1 3 Reacting Masses and Volumes Stoichiometry Limiting







- Slides: 16

1. 3 Reacting Masses and Volumes Stoichiometry, Limiting & Excess Reactants, Theoretical & Percent Yields Mrs. Page - IB SL Chemistry - 2015 -2016

Understandings • Reactants can be either limiting or excess. • The experimental yield can be different from theoretical yield. Applications & Skills • Solution of problems relating to reacting quantities, limiting and excess reactants, theoretical, experimental and percentage yields.

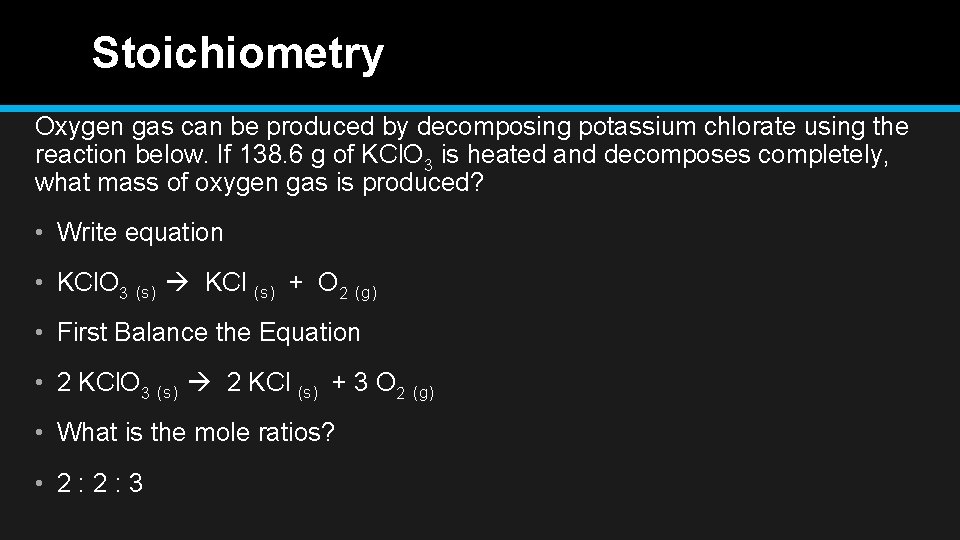
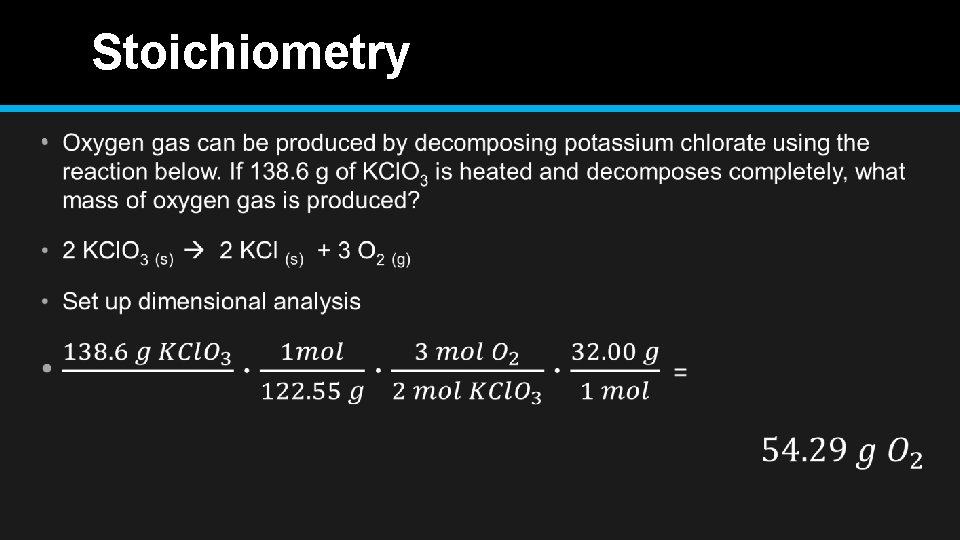
Stoichiometry Oxygen gas can be produced by decomposing potassium chlorate using the reaction below. If 138. 6 g of KCl. O 3 is heated and decomposes completely, what mass of oxygen gas is produced? • Write equation • KCl. O 3 (s) KCl (s) + O 2 (g) • First Balance the Equation • 2 KCl. O 3 (s) 2 KCl (s) + 3 O 2 (g) • What is the mole ratios? • 2: 2: 3

