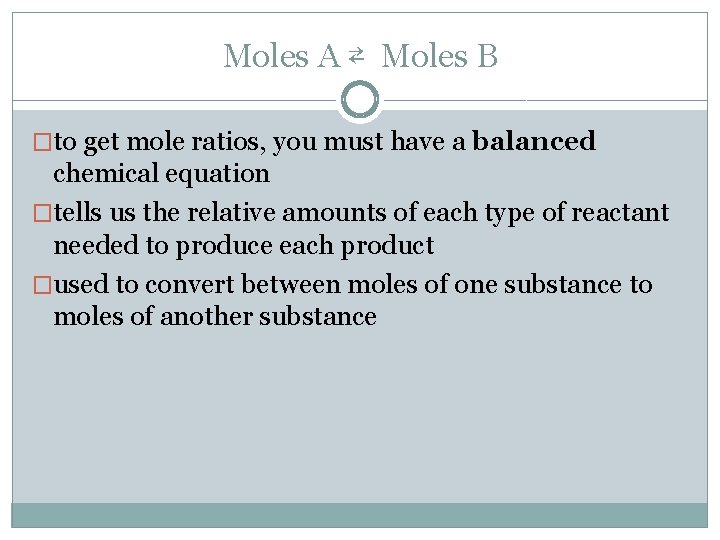
Moles A ⇄ Moles B �to get mole ratios, you must have a balanced chemical equation �tells us the relative amounts of each type of reactant needed to produce each product �used to convert between moles of one substance to moles of another substance

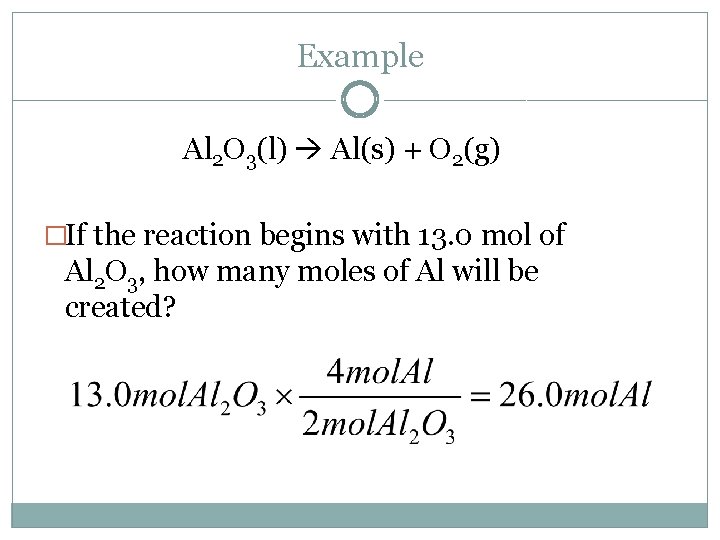
Example Al 2 O 3(l) Al(s) + O 2(g) �If the reaction begins with 13. 0 mol of Al 2 O 3, how many moles of Al will be created?

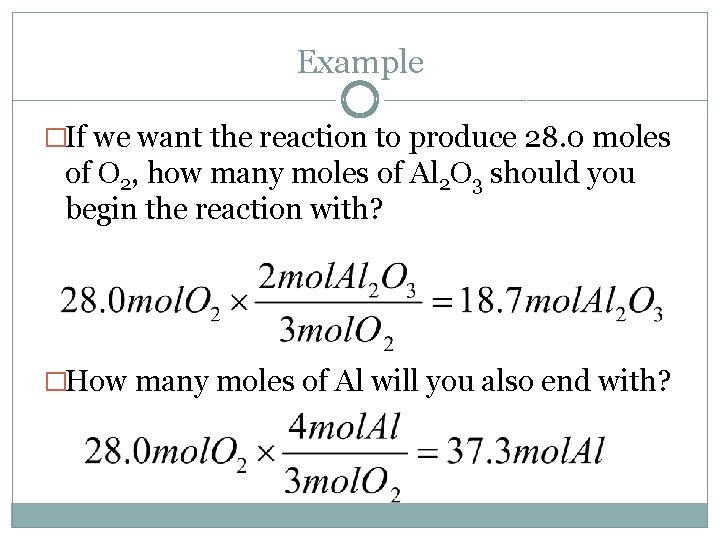
Example �If we want the reaction to produce 28. 0 moles of O 2, how many moles of Al 2 O 3 should you begin the reaction with? �How many moles of Al will you also end with?

