Pharmaceutical Calculations Percentage Ratio Strength and Other Expressions




















- Slides: 30

Pharmaceutical Calculations: Percentage, Ratio Strength, and Other Expressions of Concentration Danielle Del. Villano, Pharm. D.

Objectives • Define the expressions percent weight-involume, percent volume-in-volume, percent weight-in-weight, ratio strength • Convert percent strength to ratio strength and vice versa • Apply percent strength and ratio strength to pharmaceutical calculations

Percentage • Definition: rate per hundred • Expressed as: – 50/100 – 50% – 0. 50 • Example: express 12. 5% as an equivalent decimal fraction – 12. 5/100, or 0. 125

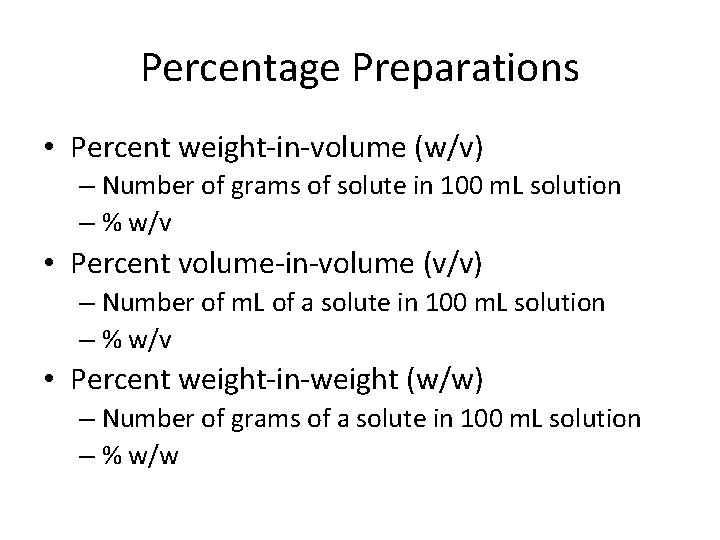
Percentage Preparations • Percent weight-in-volume (w/v) – Number of grams of solute in 100 m. L solution – % w/v • Percent volume-in-volume (v/v) – Number of m. L of a solute in 100 m. L solution – % w/v • Percent weight-in-weight (w/w) – Number of grams of a solute in 100 m. L solution – % w/w

Important notes • When to use which term – w/v – solutions, suspensions of solids in liquids – v/v – liquids in liquids – w/w – mixtures of solids (creams) • *Overall idea of this section* – Solution = Solute + Solvent

Percentage Weight-in-Volume • Number of grams of solute in 100 m. L solution • For a 3% solution… – 3 g of solute in 100 m. L solution

Problem 1 (w/v) • How many grams of dextrose are required to prepare 4000 m. L of a 5% solution? (5% solution = 5 g of dextrose in 100 m. L) 5 g = xg 100 m. L 4000 m. L x = 200 g

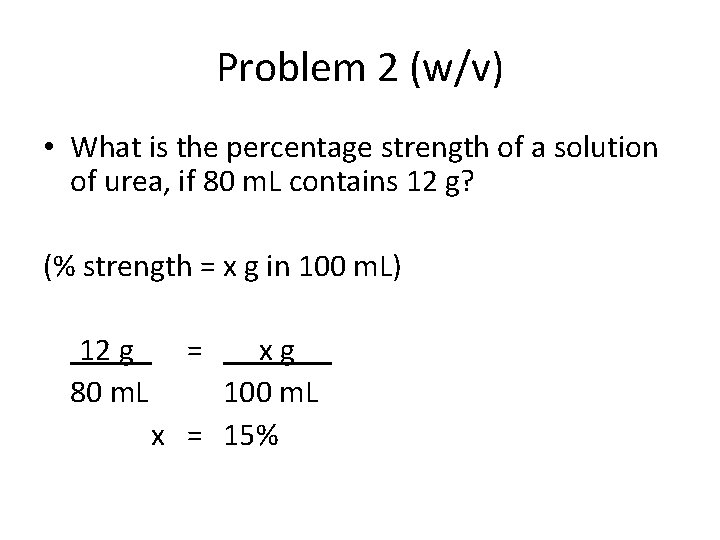
Problem 2 (w/v) • What is the percentage strength of a solution of urea, if 80 m. L contains 12 g? (% strength = x g in 100 m. L) 12 g 80 m. L = xg 100 m. L x = 15%

