REACTING TO DENSITY REACTING TO DENSITY PART 1
















- Slides: 34

REACTING TO DENSITY

REACTING TO DENSITY – PART 1 Conduct your experiment – follow your procedure Make sure you include all data and observations! Include sketches of each set-up AFTER the reaction. Step 11 – See if the gas released will extinguish a candle. Follow the procedure in your notebook! Make sure you have a BEFORE and AFTER sketch. Step 12 – Read and Take notes on important vocabulary! Step 12 a-d: Complete in your notebook Step 13 – answer in your notebook. Complete S&T #1 -4 on page 84.

REACTING TO DENSITY PART 1 P&P Step 7: Did the water bottle lose any material during the reaction? How do you know?

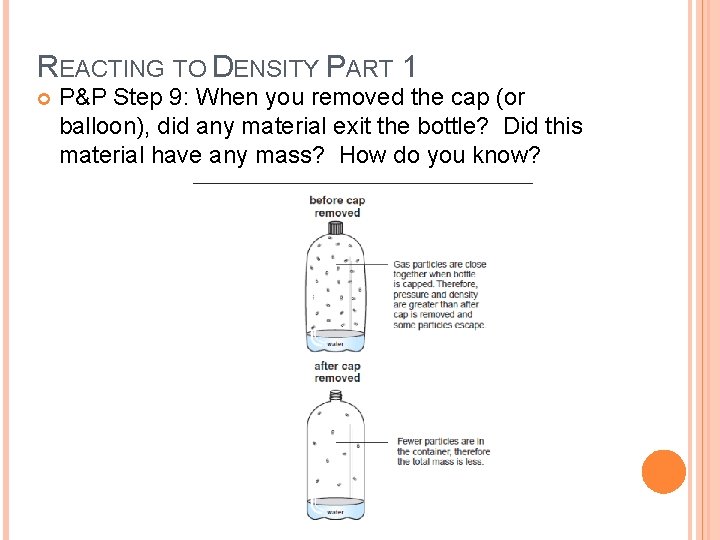
REACTING TO DENSITY PART 1 P&P Step 9: When you removed the cap (or balloon), did any material exit the bottle? Did this material have any mass? How do you know?

REACTING TO DENSITY PART 1 P&P Step 10: Did the gas that was produced extinguish a flame? What do you think the gas was?

REACTING TO DENSITY PART 1 12. Evidence For or Against a Chemical Reaction What I observed Evidence For or Against chemical Reaction How I know

REACTING TO DENSITY PART 1 13. Evidence For or Against Conservation of Matter What I observed Evidence For or Against conservatoin of matter How I know

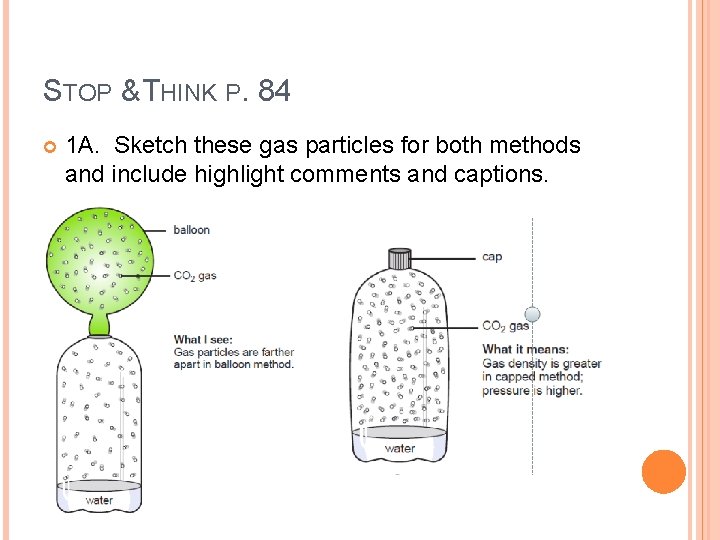
STOP & THINK P. 84 1. The gas produced was carbon dioxide, the same gas we exhale during breathing. Suppose both the cap and balloon methods captured exactly 50 molecules of carbon dioxide. � A. Sketch these gas particles for both methods and include highlight comments and captions.

STOP & THINK P. 84 1 A. Sketch these gas particles for both methods and include highlight comments and captions.