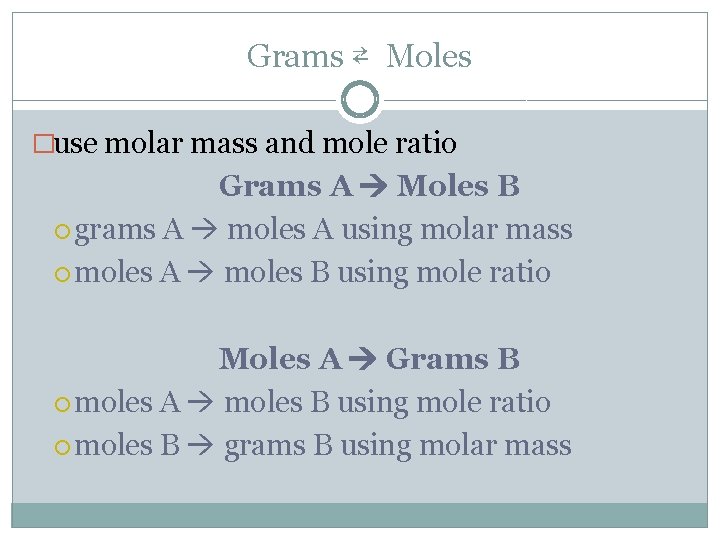
Grams ⇄ Moles �use molar mass and mole ratio Grams A Moles B grams A moles A using molar mass moles A moles B using mole ratio Moles A Grams B moles A moles B using mole ratio moles B grams B using molar mass

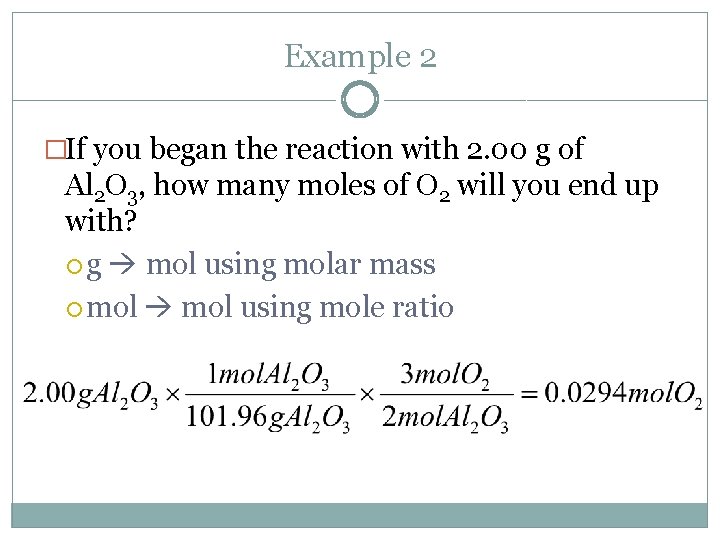
Example 2 �If you began the reaction with 2. 00 g of Al 2 O 3, how many moles of O 2 will you end up with? g mol using molar mass mol using mole ratio

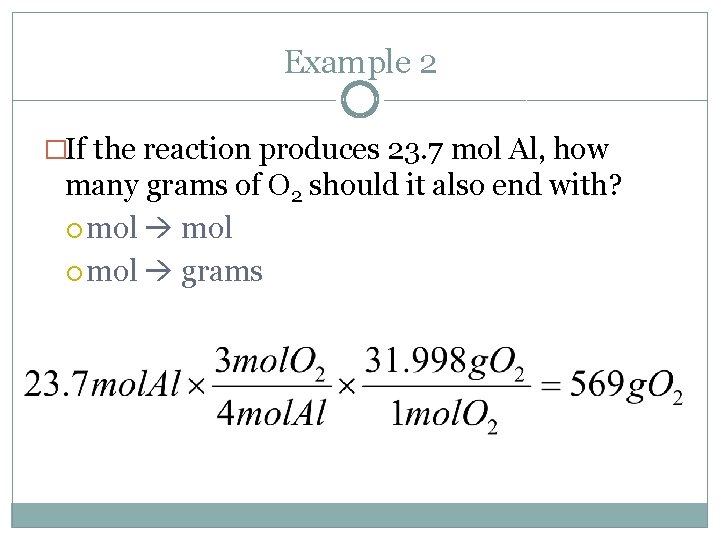
Example 2 �If the reaction produces 23. 7 mol Al, how many grams of O 2 should it also end with? mol grams

