STOICHIOMETRIC RELATIONS Reacting Masses And Volumes CHEMICAL CALCULATIONS










- Slides: 30

STOICHIOMETRIC RELATIONS Reacting Masses And Volumes

CHEMICAL CALCULATIONS • Limiting reactant determines the quantity of product. • Excess is reactant which is not fully used. • Questions 1&2

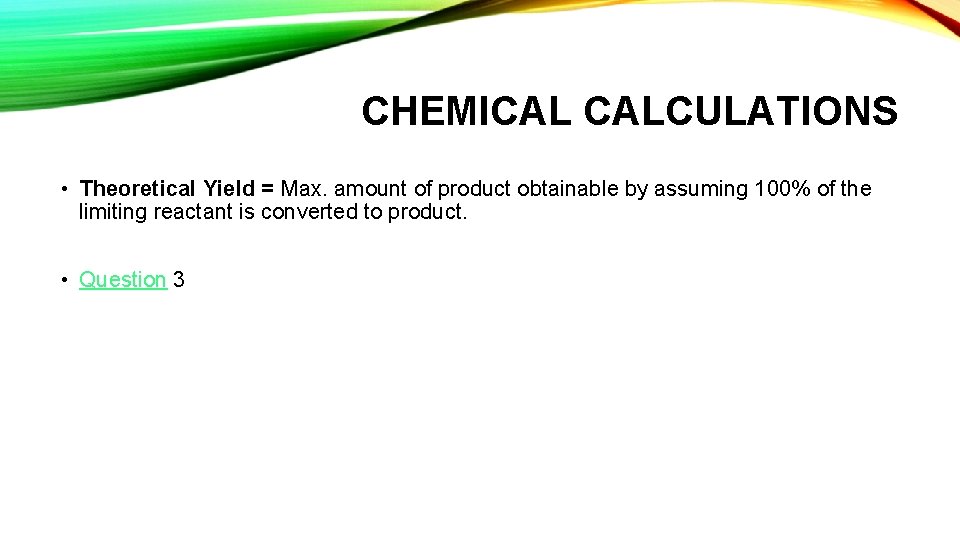
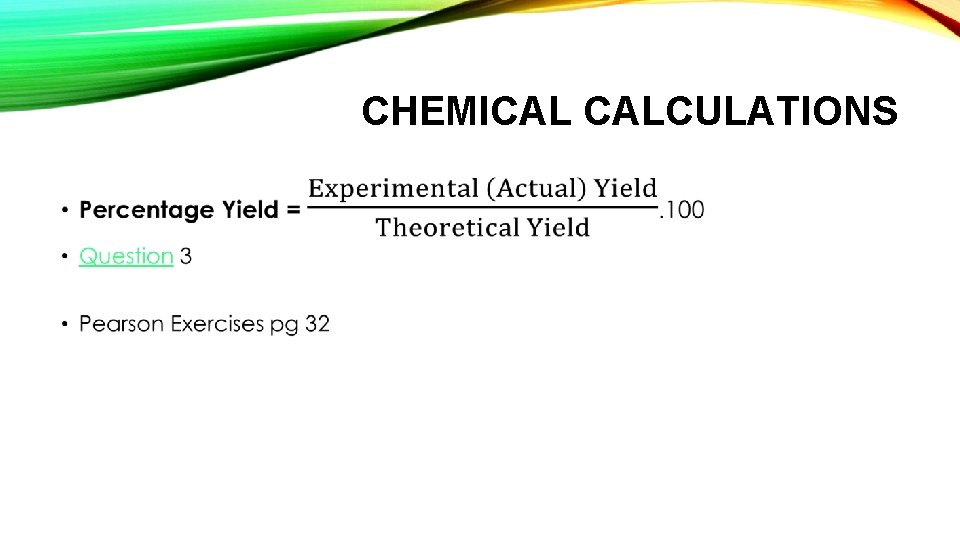
CHEMICAL CALCULATIONS • Theoretical Yield = Max. amount of product obtainable by assuming 100% of the limiting reactant is converted to product. • Question 3

CHEMICAL CALCULATIONS •

ATOM ECONOMY is the mass of product you want as a % of the mass of all the products you make. •

