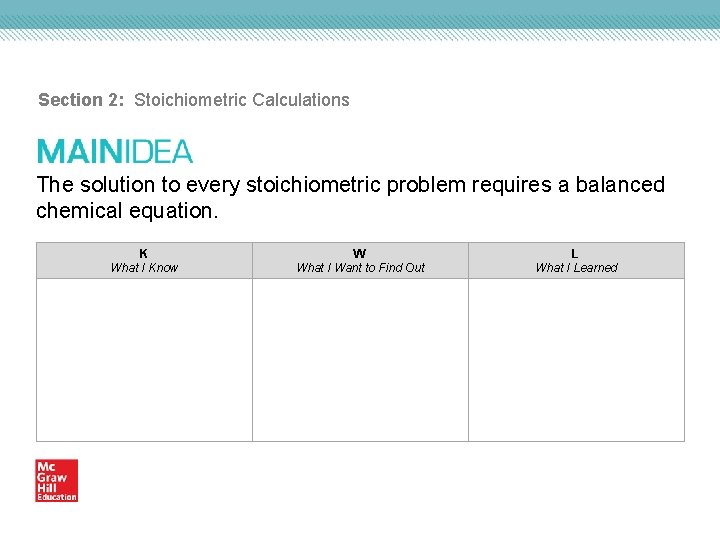
Section 2 Stoichiometric Calculations The solution to every



- Slides: 15

Section 2: Stoichiometric Calculations The solution to every stoichiometric problem requires a balanced chemical equation. K What I Know W What I Want to Find Out L What I Learned

• 8(E) Perform stoichiometric calculations, including determination of mass relationships between reactants and products, calculation of limiting reagents, and percent yield. • 2(G) Express and manipulate chemical quantities using scientific conventions and mathematical procedures, including dimensional analysis, scientific notation, and significant figures. • 2(I) Communicate valid conclusions supported by the data through methods such as lab reports, labeled drawings, graphs, journals, summaries, oral reports, and technology–based reports. • 3(A) In all fields of science, analyze, evaluate, and critique scientific explanations by using empirical evidence, logical reasoning, and experimental and observational testing, including examining all sides of scientific evidence of those scientific explanations, so as to encourage critical thinking by the student. Copyright © Mc. Graw-Hill Education Stoichiometric Calculations

• 8(A) Define and use the concept of a mole. • 8(D) Use the law of conservation of mass to write and balance chemical equations. Copyright © Mc. Graw-Hill Education Stoichiometric Calculations

Essential Questions • What is the sequence of steps used in solving stoichiometric problems? • How are these steps applied to solve stoichiometric problems? Copyright © Mc. Graw-Hill Education Stoichiometric Calculations

Vocabulary Review • chemical reaction Copyright © Mc. Graw-Hill Education Stoichiometric Calculations

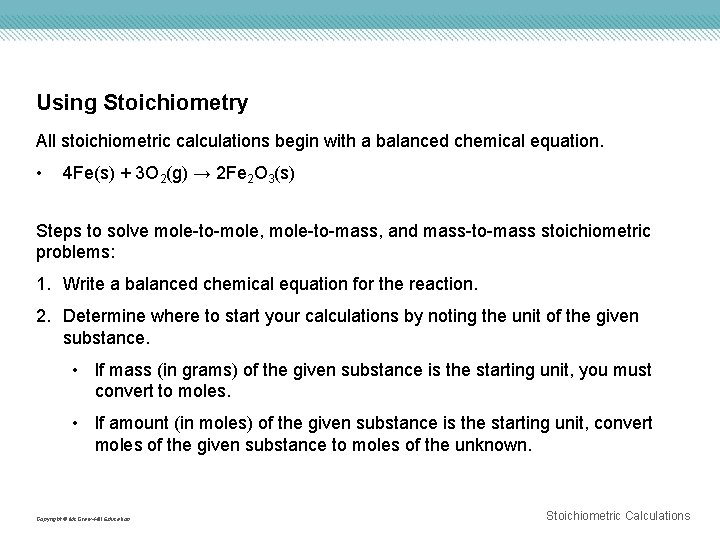
Using Stoichiometry All stoichiometric calculations begin with a balanced chemical equation. • 4 Fe(s) + 3 O 2(g) → 2 Fe 2 O 3(s) Steps to solve mole-to-mole, mole-to-mass, and mass-to-mass stoichiometric problems: 1. Write a balanced chemical equation for the reaction. 2. Determine where to start your calculations by noting the unit of the given substance. • If mass (in grams) of the given substance is the starting unit, you must convert to moles. • If amount (in moles) of the given substance is the starting unit, convert moles of the given substance to moles of the unknown. Copyright © Mc. Graw-Hill Education Stoichiometric Calculations

