1 3 Reacting Masses and Volumes Reacting Gases







- Slides: 20

1. 3 Reacting Masses and Volumes Reacting Gases Kristin Page IB SL Chemistry

Understandings • Avogadro’s law enables the mole ratio of reacting gases to be determined from volumes of the gases. • The molar volume of an ideal gas is a constant at specified temperature and pressure. • The molar concentration of a solution is determined by the amount of solute and the volume of solution. • A standard solution is one of known concentration.

Application & Skills • • Calculation of reacting volumes of gases using Avogadro’s law. Solution of problems and analysis of graphs involving the relationship between temperature, pressure and volume for a fixed mass of an ideal gas. Solution of problems relating to the ideal gas equation. Explanation of the deviation of real gases from ideal behavior at low temperature and high pressure. Obtaining and using experimental values to calculate the molar mass of a gas from the ideal gas equation. Solution of problems involving molar concentration, amount of solute and volume of solution. Use of the experimental method of titration to calculate the concentration of a solution by reference to a standard solution.

Kinetic Theory of Gases • Gas particles have high energy are separated by a lot of space • Gas particles are move rapidly in straight lines but random directions • Gas particles collide with each other and with the container but do not lose energy • There is no attractive force between gas particles • These hold true for IDEAL GASES

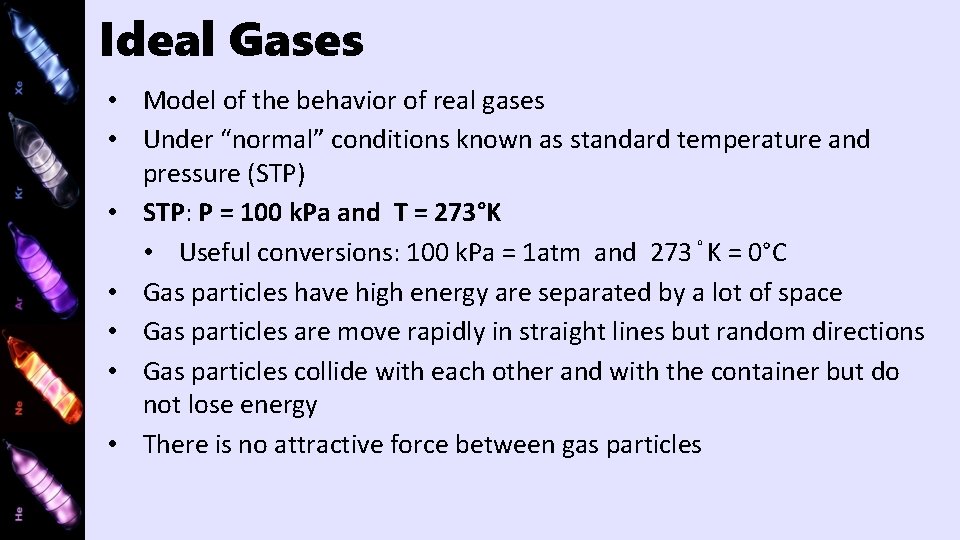
Ideal Gases • Model of the behavior of real gases • Under “normal” conditions known as standard temperature and pressure (STP) • STP: P = 100 k. Pa and T = 273°K • Useful conversions: 100 k. Pa = 1 atm and 273°K = 0°C • Gas particles have high energy are separated by a lot of space • Gas particles are move rapidly in straight lines but random directions • Gas particles collide with each other and with the container but do not lose energy • There is no attractive force between gas particles

Absolute Zero • When dealing with gases we need to use STP. This means that units of temperature must be in Kelvin (°K) • 273°K = 0°C • The Kelvin scale starts at absolute zero (0°K) • It is not actually possible to reach absolute zero since this would be the point at which particles stopped all motion* • * Actually technically not true anymore but for our purposes we will stick with this. If you are curious go to http: //www. nature. com/news/quantum-gas-goes-below-absolutezero-1. 12146