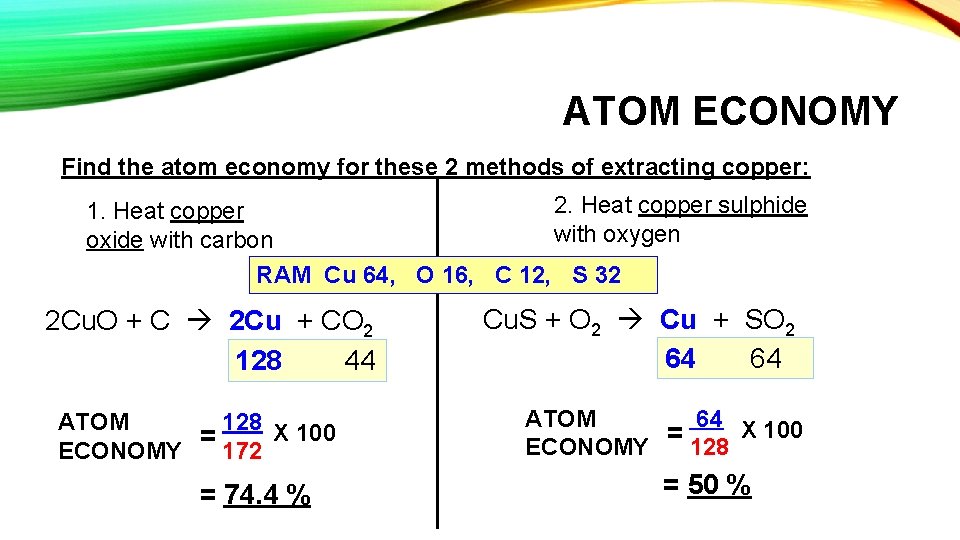
ATOM ECONOMY Find the atom economy for these 2 methods of extracting copper: 2. Heat copper sulphide 1. Heat copper with oxygen oxide with carbon RAM Cu 64, O 16, C 12, S 32 2 Cu. O + C 2 Cu + CO 2 128 44 ATOM 128 X 100 = 172 ECONOMY = 74. 4 % Cu. S + O 2 Cu + SO 2 64 64 ATOM 64 X 100 = 128 ECONOMY = 50 %

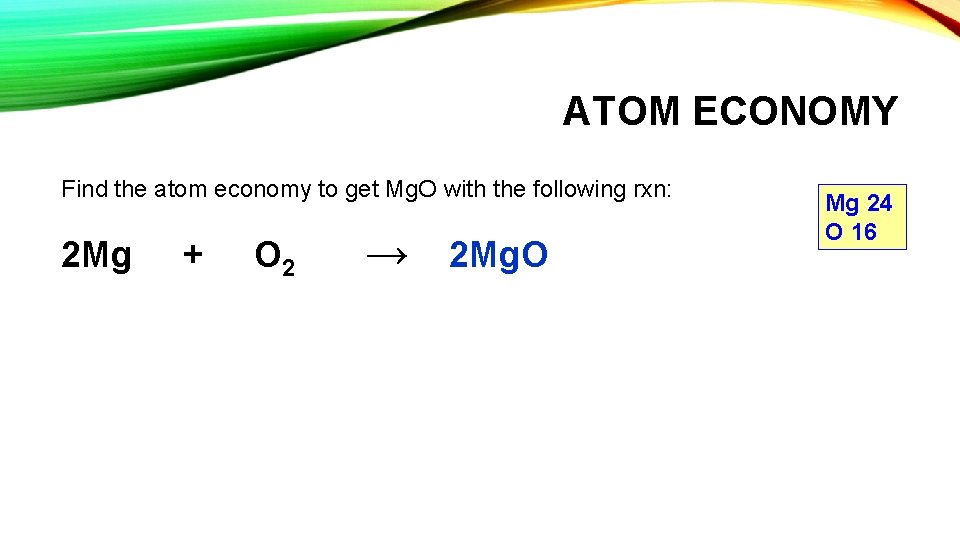
ATOM ECONOMY Find the atom economy to get Mg. O with the following rxn: 2 Mg + O 2 → 2 Mg. O Mg 24 O 16

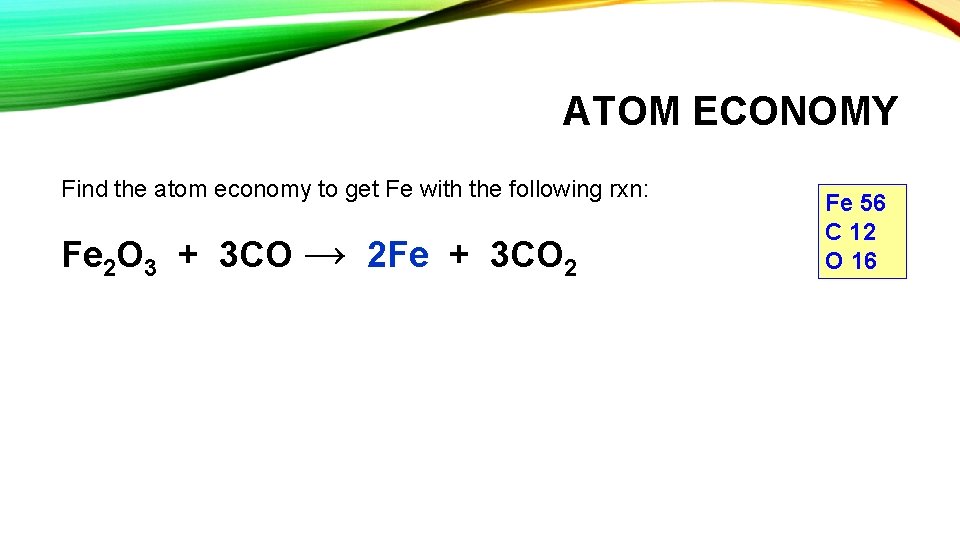
ATOM ECONOMY Find the atom economy to get Fe with the following rxn: Fe 2 O 3 + 3 CO → 2 Fe + 3 CO 2 Fe 56 C 12 O 16

IDEAL & REAL GASES Ideal gas: • The volume of gas particles is negligibly small compared to the space between gas particles. • The interactions between gas particles are negligibly weak. Real Gas: • The type of gases whose volume cannot be neglected and interactions between gas particles are not too weak to ignore. Gases deviate most from ideal behaviour at high P and low T.