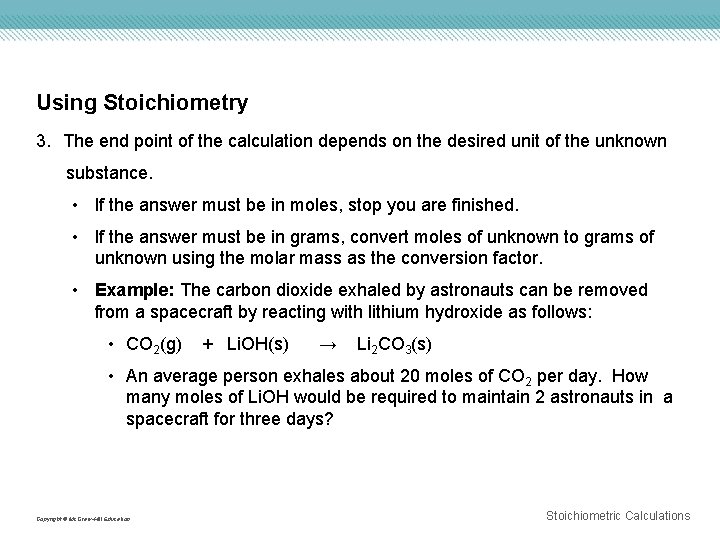
Using Stoichiometry 3. The end point of the calculation depends on the desired unit of the unknown substance. • If the answer must be in moles, stop you are finished. • If the answer must be in grams, convert moles of unknown to grams of unknown using the molar mass as the conversion factor. • Example: The carbon dioxide exhaled by astronauts can be removed from a spacecraft by reacting with lithium hydroxide as follows: • CO 2(g) + Li. OH(s) → Li 2 CO 3(s) • An average person exhales about 20 moles of CO 2 per day. How many moles of Li. OH would be required to maintain 2 astronauts in a spacecraft for three days? Copyright © Mc. Graw-Hill Education Stoichiometric Calculations

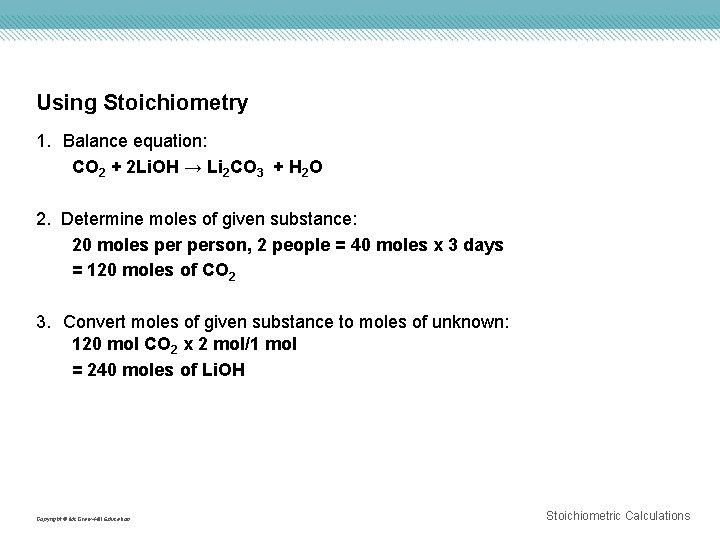
Using Stoichiometry 1. Balance equation: CO 2 + 2 Li. OH → Li 2 CO 3 + H 2 O 2. Determine moles of given substance: 20 moles person, 2 people = 40 moles x 3 days = 120 moles of CO 2 3. Convert moles of given substance to moles of unknown: 120 mol CO 2 x 2 mol/1 mol = 240 moles of Li. OH Copyright © Mc. Graw-Hill Education Stoichiometric Calculations