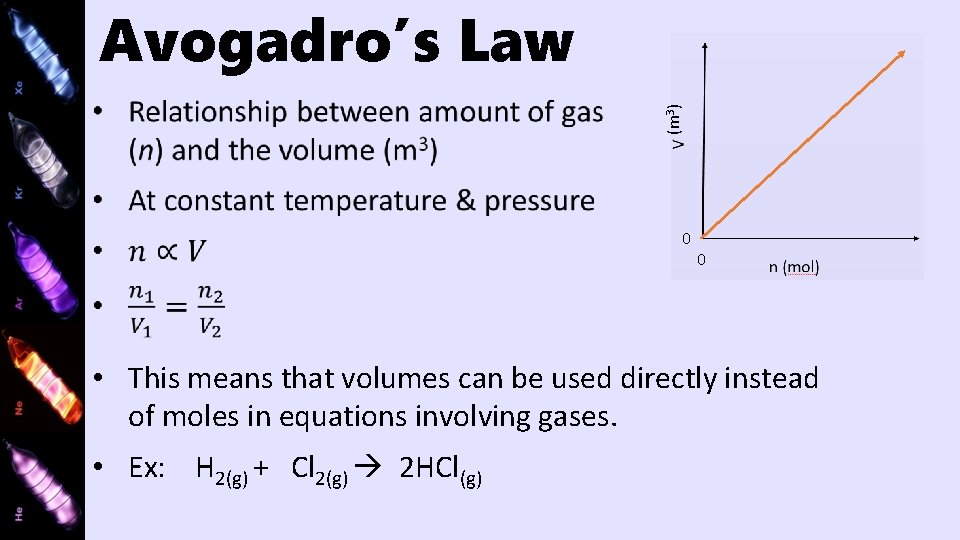
(m 3) Avogadro’s Law 0 0 • This means that volumes can be used directly instead of moles in equations involving gases. • Ex: H 2(g) + Cl 2(g) 2 HCl(g)