AVOGADRO’S LAW At the same conditions Equal volume of all gases contain Equal number of particles

AVOGADRO’S LAW At standard temperature and pressure (STP: 100 k. Pa, 273 K) 1 mole of any gas has a volume of 2, 27. 10 -2 m 3 mol-1 (22, 7 dm 3 mol-1) Pearson Exercises pg 36

AVOGADRO’S LAW •

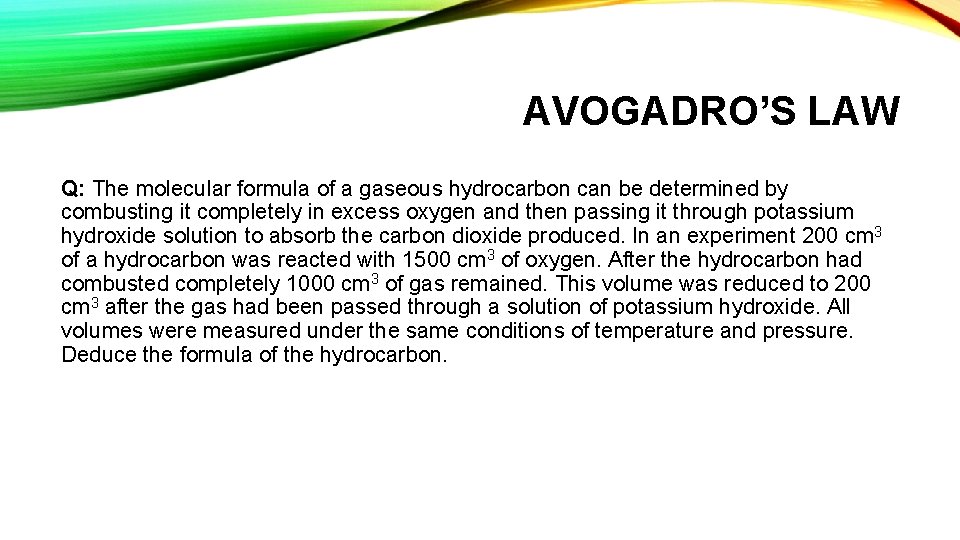
AVOGADRO’S LAW Q: The molecular formula of a gaseous hydrocarbon can be determined by combusting it completely in excess oxygen and then passing it through potassium hydroxide solution to absorb the carbon dioxide produced. In an experiment 200 cm 3 of a hydrocarbon was reacted with 1500 cm 3 of oxygen. After the hydrocarbon had combusted completely 1000 cm 3 of gas remained. This volume was reduced to 200 cm 3 after the gas had been passed through a solution of potassium hydroxide. All volumes were measured under the same conditions of temperature and pressure. Deduce the formula of the hydrocarbon.

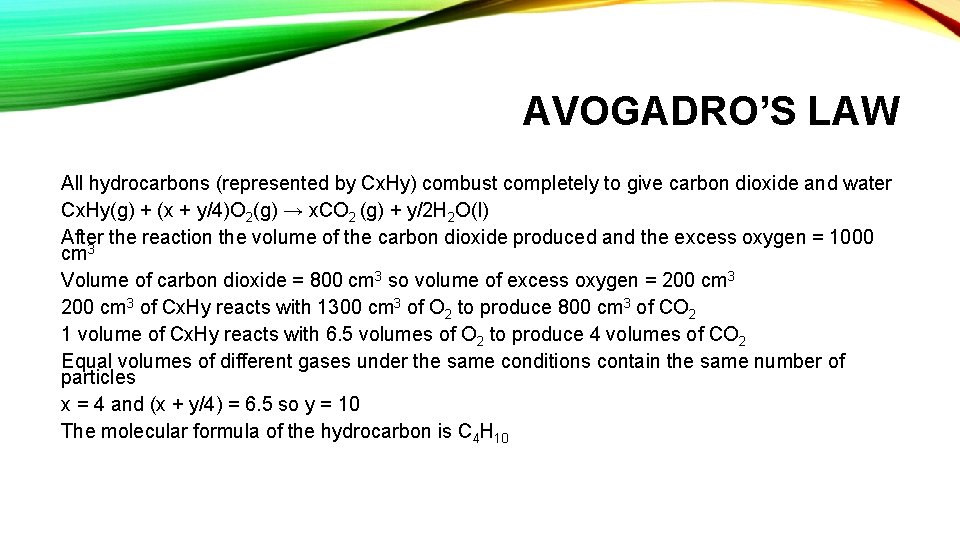
AVOGADRO’S LAW All hydrocarbons (represented by Cx. Hy) combust completely to give carbon dioxide and water Cx. Hy(g) + (x + y/4)O 2(g) → x. CO 2 (g) + y/2 H 2 O(l) After the reaction the volume of the carbon dioxide produced and the excess oxygen = 1000 cm 3 Volume of carbon dioxide = 800 cm 3 so volume of excess oxygen = 200 cm 3 of Cx. Hy reacts with 1300 cm 3 of O 2 to produce 800 cm 3 of CO 2 1 volume of Cx. Hy reacts with 6. 5 volumes of O 2 to produce 4 volumes of CO 2 Equal volumes of different gases under the same conditions contain the same number of particles x = 4 and (x + y/4) = 6. 5 so y = 10 The molecular formula of the hydrocarbon is C 4 H 10

