Density and Temperature Chemistry Ms Hughes Density and











- Slides: 22

Density and Temperature Chemistry --- Ms. Hughes

Density and Specific Gravity

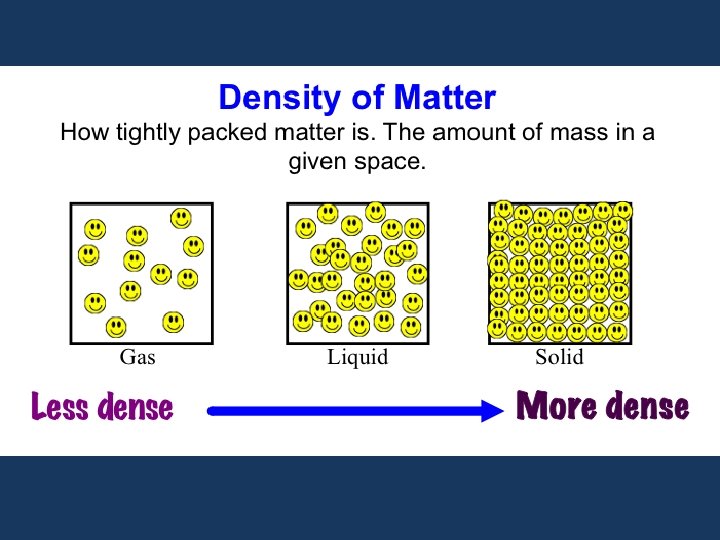
VIII. ) DENSITY AND SPECIFIC GRAVITY A. ) DENSITY is the ratio of the mass of a substance to the volume occupied by that substance. Density = mass of substance OR d= m Vol. of substance V


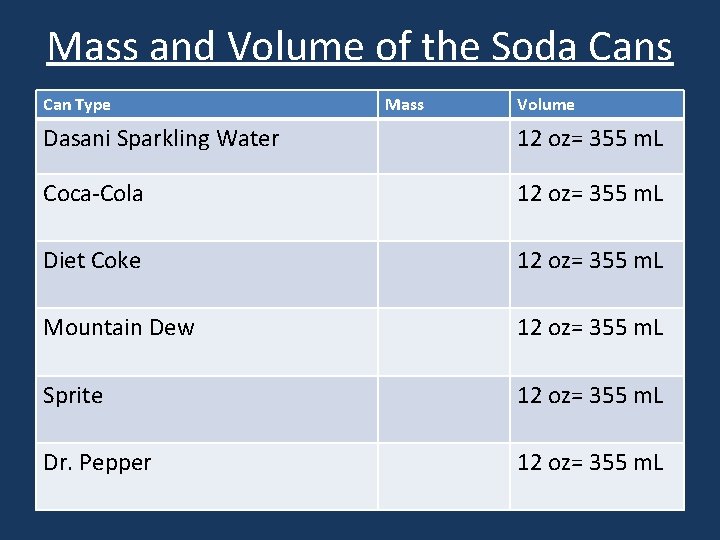
“Sinking Soda Surprise” Which can is more dense?

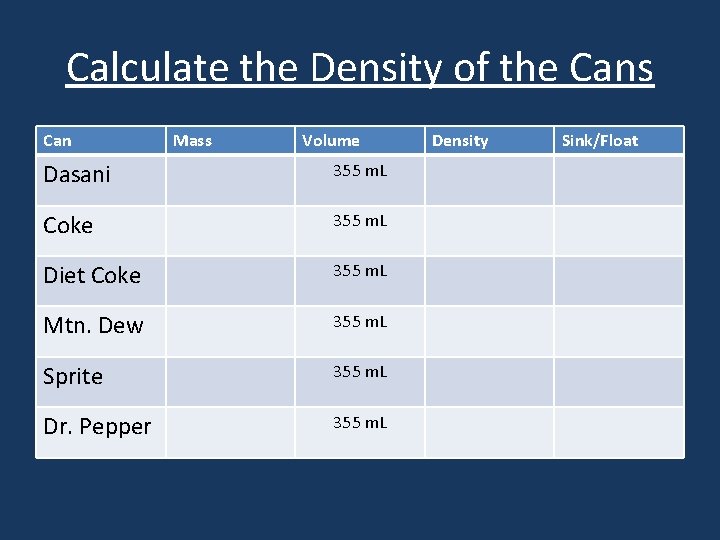
Mass and Volume of the Soda Cans Can Type Mass Volume Dasani Sparkling Water 12 oz= 355 m. L Coca-Cola 12 oz= 355 m. L Diet Coke 12 oz= 355 m. L Mountain Dew 12 oz= 355 m. L Sprite 12 oz= 355 m. L Dr. Pepper 12 oz= 355 m. L