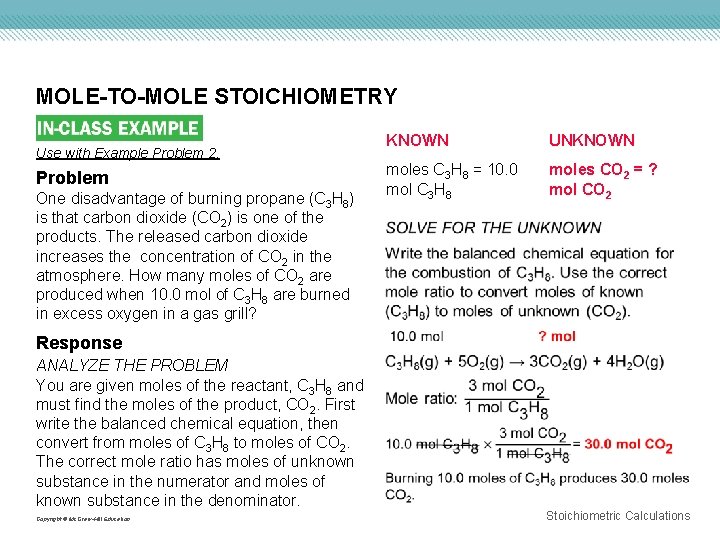
MOLE-TO-MOLE STOICHIOMETRY Use with Example Problem 2. Problem One disadvantage of burning propane (C 3 H 8) is that carbon dioxide (CO 2) is one of the products. The released carbon dioxide increases the concentration of CO 2 in the atmosphere. How many moles of CO 2 are produced when 10. 0 mol of C 3 H 8 are burned in excess oxygen in a gas grill? KNOWN UNKNOWN moles C 3 H 8 = 10. 0 mol C 3 H 8 moles CO 2 = ? mol CO 2 Response ANALYZE THE PROBLEM You are given moles of the reactant, C 3 H 8 and must find the moles of the product, CO 2. First write the balanced chemical equation, then convert from moles of C 3 H 8 to moles of CO 2. The correct mole ratio has moles of unknown substance in the numerator and moles of known substance in the denominator. Copyright © Mc. Graw-Hill Education Stoichiometric Calculations

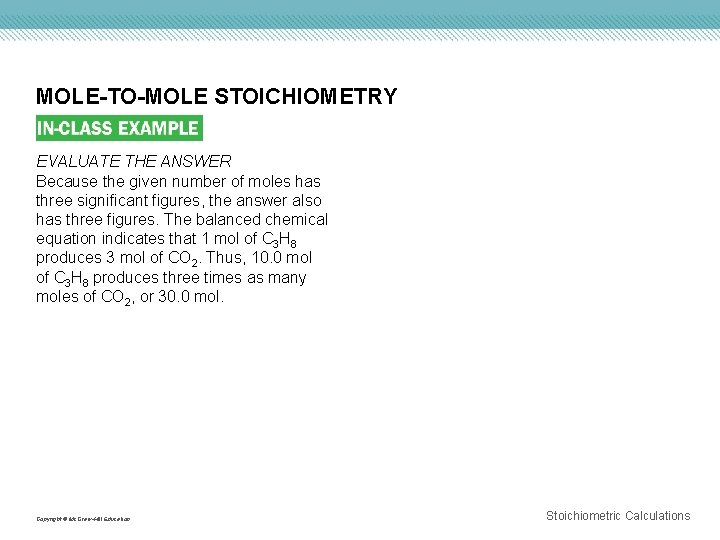
MOLE-TO-MOLE STOICHIOMETRY EVALUATE THE ANSWER Because the given number of moles has three significant figures, the answer also has three figures. The balanced chemical equation indicates that 1 mol of C 3 H 8 produces 3 mol of CO 2. Thus, 10. 0 mol of C 3 H 8 produces three times as many moles of CO 2, or 30. 0 mol. Copyright © Mc. Graw-Hill Education Stoichiometric Calculations