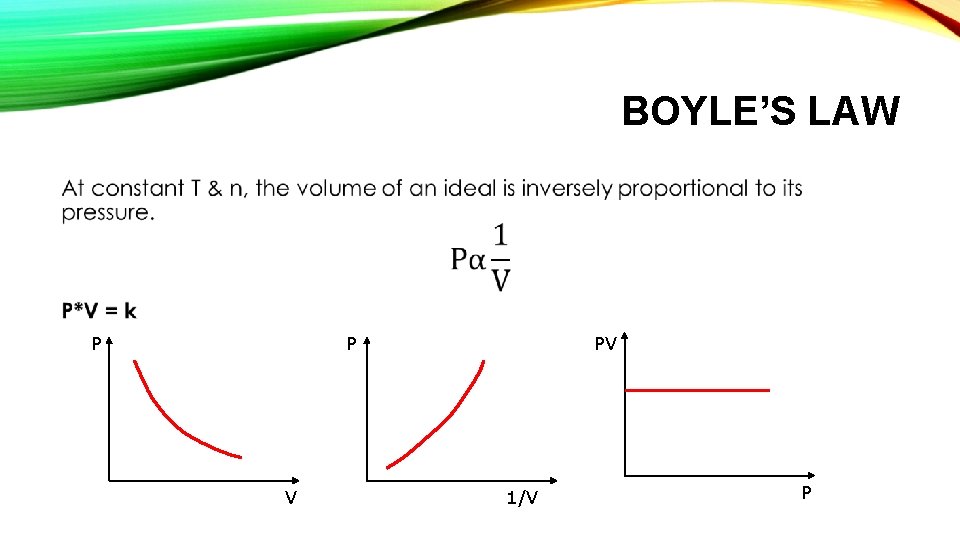
BOYLE’S LAW • P P V PV 1/V P

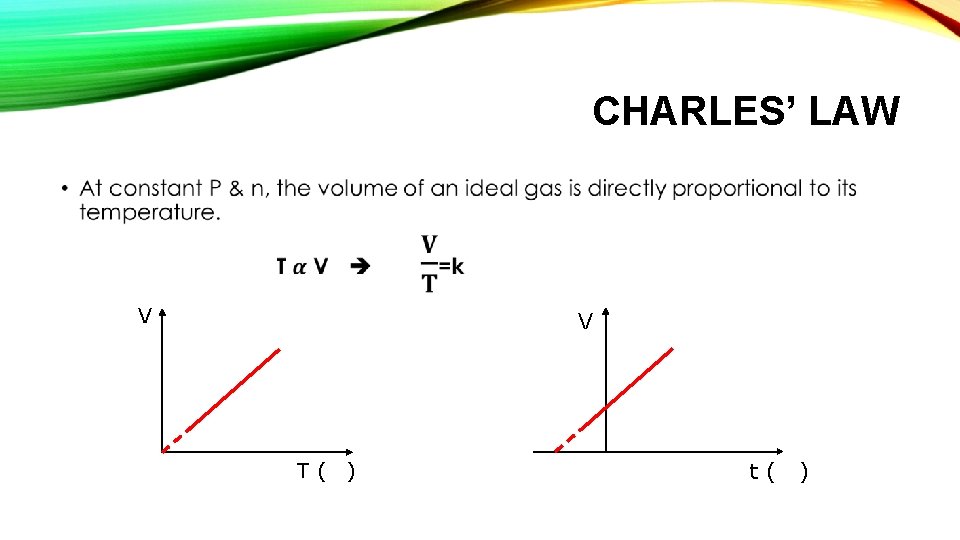
CHARLES’ LAW • V V T( ) t( )

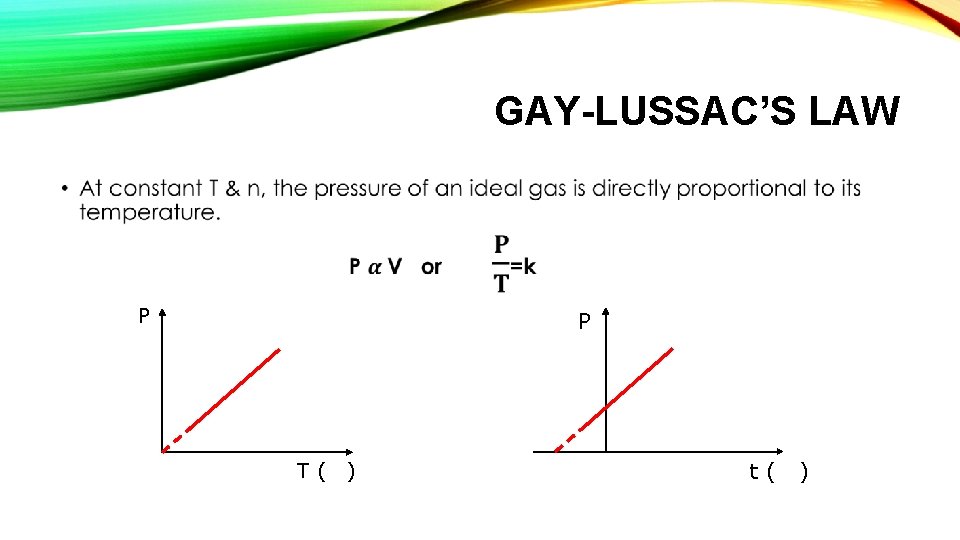
GAY-LUSSAC’S LAW • P P T( ) t( )