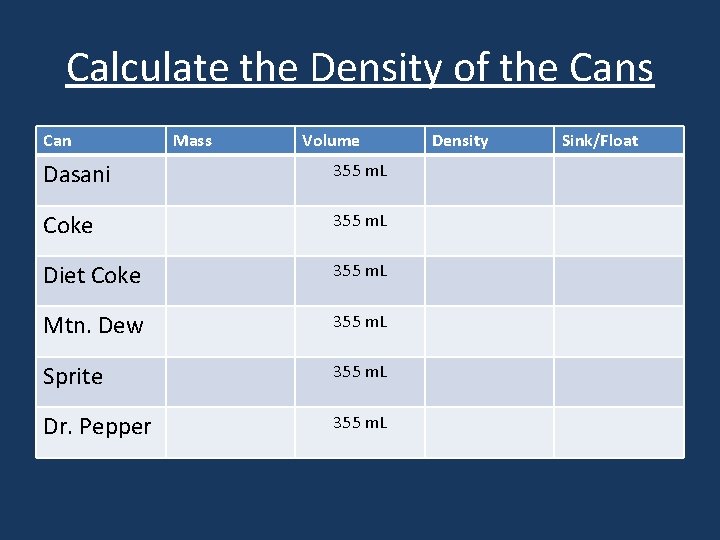
Calculate the Density of the Cans Can Mass Volume Dasani 355 m. L Coke 355 m. L Diet Coke 355 m. L Mtn. Dew 355 m. L Sprite 355 m. L Dr. Pepper 355 m. L Density Sink/Float

Calculate the Density of the Cans Can Mass Volume Dasani 355 m. L Coke 355 m. L Diet Coke 355 m. L Mtn. Dew 355 m. L Sprite 355 m. L Dr. Pepper 355 m. L Density Sink/Float

1. ) Density is expressed in different units depending on the phase of the substance. a. ) Solids are usually expressed in grams per cubic centimeter (g/cm 3)

b. ) Liquids are commonly grams per milliliter (g/m. L)

c. ) Gases are usually expressed as grams per liter (g/L)

2. ) Density can be used as a conversion factor that relates mass and volume. 3. ) Densities can be used to calculate mass if volume is given or calculate volume given mass.

WORKED EXAMPLE 1 -5 If 10. 4 m. L of a liquid has a mass of 9. 142 g, what is its density? Solution. d= m d = 9. 142 g = 0. 879 g/m. L V 10. 4 m. L