Percentage Volume-in-Volume • Number of m. L of solute in 100 m. L solution • For a 5% solution… – 5 m. L of solute in 100 m. L solution

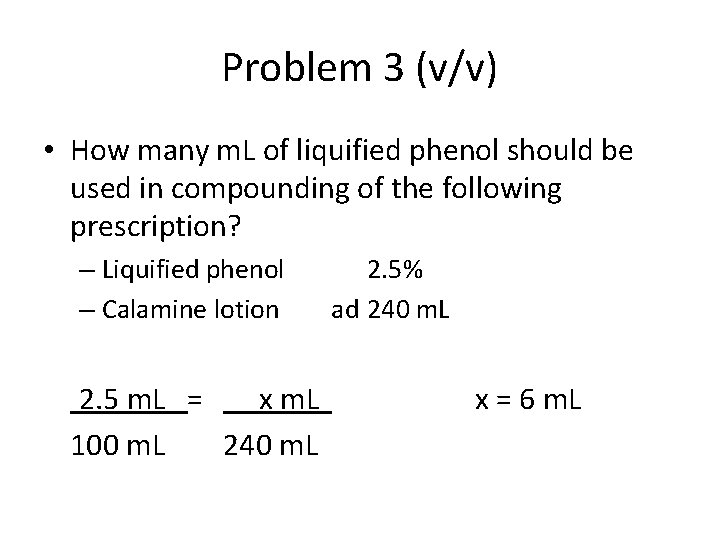
Problem 3 (v/v) • How many m. L of liquified phenol should be used in compounding of the following prescription? – Liquified phenol – Calamine lotion 2. 5 m. L = x m. L 100 m. L 240 m. L 2. 5% ad 240 m. L x = 6 m. L

Problem 4 (v/v) • What is the percentage strength (v/v) of a solution of 800 g of a liquid with a specific gravity of 0. 800 in enough water to make 4000 m. L? 800 g water = 800 m. L water 1000 m. L = x m. L 4000 m. L 1000 m. L 800 m. L / 0. 800 = 1000 m. L solute x = 25 %

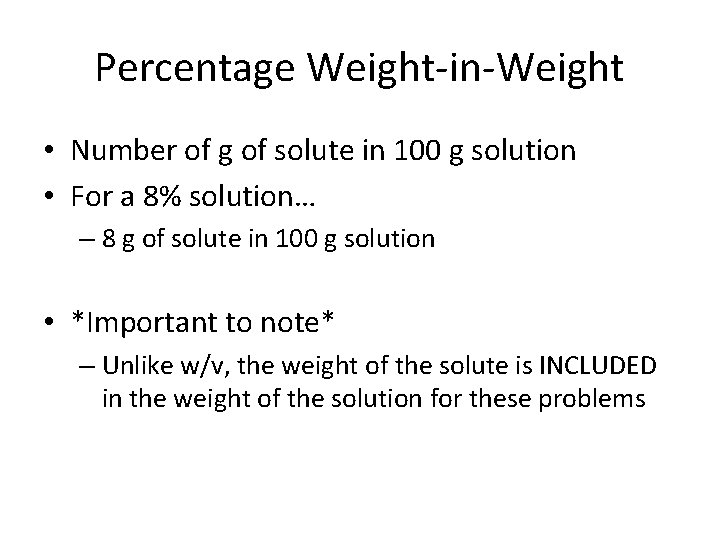
Percentage Weight-in-Weight • Number of g of solute in 100 g solution • For a 8% solution… – 8 g of solute in 100 g solution • *Important to note* – Unlike w/v, the weight of the solute is INCLUDED in the weight of the solution for these problems