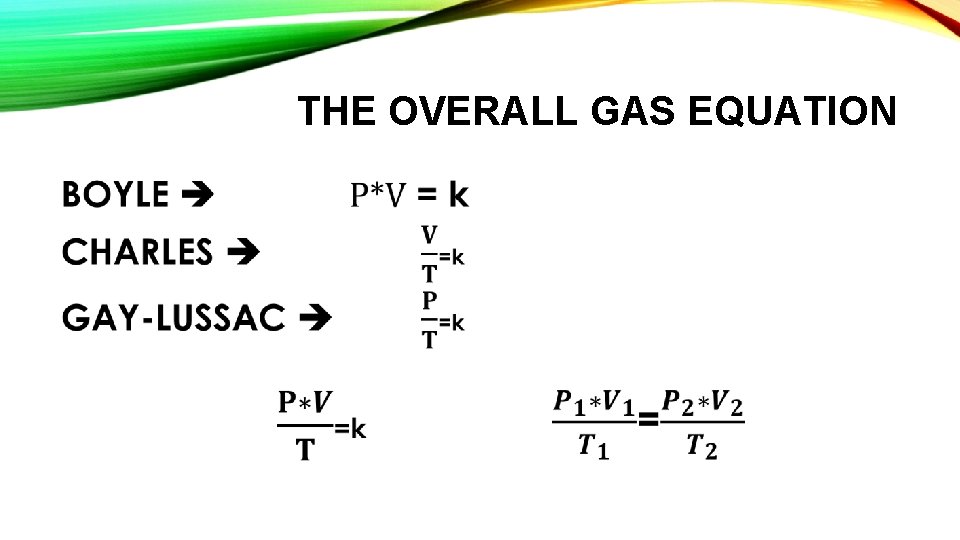
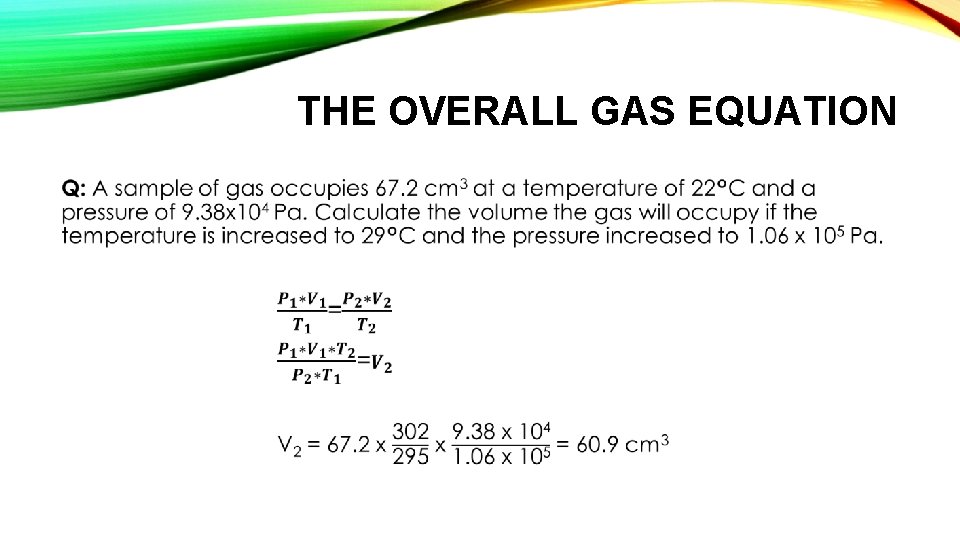
THE OVERALL GAS EQUATION •