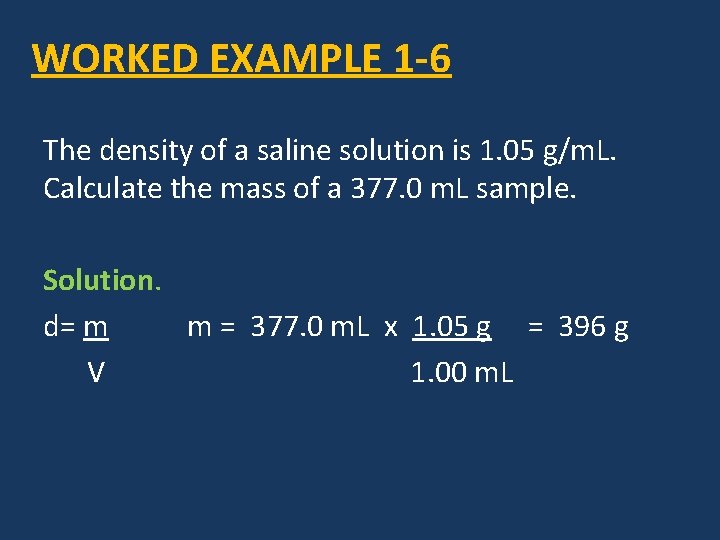
WORKED EXAMPLE 1 -6 The density of a saline solution is 1. 05 g/m. L. Calculate the mass of a 377. 0 m. L sample. Solution. d= m m = 377. 0 m. L x 1. 05 g = 396 g V 1. 00 m. L

PRACTICE 1 -8 The density of rubbing alcohol is 0. 786 g/m. L. What volume of rubbing alcohol would you use if you needed 32. 0 g? D = m 0. 786 g/m. L = 32. 0 g V V V= 32. 0 g 0. 786 g/ml V= 40. 7 m. L

B. ) SPECIFIC GRAVITY is the ratio of the density of liquid to the density of water at 4 o. C, which is 1. 00 g/m. L. Since specific gravity is a ratio of two densities, the units cancel. Specific gravity = density of sample (g/m. L) Density of water (g/m. L)

WORKED EXAMPLE 1 -7 What is the specific gravity of jet fuel if the density is 0. 775 g/m. L? Solution. Specific gravity= 0. 775 g/m. L = 0. 775 1. 00 g/m. L

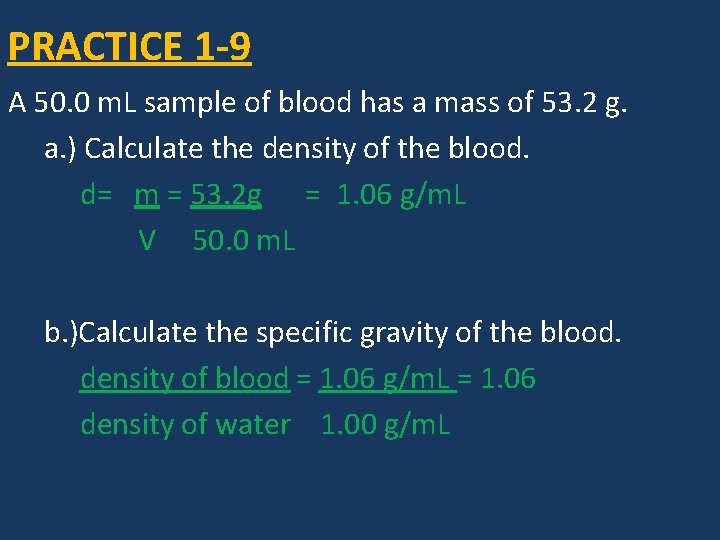
PRACTICE 1 -9 A 50. 0 m. L sample of blood has a mass of 53. 2 g. a. ) Calculate the density of the blood. d= m = 53. 2 g = 1. 06 g/m. L V 50. 0 m. L b. )Calculate the specific gravity of the blood. density of blood = 1. 06 g/m. L = 1. 06 density of water 1. 00 g/m. L

