Calculating gasgas volumes and concentrations Calculating volumes and




- Slides: 7

Calculating gasgas volumes and concentrations Calculating volumes and concentrations of solutions d. calculate the amount of substance in a solution of known concentration (excluding titration calculations at this stage) in mol dm-3 f. use chemical equations to calculate volumes of gases and vice versa using the concepts of amount of substance and molar volume of gases Connector: 1. When 10 g of zinc powder was added to a solution of copper(II) sulphate a displacement reaction took place and copper metal was formed along with zinc sulphate solution. a. Write the balanced symbol equation including states of matter for this reaction. b. What is the maximum mass of copper that could be formed? c. What is the atom efficiency with respect to copper?

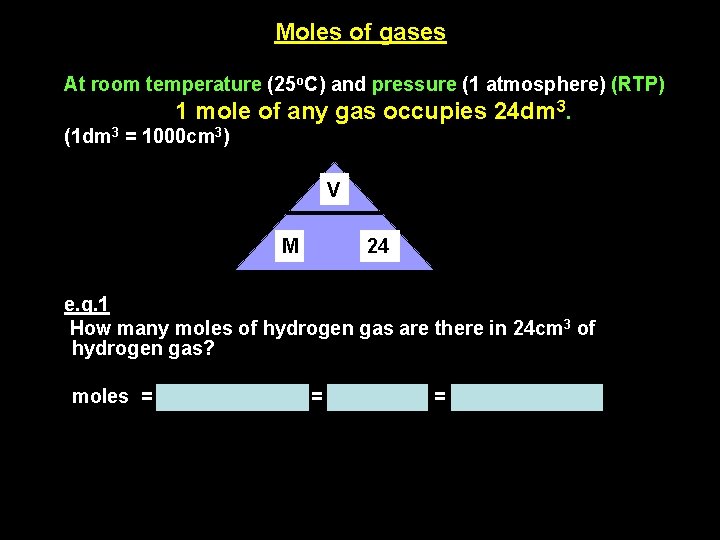
Moles of gases At room temperature (25 o. C) and pressure (1 atmosphere) (RTP) 1 mole of any gas occupies 24 dm 3. (1 dm 3 = 1000 cm 3) V M 24 e. g. 1 How many moles of hydrogen gas are there in 24 cm 3 of hydrogen gas? moles = volume/24, 000 = 24/24, 000 = 0. 001 mole

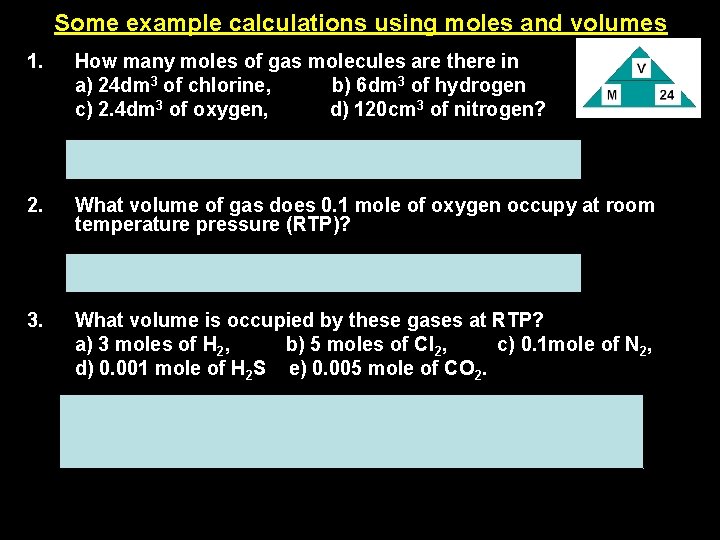
Some example calculations using moles and volumes 1. How many moles of gas molecules are there in a) 24 dm 3 of chlorine, b) 6 dm 3 of hydrogen c) 2. 4 dm 3 of oxygen, d) 120 cm 3 of nitrogen? a= 1 mole, b= 0. 25 mole, c= 0. 1 mole, d= 0. 005 mole 2. What volume of gas does 0. 1 mole of oxygen occupy at room temperature pressure (RTP)? V = moles x 24 = 0. 1 x 24 = 2. 4 dm 3 3. What volume is occupied by these gases at RTP? a) 3 moles of H 2, b) 5 moles of Cl 2, c) 0. 1 mole of N 2, d) 0. 001 mole of H 2 S e) 0. 005 mole of CO 2. a= 72 dm 3, d= 0. 024 dm 3 or 24 cm 3, b= 120 dm 3, c= 2. 4 dm 3, e= 0. 12 dm 3 or 120 cm 3