THE OVERALL GAS EQUATION •

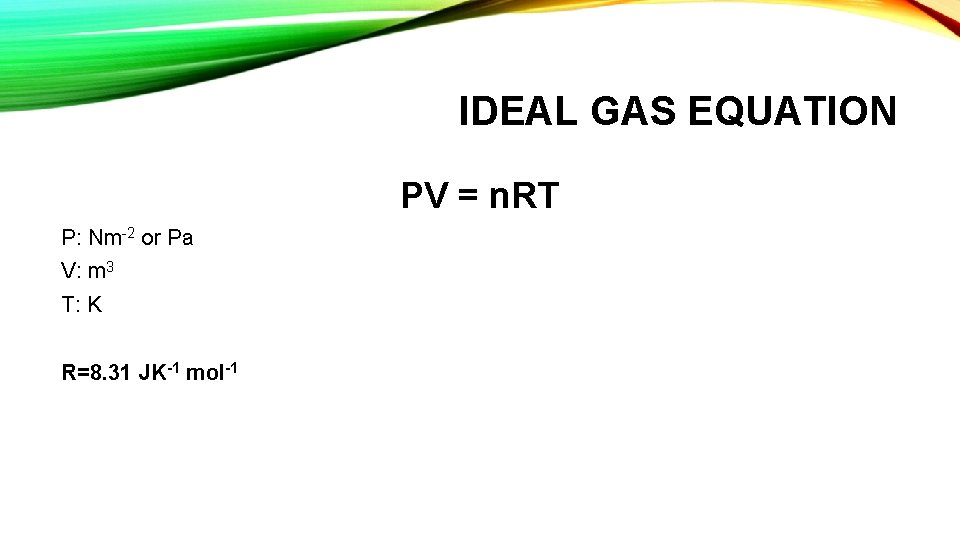
IDEAL GAS EQUATION PV = n. RT P: Nm-2 or Pa V: m 3 T: K R=8. 31 JK-1 mol-1

IDEAL GAS EQUATION Q: 2. 50 dm 3 of gas at a temperature of 19°C and a pressure of 1. 01 x 105 Pa has a mass of 4. 59 g. Determine the molar mass of the gas. n = PV/RT n = (1. 01 x 105) x (2. 50 x 10 -3)/ (8. 31 x 292) = 1. 04 x 10 -1 mol Molar mass of gas = 4. 59/1. 04 x 10 -1 = 44. 1 g mol-1 Test yourself pg 39

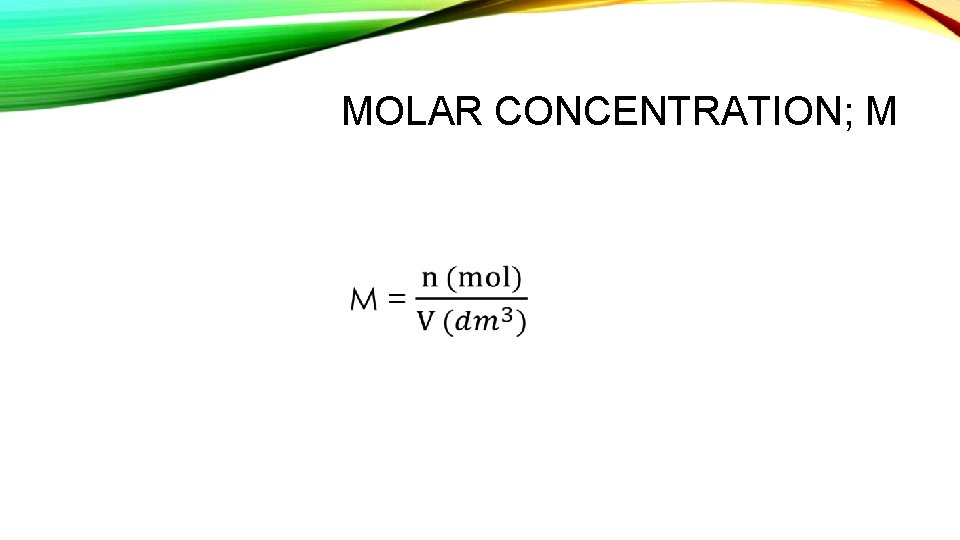
SOLUTIONS Solution = Solute + Solvent Concentration: The amount of solute dissolved in a unit volume of solution.