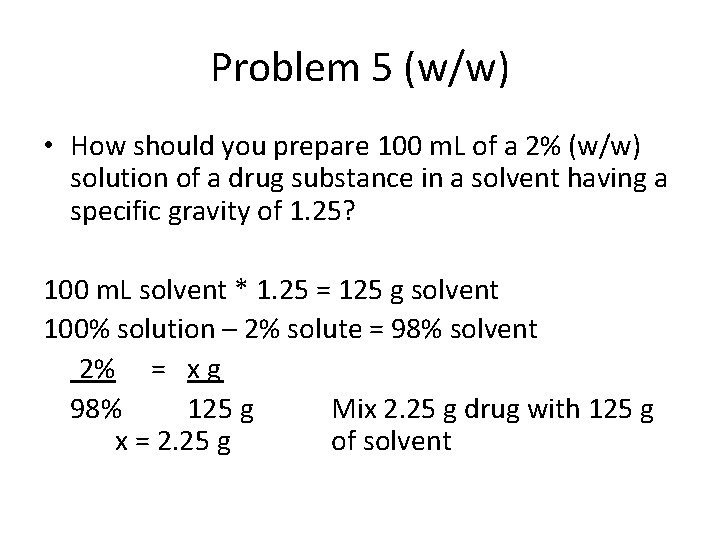
Problem 5 (w/w) • How should you prepare 100 m. L of a 2% (w/w) solution of a drug substance in a solvent having a specific gravity of 1. 25? 100 m. L solvent * 1. 25 = 125 g solvent 100% solution – 2% solute = 98% solvent 2% = x g 98% 125 g Mix 2. 25 g drug with 125 g x = 2. 25 g of solvent

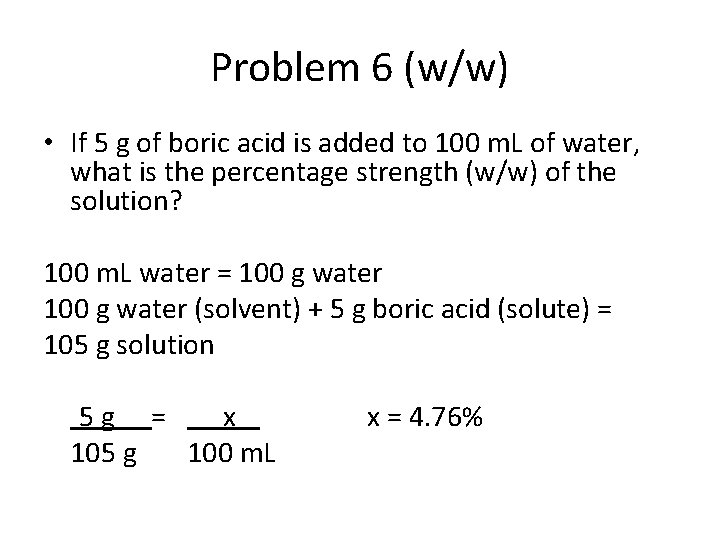
Problem 6 (w/w) • If 5 g of boric acid is added to 100 m. L of water, what is the percentage strength (w/w) of the solution? 100 m. L water = 100 g water (solvent) + 5 g boric acid (solute) = 105 g solution 5 g = x 105 g 100 m. L x = 4. 76%

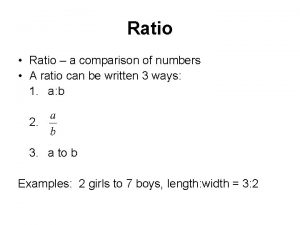
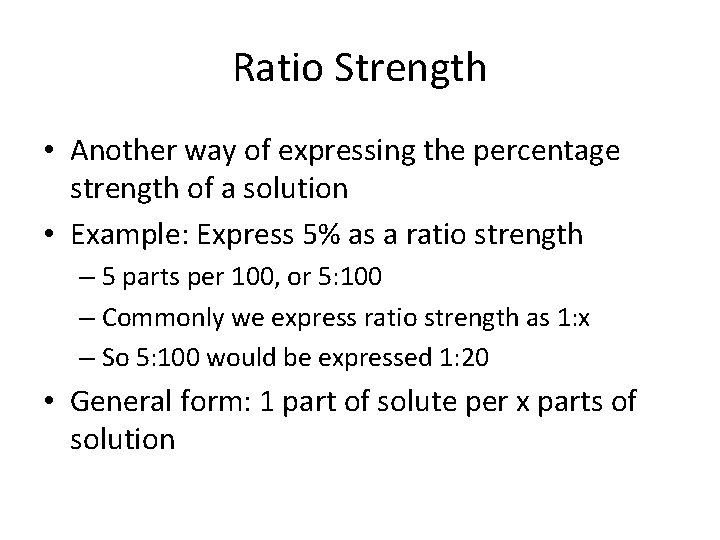
Ratio Strength • Another way of expressing the percentage strength of a solution • Example: Express 5% as a ratio strength – 5 parts per 100, or 5: 100 – Commonly we express ratio strength as 1: x – So 5: 100 would be expressed 1: 20 • General form: 1 part of solute per x parts of solution