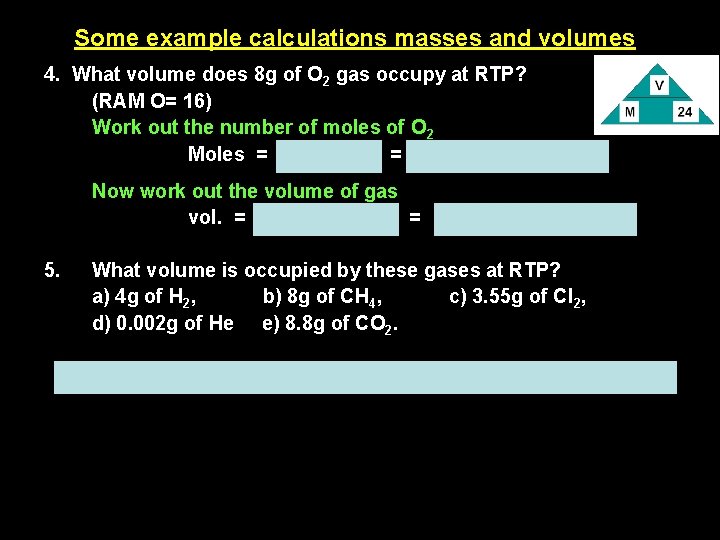
Some example calculations masses and volumes 4. What volume does 8 g of O 2 gas occupy at RTP? (RAM O= 16) Work out the number of moles of O 2 Moles = mass/RFM = 8/32 = 0. 25 mole Now work out the volume of gas vol. = moles x 24 dm 3 = 0. 25 x 24 = 6 dm 3 5. What volume is occupied by these gases at RTP? a) 4 g of H 2, b) 8 g of CH 4, c) 3. 55 g of Cl 2, d) 0. 002 g of He e) 8. 8 g of CO 2. a) 48 dm 3, b) 12 dm 3, c) 1. 2 dm 3, d) 0. 012 dm 3, e) 4. 8 dm 3

Calculating products and reactants Consider the reaction: Ca. CO 3 Ca. O + CO 2 The equation is balanced, this means that when 1 mole of calcium carbonate is heated it decomposes to form 1 mole of calcium oxide and 1 mole of carbon dioxide. If 10 g of calcium carbonate was used, what mass of what volume of carbon dioxide (measured at RTP) would be released? (RAMs Ca= 40, C=12, O=16) Ca. CO 3 = 100 = 1 mole So 10 g = 0. 1 mol and so 0. 1 mol of CO 2 made 1 mol of CO 2 occupies 24 dm 3 So 0. 1 mol occupies 2. 4 dm 3

Moles in solutions Concentration is measured in mol dm-3 1. 2. 3. How many moles of Na. Cl are there in 100 cm 3 of a 2 mol dm-3 solution? How many moles of Na. Cl are there in 22 cm 3 of a 2 mol dm-3 solution? What mass of sodium nitrate must be dissolved in 250 cm 3 of water to make a 1 mol dm-3 solution? (RAMs Na= 23, Cl= 35. 5, N= 14, O= 16)