MOLAR CONCENTRATION; M •

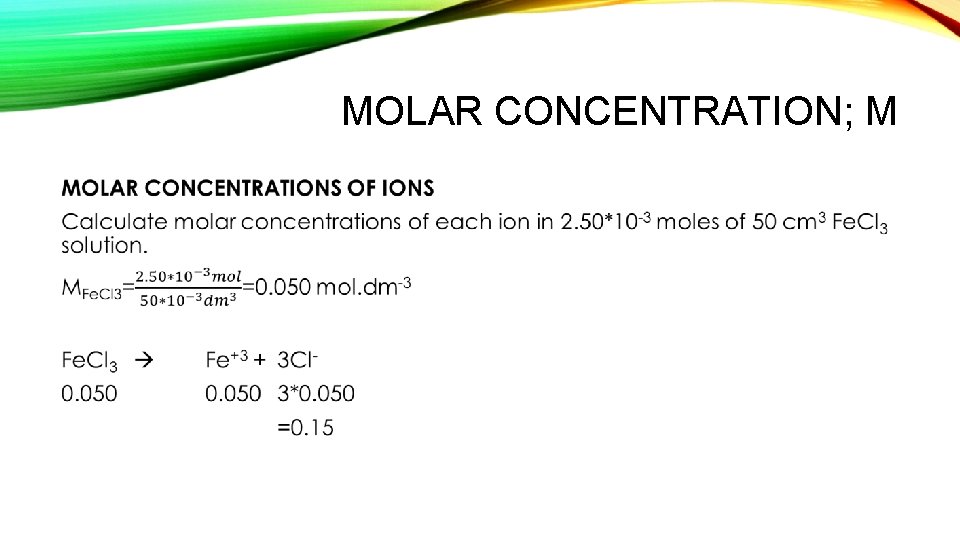
MOLAR CONCENTRATION; M •

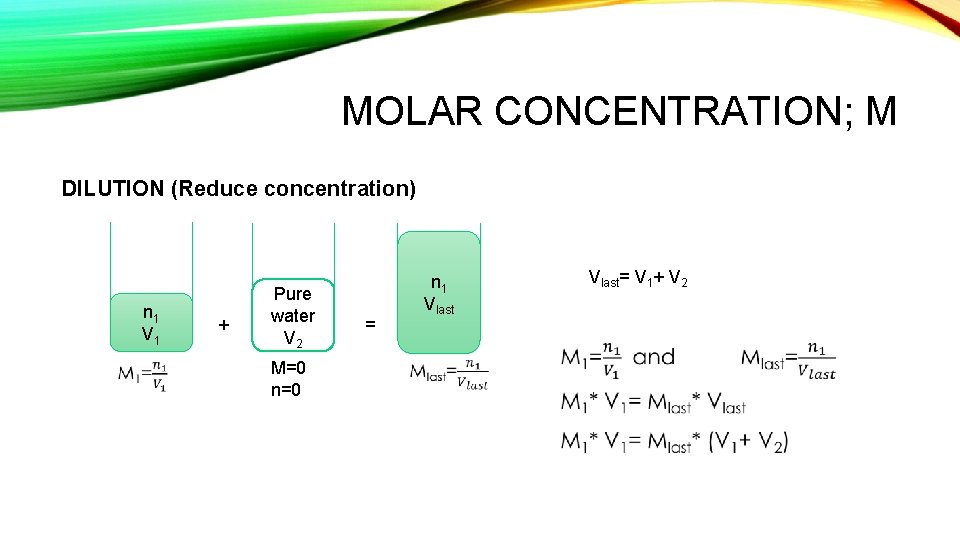
MOLAR CONCENTRATION; M DILUTION (Reduce concentration) n 1 V 1 + Pure water V 2 M=0 n=0 = n 1 Vlast= V 1+ V 2

Dilution (Reduce concentration) Q: Determine the final concentration of a 73 cm 3 solution of HCl with concentration 0. 40 mol. dm-3, which is diluted to a volume of 300 cm 3. 0. 10 mol. dm-3

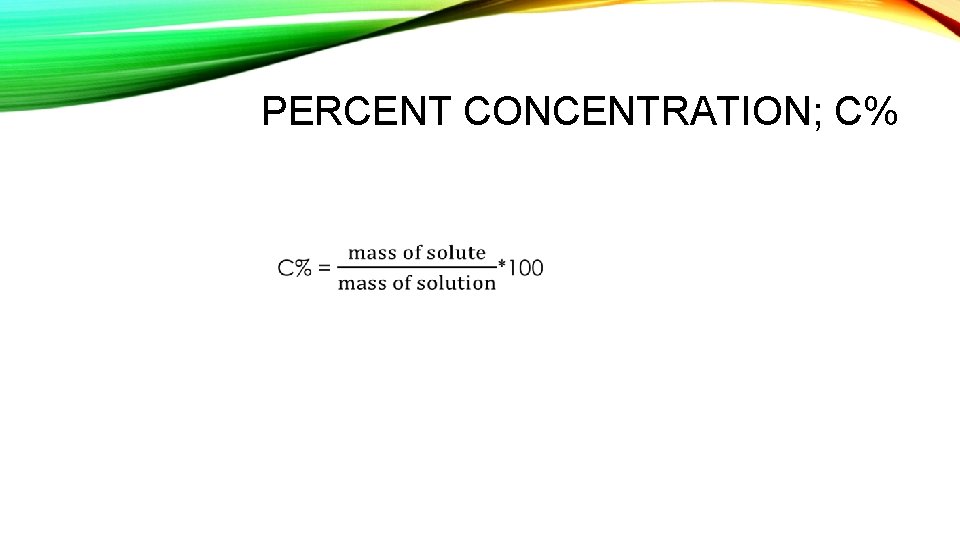
PERCENT CONCENTRATION; C% •

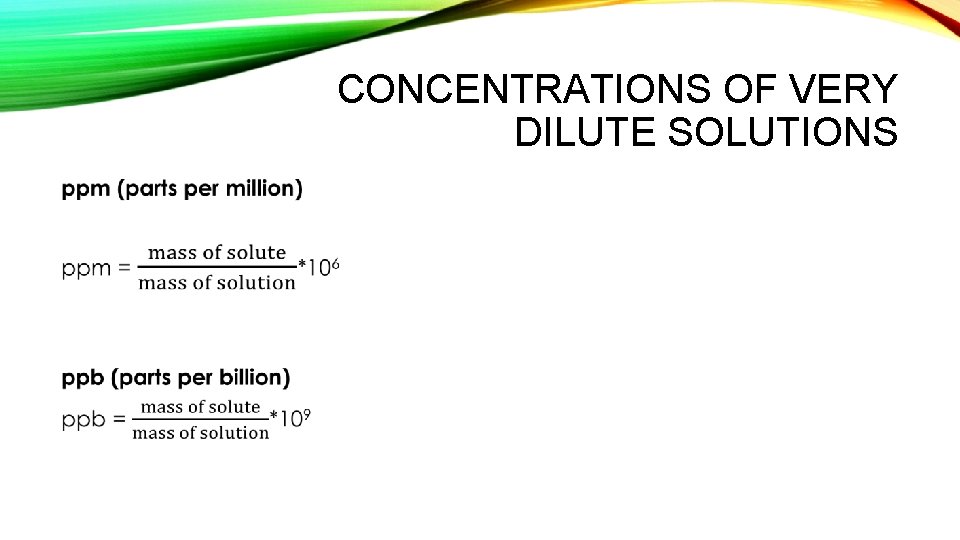
CONCENTRATIONS OF VERY DILUTE SOLUTIONS •