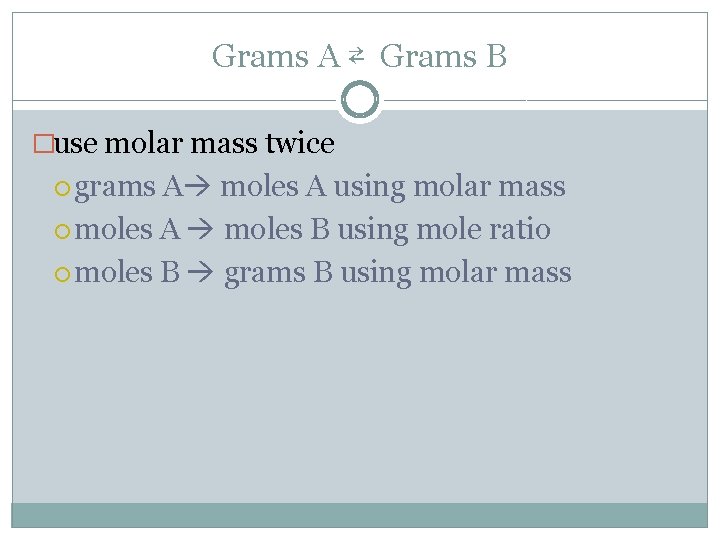
Grams A ⇄ Grams B �use molar mass twice grams A moles A using molar mass moles A moles B using mole ratio moles B grams B using molar mass

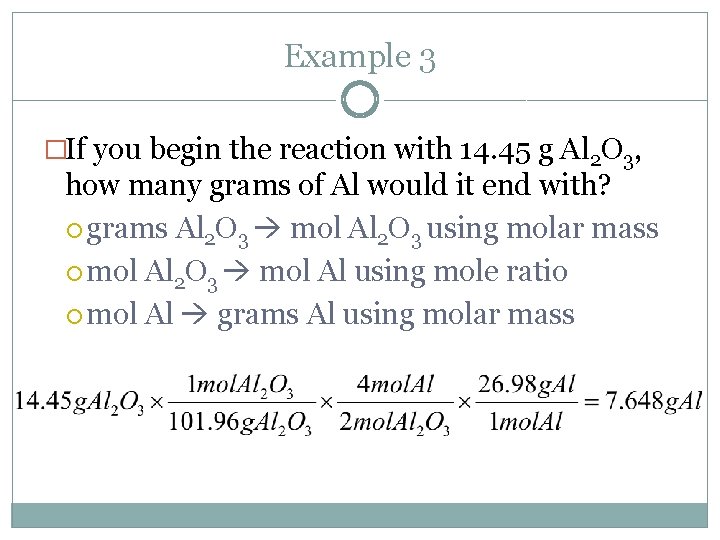
Example 3 �If you begin the reaction with 14. 45 g Al 2 O 3, how many grams of Al would it end with? grams Al 2 O 3 mol Al 2 O 3 using molar mass mol Al 2 O 3 mol Al using mole ratio mol Al grams Al using molar mass

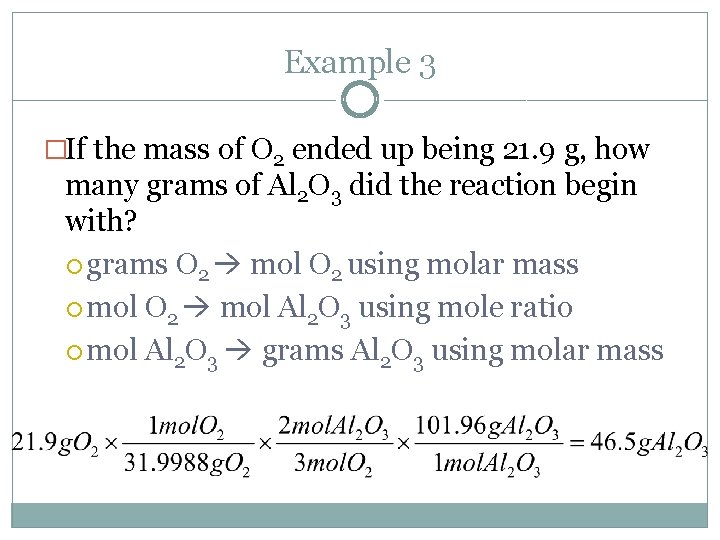
Example 3 �If the mass of O 2 ended up being 21. 9 g, how many grams of Al 2 O 3 did the reaction begin with? grams O 2 mol O 2 using molar mass mol O 2 mol Al 2 O 3 using mole ratio mol Al 2 O 3 grams Al 2 O 3 using molar mass