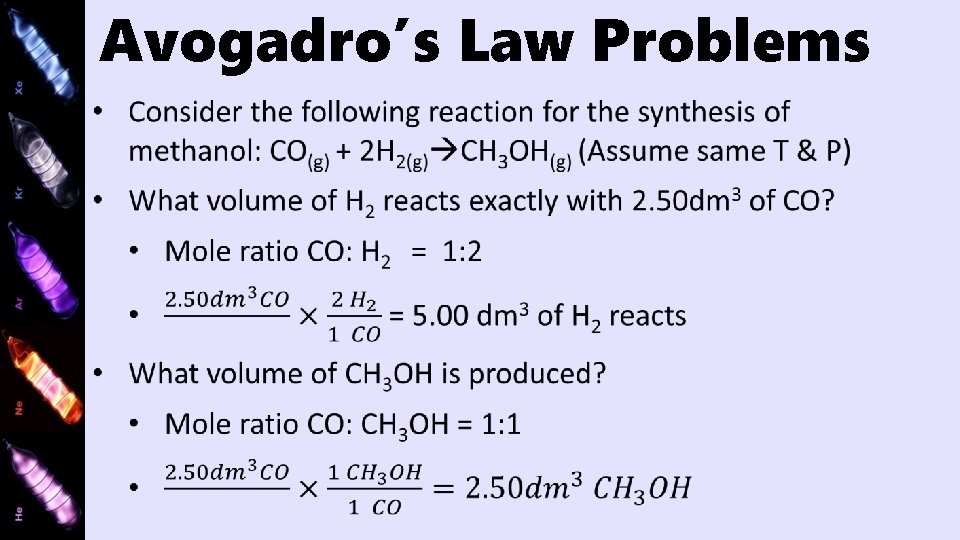
Avogadro’s Law Problems

Avogadro’s Law Problems

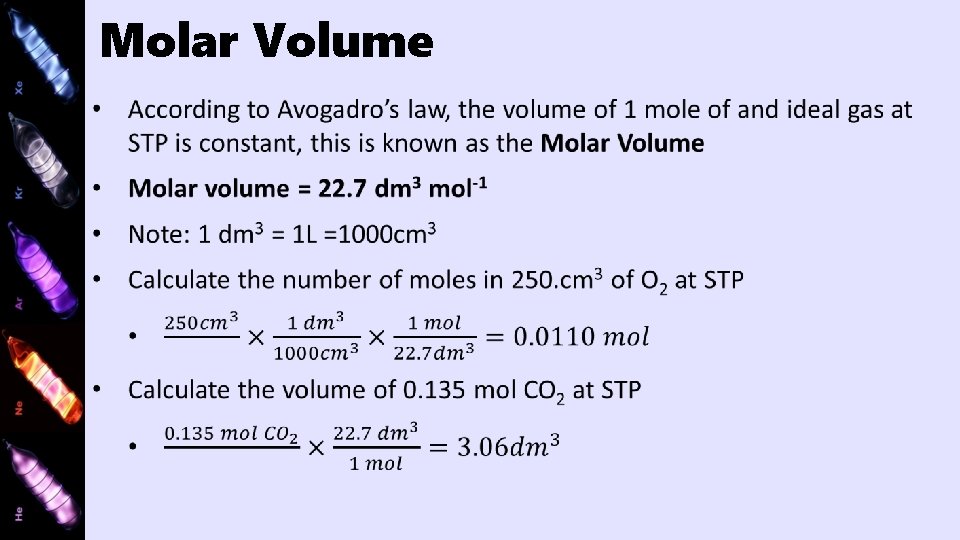
Molar Volume

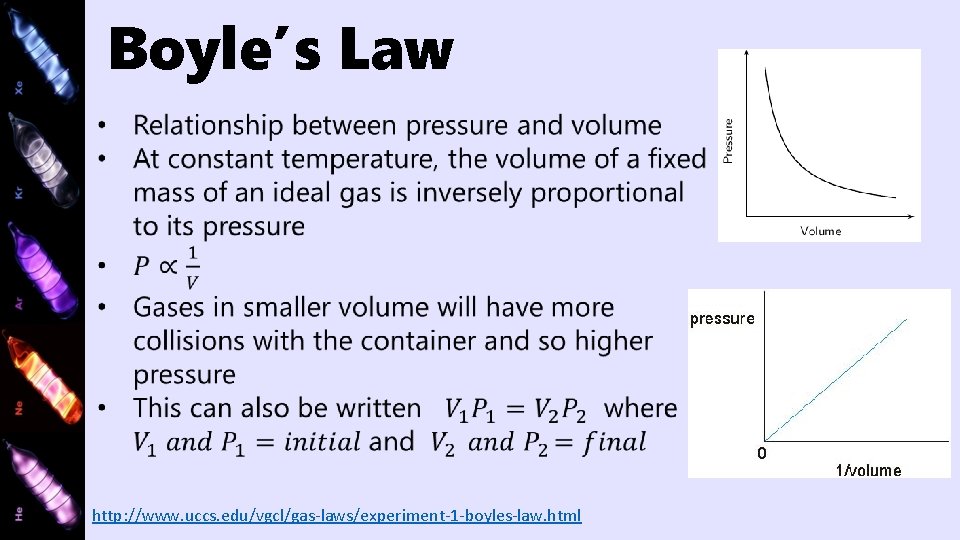
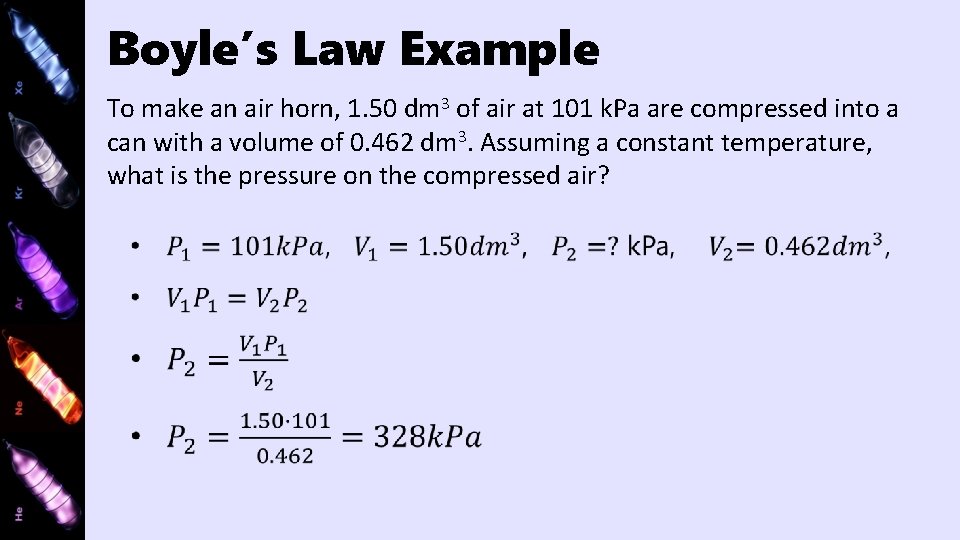
Boyle’s Law http: //www. uccs. edu/vgcl/gas-laws/experiment-1 -boyles-law. html