STOP & THINK P. 84 1 b. Why should there be the same number of gas particles in each bottle, provided the amount of water and the size of the tablet were the same? If the law of conservation of matter is true, then the amount of gas produced must be the same if the amounts of the reactants are the same.

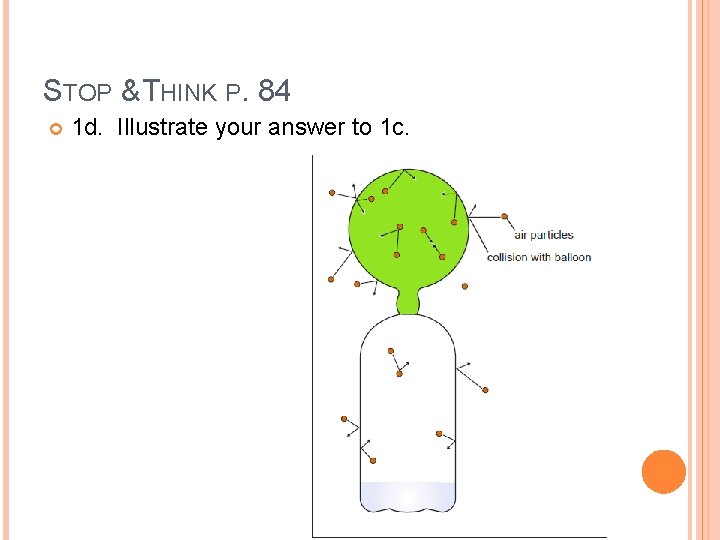
STOP & THINK P. 84 1 c. Are the carbon dioxide particles moving or stationary? How do you know? The carbon dioxide particles must be moving, otherwise the balloon would not inflate and stay inflated. The only way the gas could generate a pressure on the inside of the balloon is if those gas particles on the inside were moving around and colliding with the balloon walls to maintain the pressure.

STOP & THINK P. 84 1 d. Illustrate your answer to 1 c.

STOP & THINK P. 84 2. In which method is the pressure inside the bottle the greatest? What evidence supports your answer? The capped bottle has more pressure inside. If you squeezed both bottles, you would have seen that the capped bottle was more difficult to squeeze.

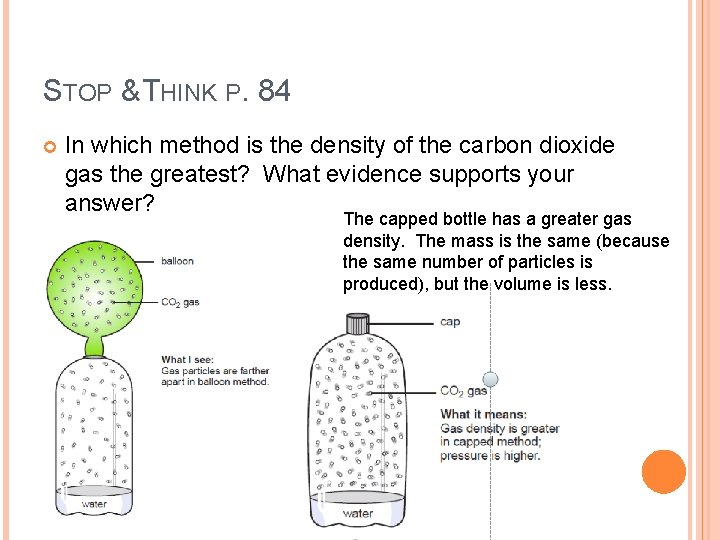
STOP & THINK P. 84 In which method is the density of the carbon dioxide gas the greatest? What evidence supports your answer? The capped bottle has a greater gas density. The mass is the same (because the same number of particles is produced), but the volume is less.