Converting between the Strength Expressions • Express 0. 02% as a ratio strength 0. 02 = 1 part 100 x parts x = 5000 Answer: 1: 5000 • Express 1: 4000 as a percentage strength 1 part = x 4000 parts 100 x = 0. 025%

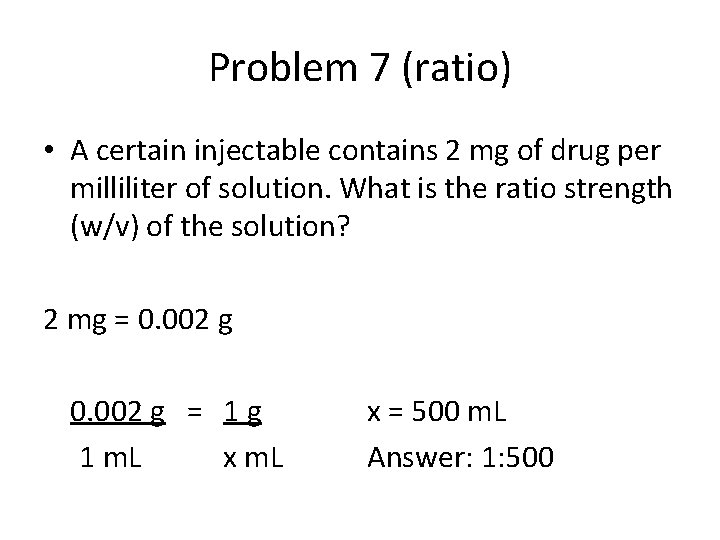
Problem 7 (ratio) • A certain injectable contains 2 mg of drug per milliliter of solution. What is the ratio strength (w/v) of the solution? 2 mg = 0. 002 g = 1 g 1 m. L x = 500 m. L Answer: 1: 500

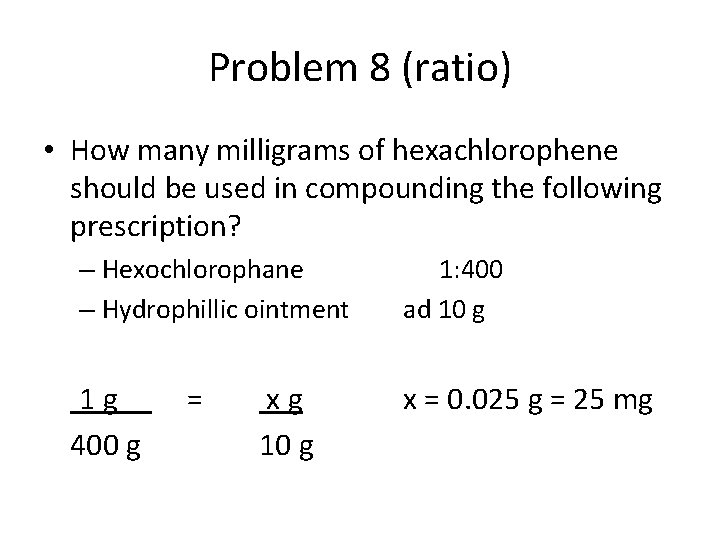
Problem 8 (ratio) • How many milligrams of hexachlorophene should be used in compounding the following prescription? – Hexochlorophane – Hydrophillic ointment 1 g 400 g = xg 10 g 1: 400 ad 10 g x = 0. 025 g = 25 mg