Boyle’s Law Example To make an air horn, 1. 50 dm 3 of air at 101 k. Pa are compressed into a can with a volume of 0. 462 dm 3. Assuming a constant temperature, what is the pressure on the compressed air?

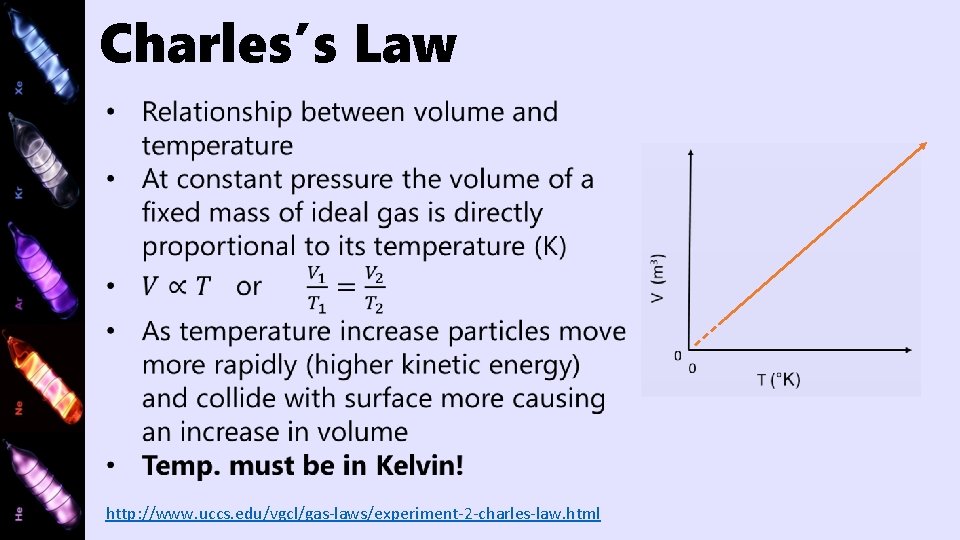
Charles’s Law http: //www. uccs. edu/vgcl/gas-laws/experiment-2 -charles-law. html

Charles’s Law Example On hot days, you may have noticed that potato chip bags seem to “inflate”, even though they have not been opened. If I have a 250 m. L bag at a temperature of 19°C, and I leave it in my car which has a temperature of 45°C, what will the new volume of the bag be?