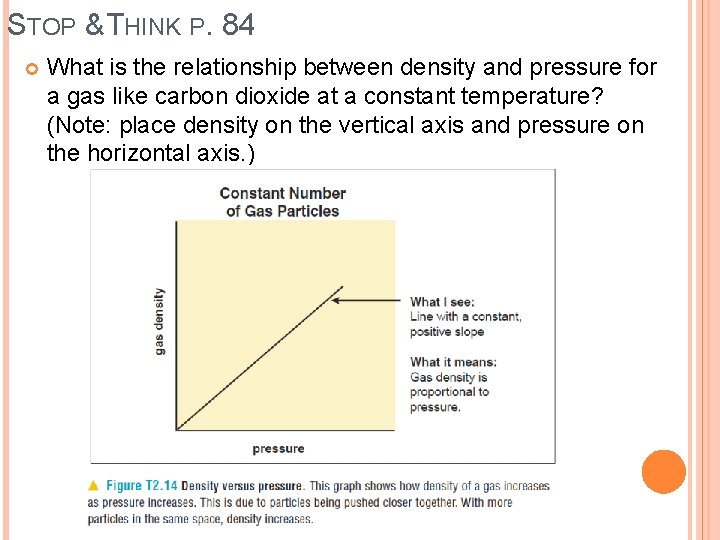
STOP & THINK P. 84 What is the relationship between density and pressure for a gas like carbon dioxide at a constant temperature? (Note: place density on the vertical axis and pressure on the horizontal axis. )

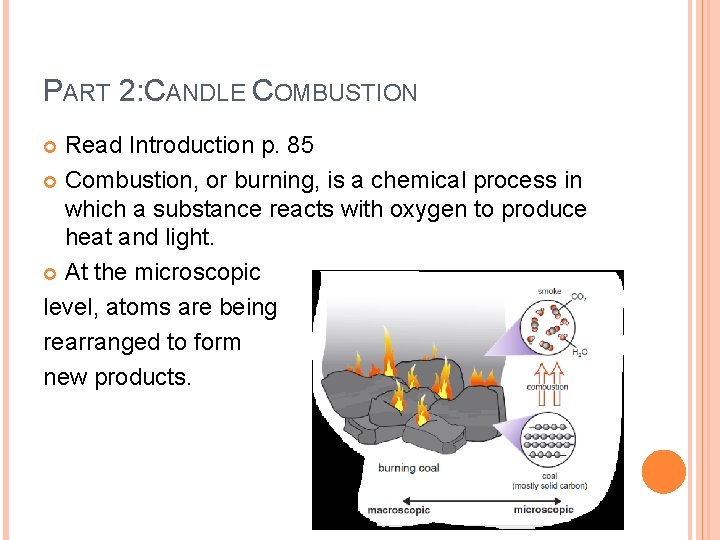
PART 2: CANDLE COMBUSTION Read Introduction p. 85 Combustion, or burning, is a chemical process in which a substance reacts with oxygen to produce heat and light. At the microscopic level, atoms are being rearranged to form new products.

PART 2: CANDLE COMBUSTION How can we demonstrate that a candle burning for 2 minutes represents a chemical reaction? Draw a sketch of the candle before and after, and label your sketch with the words: solid, liquid, gas, wick, flame, temperature change.

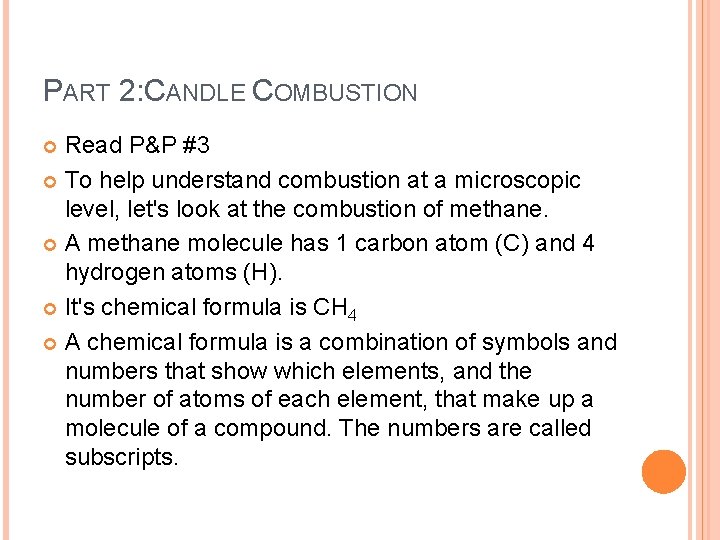
PART 2: CANDLE COMBUSTION Read P&P #3 To help understand combustion at a microscopic level, let's look at the combustion of methane. A methane molecule has 1 carbon atom (C) and 4 hydrogen atoms (H). It's chemical formula is CH 4 A chemical formula is a combination of symbols and numbers that show which elements, and the number of atoms of each element, that make up a molecule of a compound. The numbers are called subscripts.