TEMPERATURE

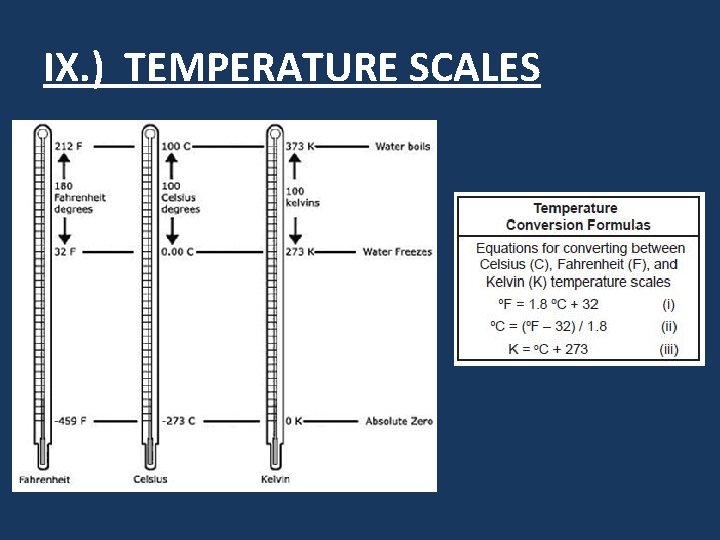
IX. ) TEMPERATURE SCALES

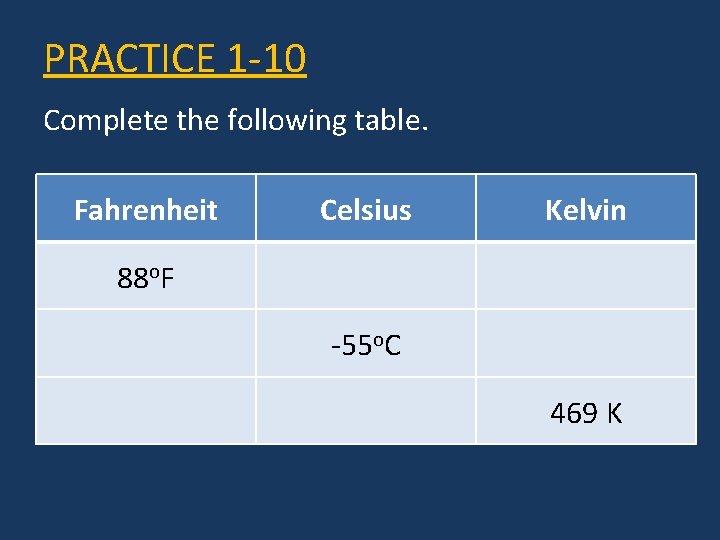
PRACTICE 1 -10 Complete the following table. Fahrenheit Celsius Kelvin 88 o. F -55 o. C 469 K

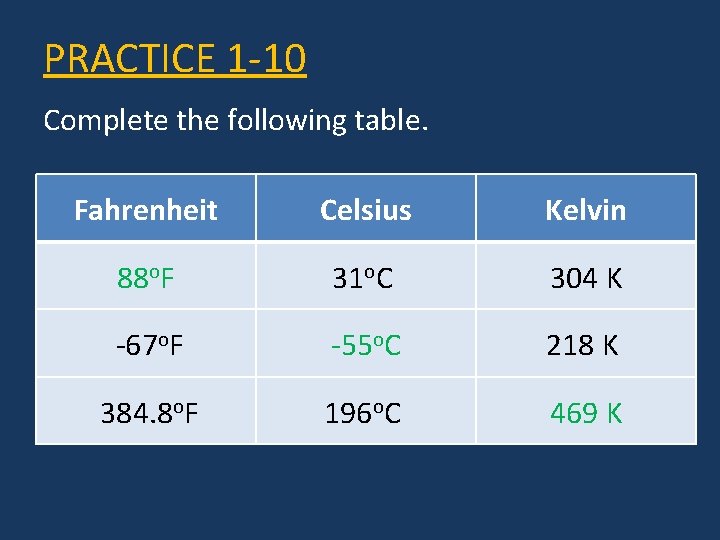
PRACTICE 1 -10 Complete the following table. Fahrenheit Celsius Kelvin 88 o. F 31 o. C 304 K -67 o. F -55 o. C 218 K 384. 8 o. F 196 o. C 469 K