Stoichiometry •

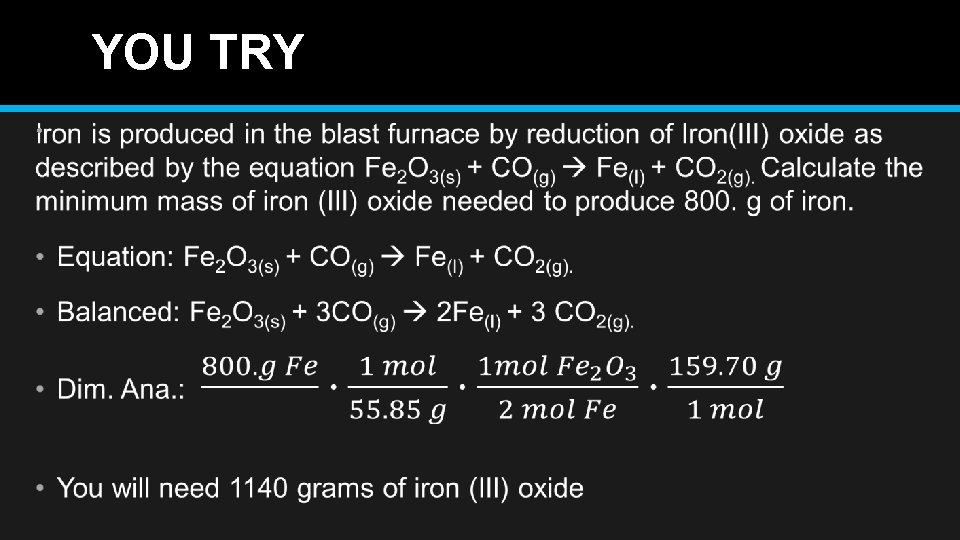
YOU TRY •

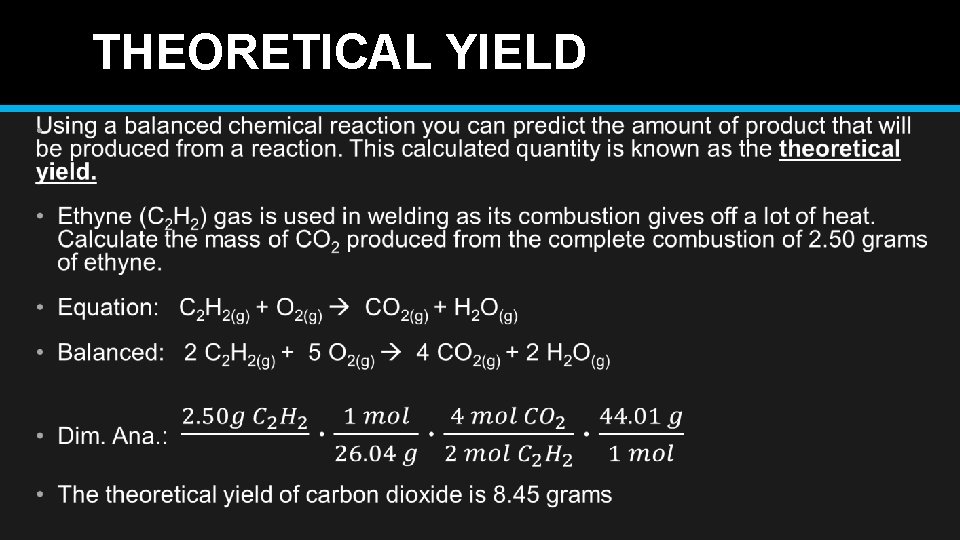
THEORETICAL YIELD •

Limiting Reactant • The limiting reactant in a chemical reaction is the reactant that determines theoretical yield of a product. This is the reactant that will run out first in an equation and therefore determine how much product can be produced. • A substance that is not used up completely in a reaction. It’s what is left over after you run out of the limiting reactant is said to be in excess.