PART 2: CANDLE COMBUSTION Chemical equations describe the chemical reaction of elements and compounds to form new compounds. The structure of a chemical equation is reactants ------- products The arrow means “yields” and tells which direction the reaction goes

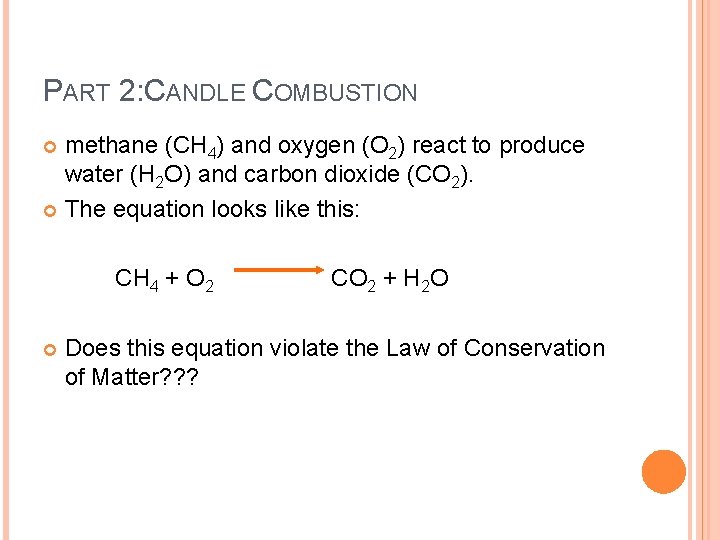
PART 2: CANDLE COMBUSTION methane (CH 4) and oxygen (O 2) react to produce water (H 2 O) and carbon dioxide (CO 2). The equation looks like this: CH 4 + O 2 CO 2 + H 2 O Does this equation violate the Law of Conservation of Matter? ? ?

PART 2: CANDLE COMBUSTION We need to balance the equation. Why? The number of atoms of each type must be equal on both sides of the arrow. Why? You CANNOT change the chemical formula. You CANNOT add new products or reactants You CAN change the coefficients in front of the existing reactants and products to make the equation balance. (This is the same as adding another molecule of a product or reactant. )

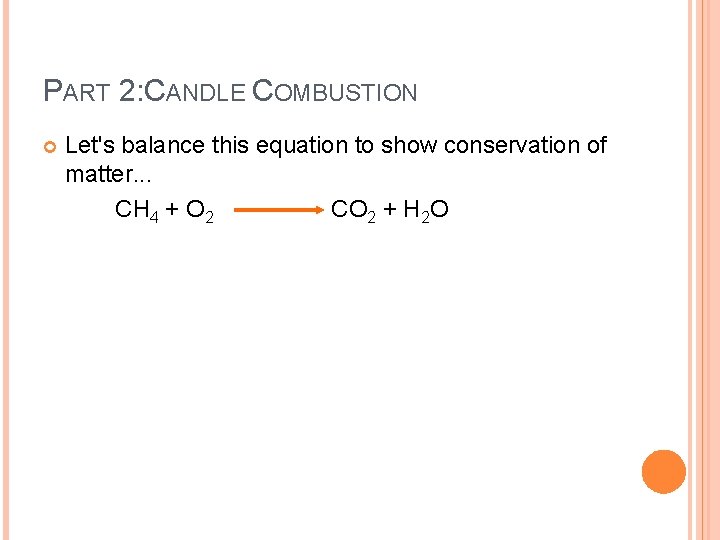
PART 2: CANDLE COMBUSTION Let's balance this equation to show conservation of matter. . . CH 4 + O 2 CO 2 + H 2 O