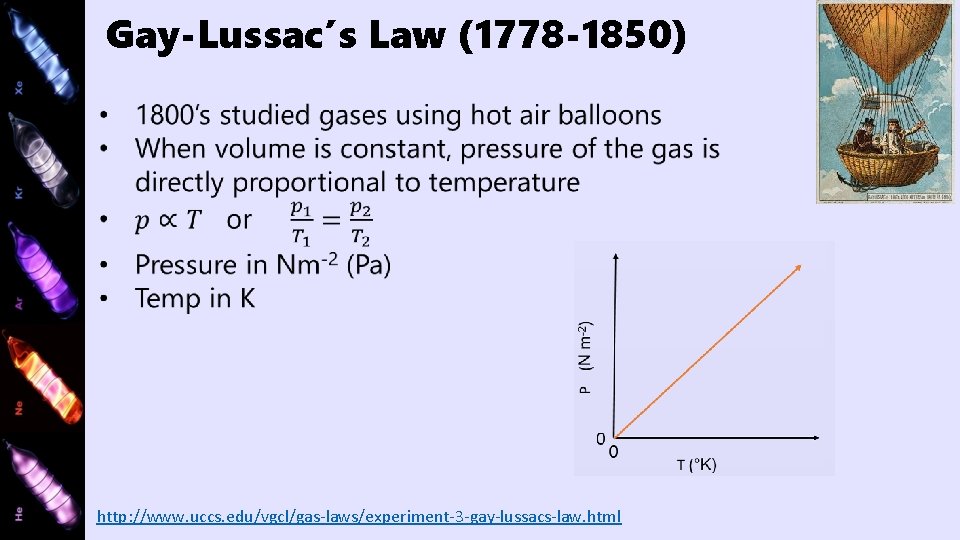
Gay-Lussac’s Law (1778 -1850) http: //www. uccs. edu/vgcl/gas-laws/experiment-3 -gay-lussacs-law. html

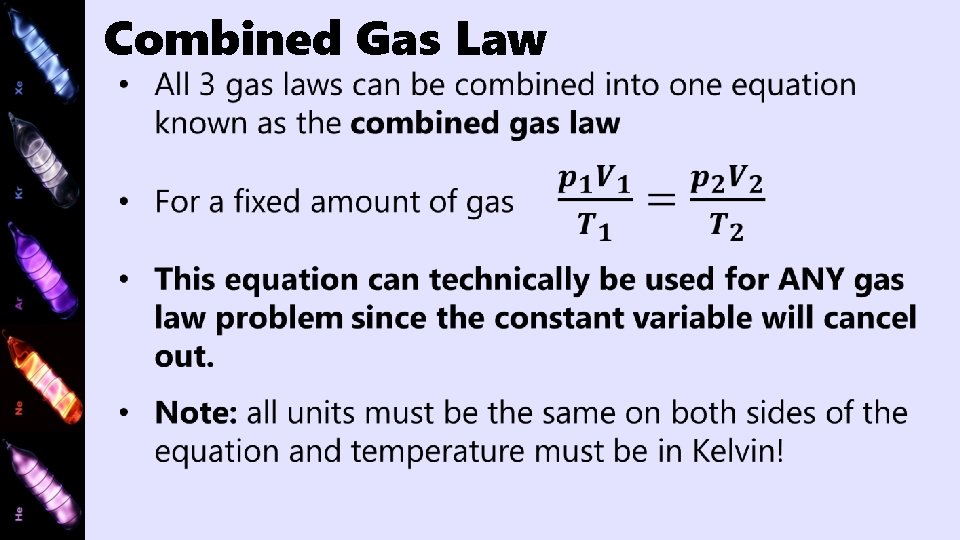
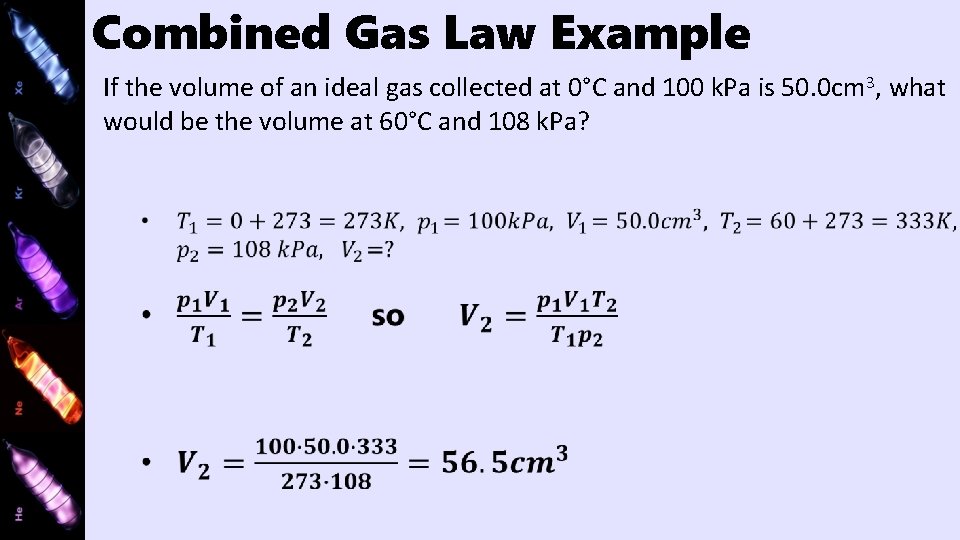
Combined Gas Law