Limiting & Excess Reactants S’mores Recipe 20 choc. chips 30 marshmallows 40 cookies You can make 10 s’mores based on the chocolate chips You can make 15 s’mores based on the marshmallows. You can make 40 s’mores based on the cookies. How many s’ mores can you make? What do you run out first? 10 YIELD Chocolate chips LIMITING REACTANT How much of the other substance are left over? If you make 10 s’mores, you use 20 marshmallows and have 10 left. You use 10 cookies and have 30 left. EXCESS REACTANT

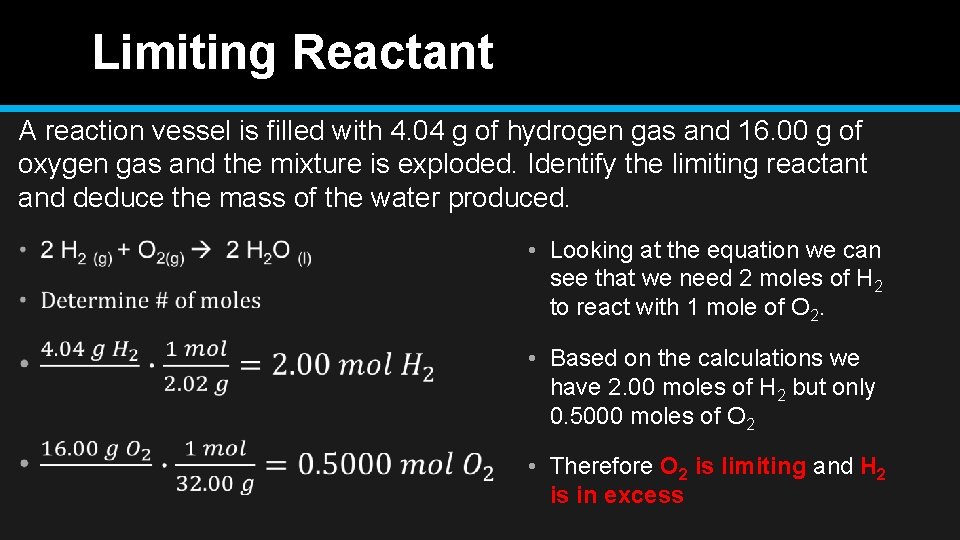
Limiting Reactant A reaction vessel is filled with 4. 04 g of hydrogen gas and 16. 00 g of oxygen gas and the mixture is exploded. Identify the limiting reactant and deduce the mass of the water produced. • Looking at the equation we can see that we need 2 moles of H 2 to react with 1 mole of O 2. • Based on the calculations we have 2. 00 moles of H 2 but only 0. 5000 moles of O 2 • Therefore O 2 is limiting and H 2 is in excess

Limiting Reactant A reaction vessel is filled with 4. 04 g of hydrogen gas and 16. 00 g of oxygen gas and the mixture is exploded. Identify the limiting reactant and deduce the mass of the water produced. 2 H 2 (g) + O 2(g) 2 H 2 O (l)

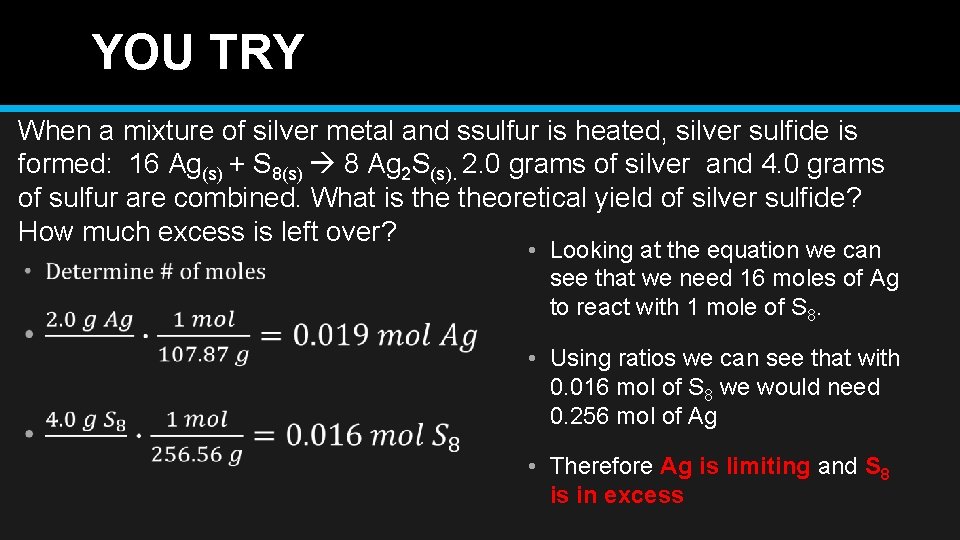
YOU TRY When a mixture of silver metal and ssulfur is heated, silver sulfide is formed: 16 Ag(s) + S 8(s) 8 Ag 2 S(s). 2. 0 grams of silver and 4. 0 grams of sulfur are combined. What is theoretical yield of silver sulfide? How much excess is left over? • Looking at the equation we can see that we need 16 moles of Ag to react with 1 mole of S 8. • Using ratios we can see that with 0. 016 mol of S 8 we would need 0. 256 mol of Ag • Therefore Ag is limiting and S 8 is in excess