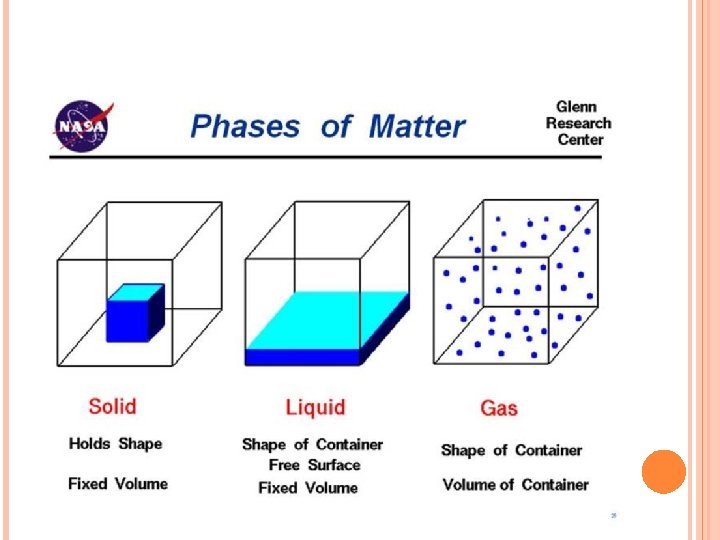
PART 3: PHASES OF MATTER Read Intro p. 87

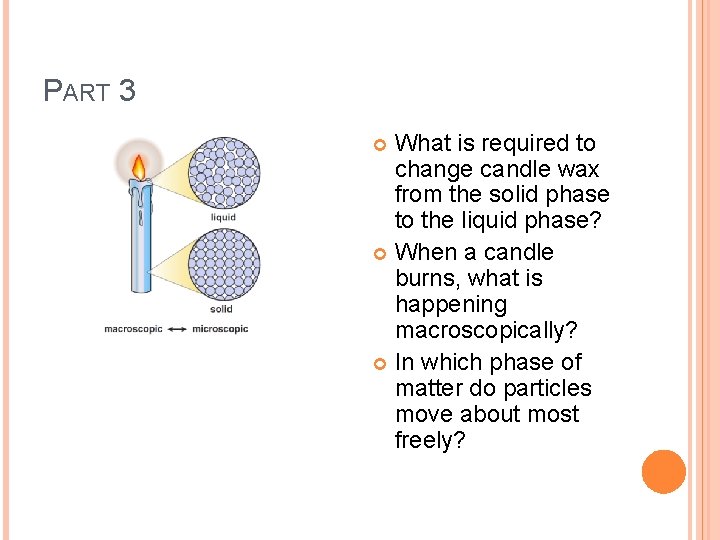
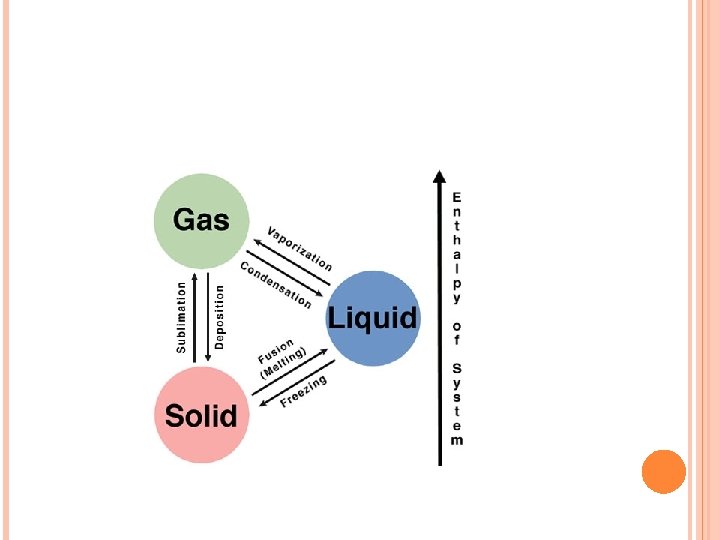
PHYSICAL CHANGE A change where the composition of the substance remains the same, even if the appearance does not. � Examples: changes of state: solid – liquid – gas What is happening at the atomic level? Let’s look at a burning candle to find out…

PART 3 What is required to change candle wax from the solid phase to the liquid phase? When a candle burns, what is happening macroscopically? In which phase of matter do particles move about most freely?