Combined Gas Law Example If the volume of an ideal gas collected at 0°C and 100 k. Pa is 50. 0 cm 3, what would be the volume at 60°C and 108 k. Pa?

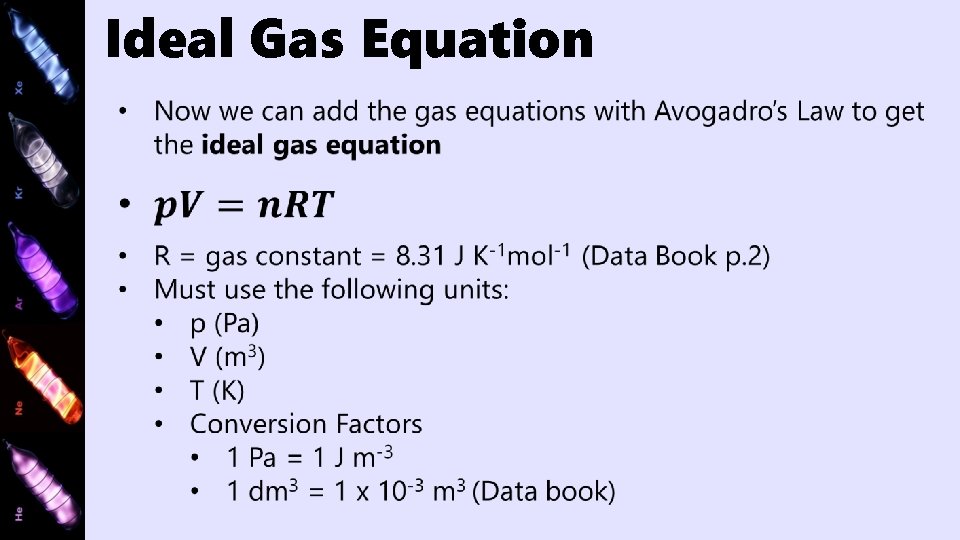
Ideal Gas Equation

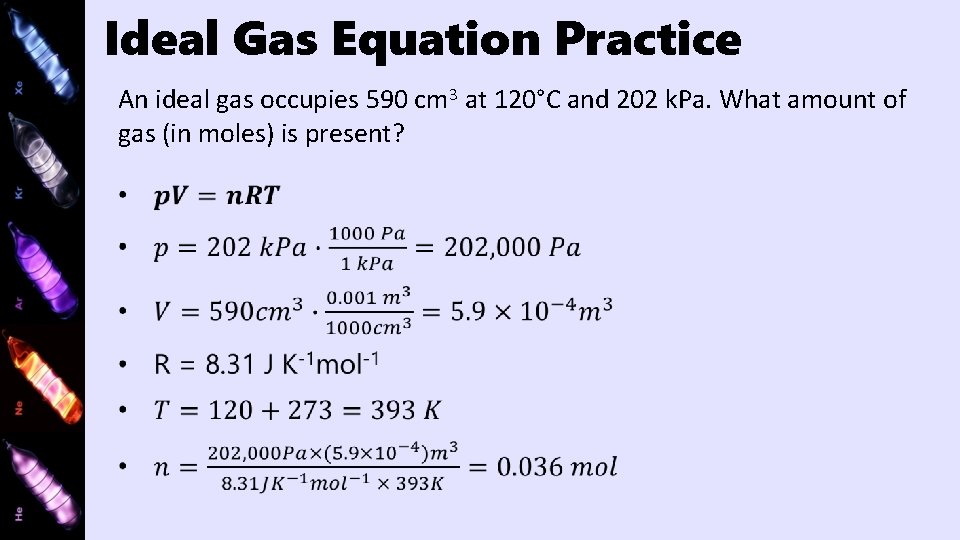
Ideal Gas Equation Practice An ideal gas occupies 590 cm 3 at 120°C and 202 k. Pa. What amount of gas (in moles) is present?