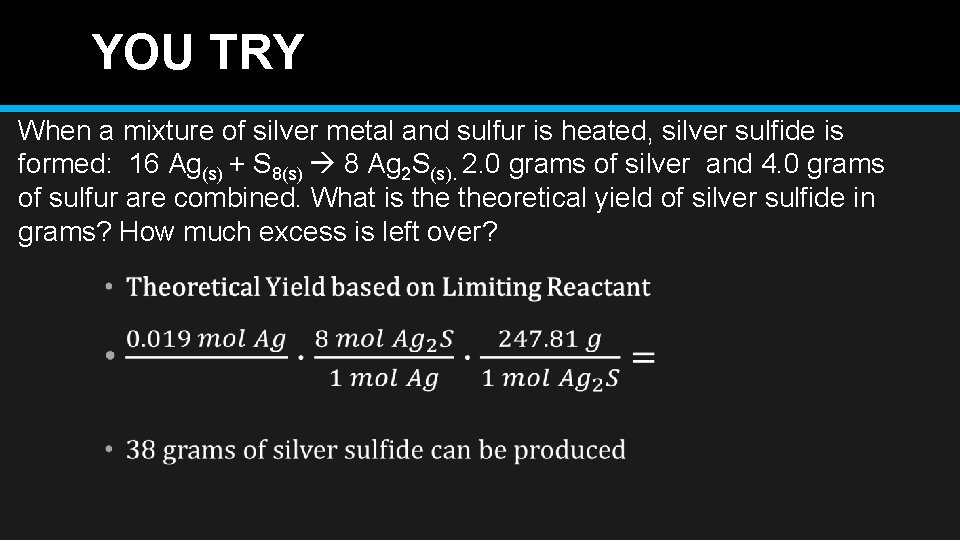
YOU TRY When a mixture of silver metal and sulfur is heated, silver sulfide is formed: 16 Ag(s) + S 8(s) 8 Ag 2 S(s). 2. 0 grams of silver and 4. 0 grams of sulfur are combined. What is theoretical yield of silver sulfide in grams? How much excess is left over?

YOU TRY When a mixture of silver metal and sulfur is heated, silver sulfide is formed: 16 Ag(s) + S 8(s) 8 Ag 2 S(s). 2. 0 grams of silver and 4. 0 grams of sulfur are combined. What is theoretical yield of silver sulfide in grams? How much excess is left over?