PART 2&3: CANDLE COMBUSTION Complete Stop & Think #1 -4 p. 87 Stop & Think #1 -4 p. 89 Reflect & Connect #1 -4 p. 89 -90

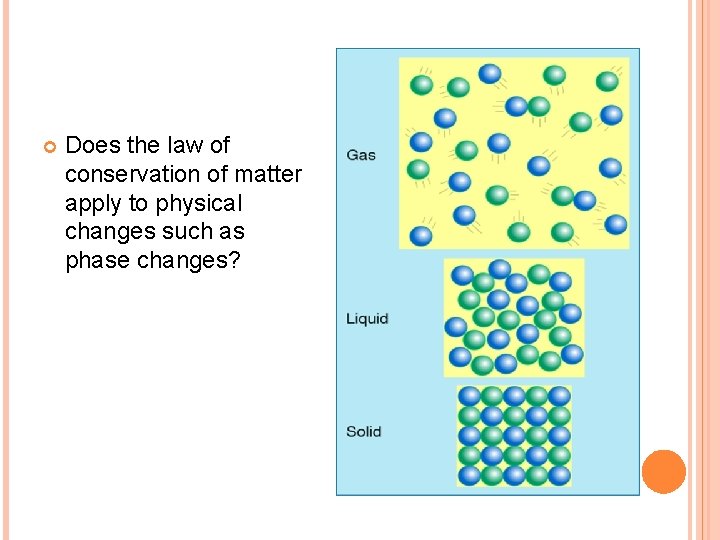
Does the law of conservation of matter apply to physical changes such as phase changes?


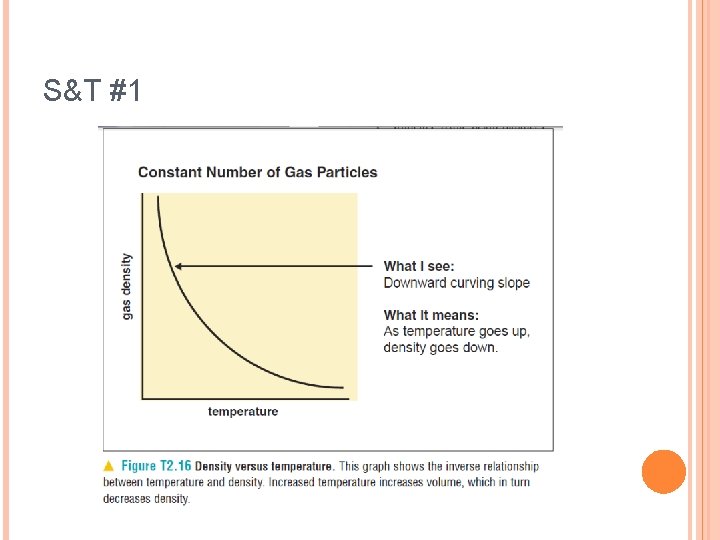
S&T #1 P. 89 In which phase of matter does a CONSTANT number of particles have a higher temp than its other phases? How does increased temperature for a constant number of particles affect density? Sketch the relationship between density and temperature at a constant pressure and constant number of particles.

S&T #1

S&T #2 In which phase of matter do attractions between particles affect motion the most? In which phase of matter do attractions between particles affect motion least?

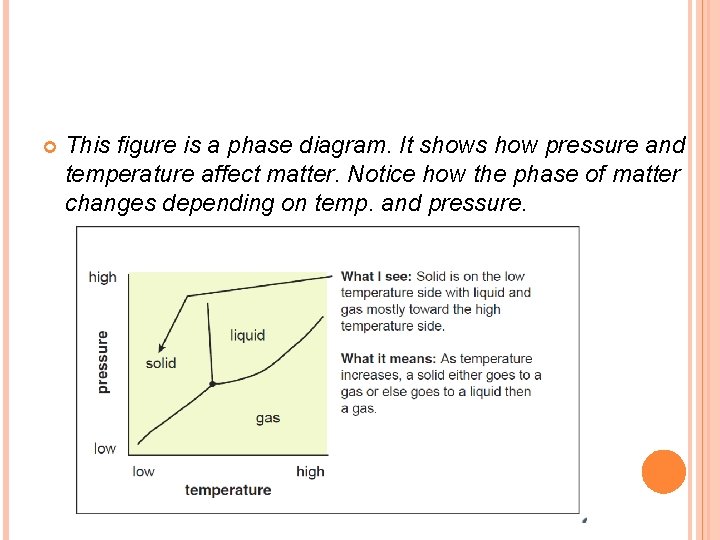
This figure is a phase diagram. It shows how pressure and temperature affect matter. Notice how the phase of matter changes depending on temp. and pressure.