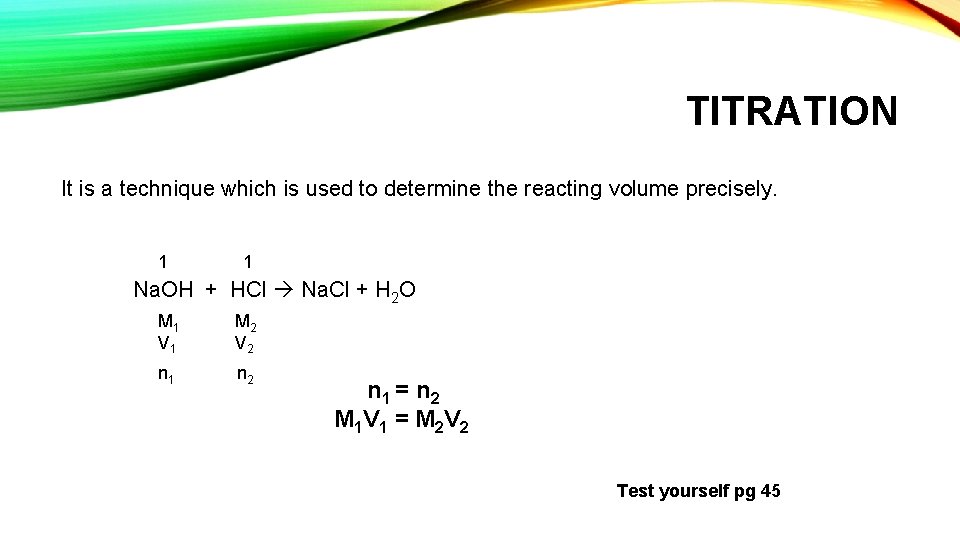
TITRATION It is a technique which is used to determine the reacting volume precisely. 1 1 Na. OH + HCl Na. Cl + H 2 O M 1 V 1 M 2 V 2 n 1 n 2 n 1 = n 2 M 1 V 1 = M 2 V 2 Test yourself pg 45

FINISH • Exam-Style questions pg 51 – 53
 Reacting masses and volumes
Reacting masses and volumes Reacting masses questions
Reacting masses questions Stoichiometry and stoichiometric calculations
Stoichiometry and stoichiometric calculations Mass to mass stoichiometry formula
Mass to mass stoichiometry formula Defining stoichiometry
Defining stoichiometry Types of connections in steel structures
Types of connections in steel structures Lithium and oxygen reaction
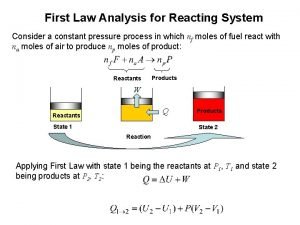
Lithium and oxygen reaction First law analysis of combustion reaction
First law analysis of combustion reaction Magnesium reacting with nitric acid equation
Magnesium reacting with nitric acid equation Hcl + naco3
Hcl + naco3 Magnesium reacting with oxygen
Magnesium reacting with oxygen Alkali metals reacting with water
Alkali metals reacting with water React to indirect fire
React to indirect fire Reacting to indirect fire
Reacting to indirect fire Copper react with oxygen
Copper react with oxygen Alkali metals reacting with water
Alkali metals reacting with water Alkali metals reacting with water
Alkali metals reacting with water The calculation of quantities in chemical reactions
The calculation of quantities in chemical reactions Employee relations in public relations
Employee relations in public relations What is stoichiometry
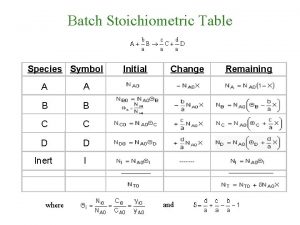
What is stoichiometry Stoichiometric table
Stoichiometric table Stoichiometric table for flow system
Stoichiometric table for flow system Stoichiometric gasoline
Stoichiometric gasoline Stoichiometric table for flow system
Stoichiometric table for flow system Me 322
Me 322 Symbolaab
Symbolaab Stoichiometric point definition
Stoichiometric point definition Stoichiometric table for flow system
Stoichiometric table for flow system Stoichiometric coefficient
Stoichiometric coefficient Stoichiometry
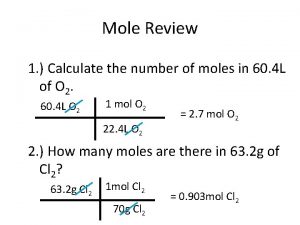
Stoichiometry Mole ratio examples
Mole ratio examples