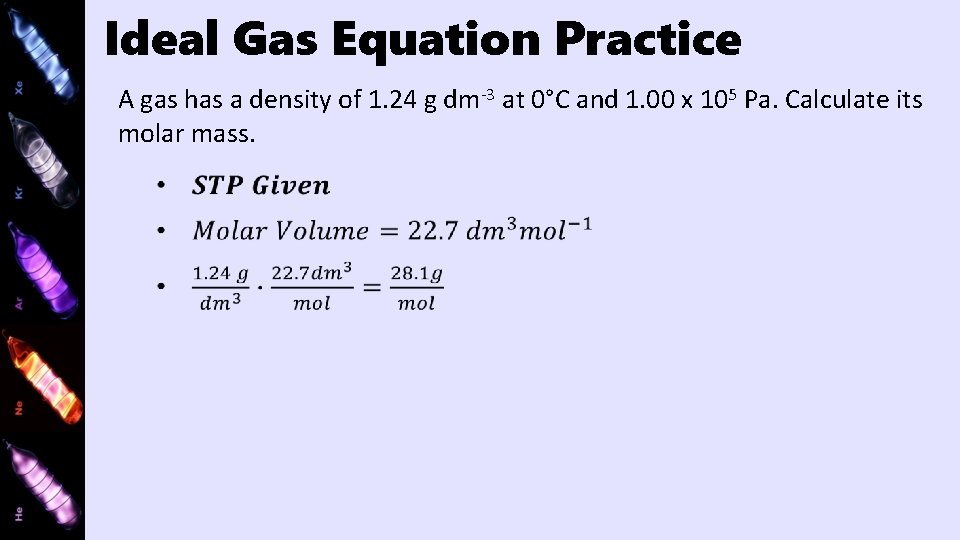
Ideal Gas Equation Practice A gas has a density of 1. 24 g dm-3 at 0°C and 1. 00 x 105 Pa. Calculate its molar mass.
 Reacting masses and volumes
Reacting masses and volumes Zinc carbonate
Zinc carbonate Lithium react with oxygen equation
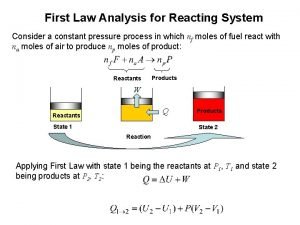
Lithium react with oxygen equation First law analysis
First law analysis Magnesium reacting with nitric acid equation
Magnesium reacting with nitric acid equation Hydrochloric acid and sodium bicarbonate
Hydrochloric acid and sodium bicarbonate Magnesium reacting with oxygen
Magnesium reacting with oxygen Alkali metals reacting with water
Alkali metals reacting with water React to direct fire while mounted
React to direct fire while mounted Reacting to indirect fire
Reacting to indirect fire Reaction of copper with air
Reaction of copper with air Alkali metals reacting with water
Alkali metals reacting with water Alkali metals reacting with water
Alkali metals reacting with water Practice 10-6 volumes of pyramids and cones
Practice 10-6 volumes of pyramids and cones Fictional character
Fictional character 11-3 reteach volume of pyramids and cones
11-3 reteach volume of pyramids and cones Geometry
Geometry 1 inch
1 inch Two astronauts of masses 60 kg and 80 kg
Two astronauts of masses 60 kg and 80 kg 12-6 surface areas and volumes of spheres answers
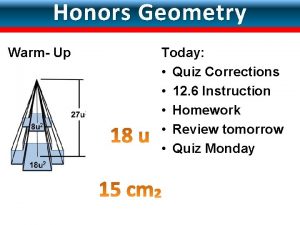
12-6 surface areas and volumes of spheres answers Lesson 12-5 volumes of pyramids and cones
Lesson 12-5 volumes of pyramids and cones