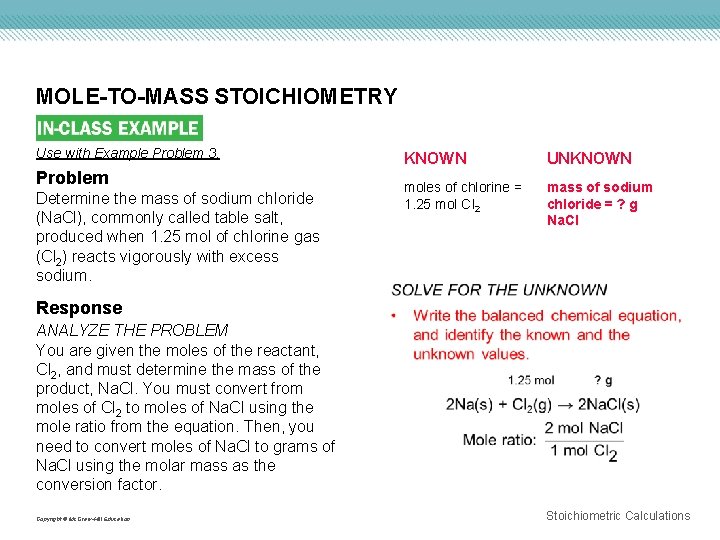
MOLE-TO-MASS STOICHIOMETRY Use with Example Problem 3. Problem Determine the mass of sodium chloride (Na. Cl), commonly called table salt, produced when 1. 25 mol of chlorine gas (Cl 2) reacts vigorously with excess sodium. Response KNOWN UNKNOWN moles of chlorine = 1. 25 mol Cl 2 mass of sodium chloride = ? g Na. Cl ANALYZE THE PROBLEM You are given the moles of the reactant, Cl 2, and must determine the mass of the product, Na. Cl. You must convert from moles of Cl 2 to moles of Na. Cl using the mole ratio from the equation. Then, you need to convert moles of Na. Cl to grams of Na. Cl using the molar mass as the conversion factor. Copyright © Mc. Graw-Hill Education Stoichiometric Calculations

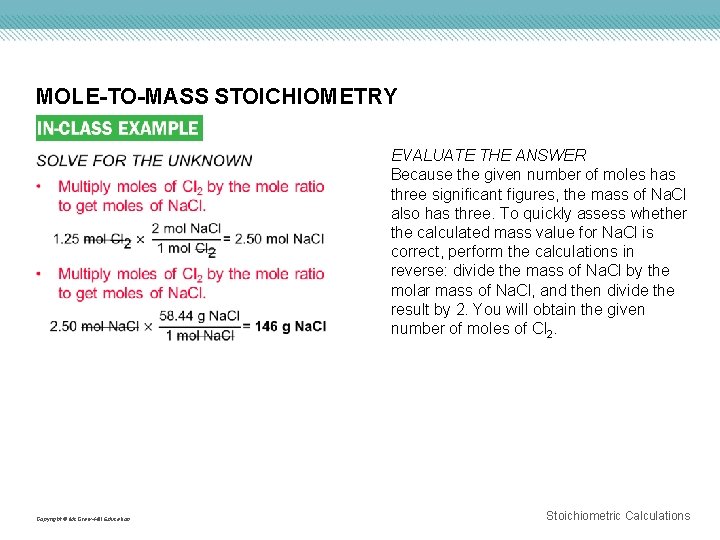
MOLE-TO-MASS STOICHIOMETRY Copyright © Mc. Graw-Hill Education EVALUATE THE ANSWER Because the given number of moles has three significant figures, the mass of Na. Cl also has three. To quickly assess whether the calculated mass value for Na. Cl is correct, perform the calculations in reverse: divide the mass of Na. Cl by the molar mass of Na. Cl, and then divide the result by 2. You will obtain the given number of moles of Cl 2. Stoichiometric Calculations