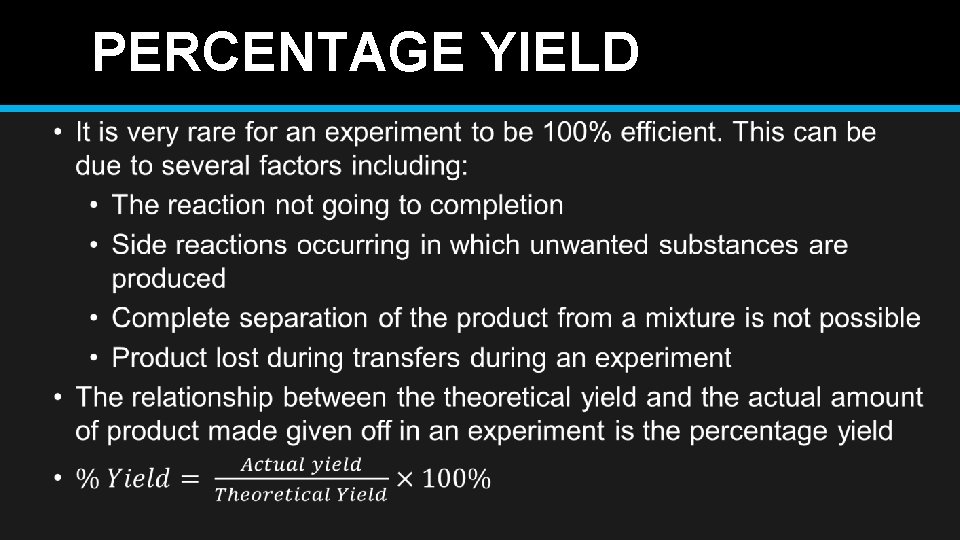
PERCENTAGE YIELD

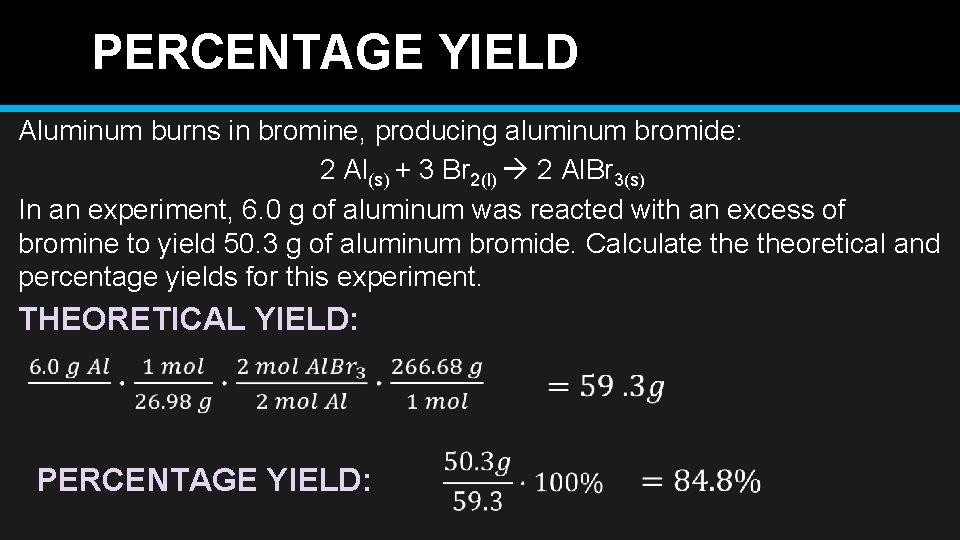
PERCENTAGE YIELD Aluminum burns in bromine, producing aluminum bromide: 2 Al(s) + 3 Br 2(l) 2 Al. Br 3(s) In an experiment, 6. 0 g of aluminum was reacted with an excess of bromine to yield 50. 3 g of aluminum bromide. Calculate theoretical and percentage yields for this experiment. THEORETICAL YIELD: PERCENTAGE YIELD:

YOUR TURN Aspirin, C 9 H 8 O 4, is made by reacting ethanoic anhydride C 4 H 6 O 3, with 2 -hydroxybenzoic acid, C 7 H 6 O 3, according to the equation: 2 C 7 H 6 O 3 + C 4 H 6 O 3 2 C 9 H 8 O 4 + H 2 O 13. 80 g of 2 -hydroxybenzoic acid is reacted with 10. 26 g of ethanoic anhydride. a) Determine the limiting reagent. b) The mass obtained in the experiment was 10. 90 g. Calculate theoretical and percentage yield of aspirin.
 Reacting masses and volumes
Reacting masses and volumes Reacting masses questions
Reacting masses questions Reactant
Reactant What is limiting reactant
What is limiting reactant Colour of alkali metals
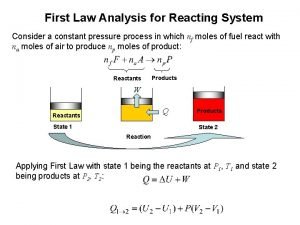
Colour of alkali metals Reacting system
Reacting system Magnesium reacting with nitric acid equation
Magnesium reacting with nitric acid equation Sodium bicarbonate reacting with hydrochloric acid
Sodium bicarbonate reacting with hydrochloric acid Magnesium reacting with oxygen
Magnesium reacting with oxygen Alkali metals reacting with water
Alkali metals reacting with water React to direct fire while mounted
React to direct fire while mounted React to direct fire while dismounted
React to direct fire while dismounted Preparation of co2 gas
Preparation of co2 gas Alkali metals reacting with water
Alkali metals reacting with water Alkali metals reacting with water
Alkali metals reacting with water Practice 10-6 volumes of pyramids and cones answers
Practice 10-6 volumes of pyramids and cones answers Fictional character
Fictional character