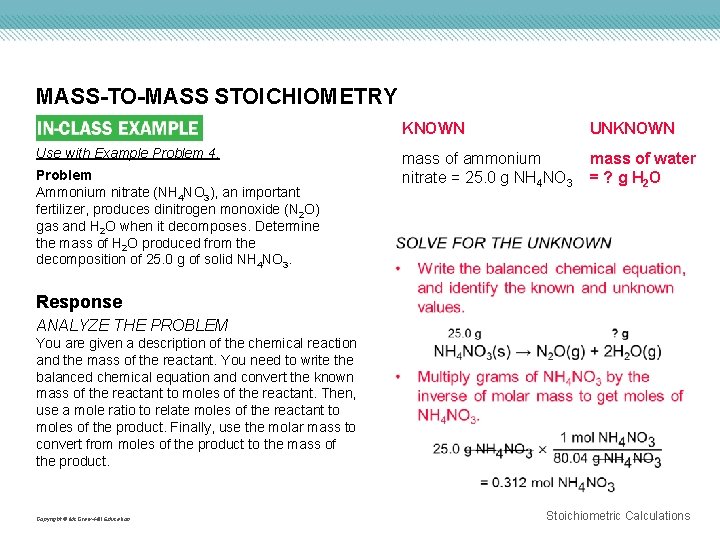
MASS-TO-MASS STOICHIOMETRY Use with Example Problem 4. Problem Ammonium nitrate (NH 4 NO 3), an important fertilizer, produces dinitrogen monoxide (N 2 O) gas and H 2 O when it decomposes. Determine the mass of H 2 O produced from the decomposition of 25. 0 g of solid NH 4 NO 3. KNOWN UNKNOWN mass of ammonium nitrate = 25. 0 g NH 4 NO 3 mass of water = ? g H 2 O Response ANALYZE THE PROBLEM You are given a description of the chemical reaction and the mass of the reactant. You need to write the balanced chemical equation and convert the known mass of the reactant to moles of the reactant. Then, use a mole ratio to relate moles of the reactant to moles of the product. Finally, use the molar mass to convert from moles of the product to the mass of the product. Copyright © Mc. Graw-Hill Education Stoichiometric Calculations

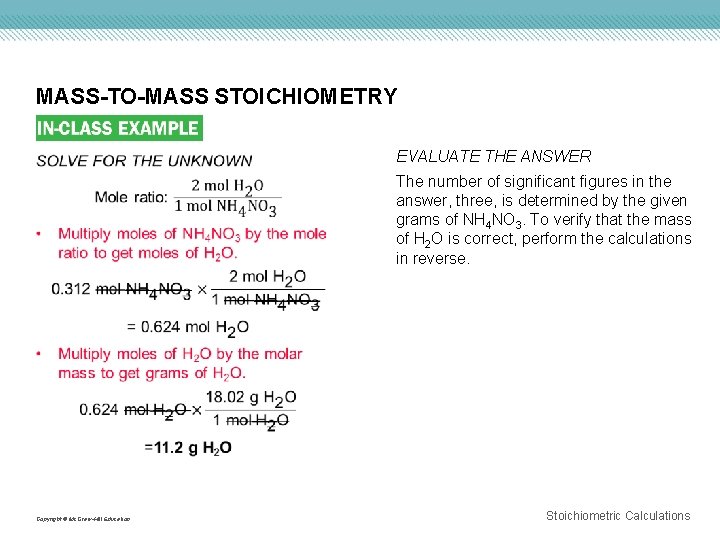
MASS-TO-MASS STOICHIOMETRY Copyright © Mc. Graw-Hill Education EVALUATE THE ANSWER The number of significant figures in the answer, three, is determined by the given grams of NH 4 NO 3. To verify that the mass of H 2 O is correct, perform the calculations in reverse. Stoichiometric Calculations

Review Essential Questions • What is the sequence of steps used in solving stoichiometric problems? • How are these steps applied to solve stoichiometric problems? Copyright © Mc. Graw-Hill Education Stoichiometric Calculations