Parts Per Million (PPM) and Parts Per Billion (PPB) • Strengths of very dilute solutions • Example: 1 -4 PPM flouride in drinking water – 1 PPM – 4 PPM – 1: 1000000 – 4: 1000000

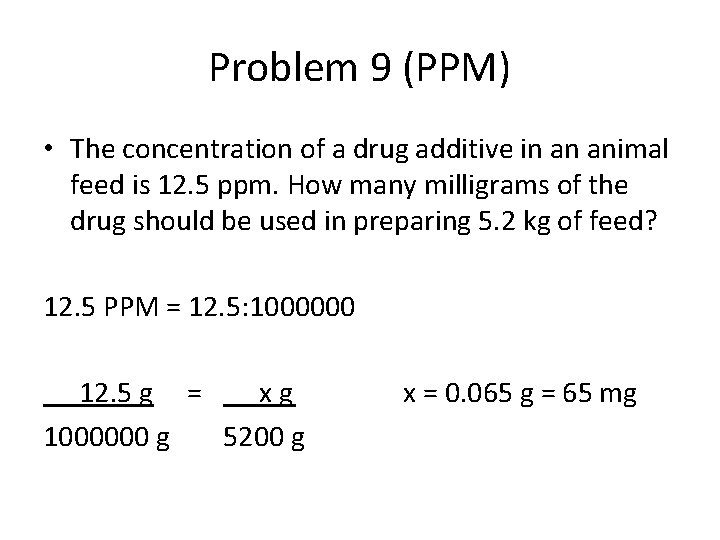
Problem 9 (PPM) • The concentration of a drug additive in an animal feed is 12. 5 ppm. How many milligrams of the drug should be used in preparing 5. 2 kg of feed? 12. 5 PPM = 12. 5: 1000000 12. 5 g = xg 1000000 g 5200 g x = 0. 065 g = 65 mg

Questions

Reference • Ansel, H. C. (2009) Phamaceutical Calculations (13 th Ed. ). Philadelphia: Lippincott Williams & Wilkins, and Wolters Kluwer Publishers

Additional Problems

Chapter 6 Problem 7 • If a pharmacist dissolved the contents of eight capsules, each containing 300 mg of clindamycin phosphate, into a sufficient amount of an astringent liquid base to prepare 60 m. L of topical solution, what would be the percentage strength (w/v) of clindamycin phosphate in the prescription?

Chapter 6 Problem 29 • A topical solution contains 3% w/v hydroquinone. How many liters of the solution can be prepared from 30 g of hydroquinone?

Chapter 6 Problem 30 • What is the percentage strength (v/v) if 225 g of a liquid having a specific gravity of 0. 8 is added to enough water to make 1. 5 liters of the solution?

Chapter 6 Problem 38 • If 500 g of dextrose are dissolved in 600 m. L of water with a resultant final volume of 1 liter, what is the percentage strength of dextrose in the solution on a w/w basis?

Chapter 6 Problem 57 • A sample of white petrolatum contains 10 mg of tocopherol per kilogram as a preservative. Express the amount of tocopherol as a ratio strength.

Chapter 6 Problem 65 • In acute hypersensitivity reactions, 0. 5 m. L of a 1: 1000 (w/v) solution of epinephrine may be administered subcutaneously or intramuscularly. Calculate the milligrams of epinephrine given.

Chapter 6 Problem 70 • If a commercially available insulin preparation contains 1 ppm of proinsulin, how many micrograms of proinsulin would be contained in a 10 -m. L vial of insulin?
 Percentage by strength
Percentage by strength Pharmaceutical calculations percentage strength
Pharmaceutical calculations percentage strength What is the percentage strength of 1:1000
What is the percentage strength of 1:1000 Pharmaceutical measurements and calculations
Pharmaceutical measurements and calculations Household measurements in pharmacy
Household measurements in pharmacy Pharmaceutical compounding calculations
Pharmaceutical compounding calculations Types of connections in steel structures
Types of connections in steel structures Percentage calculations worksheet
Percentage calculations worksheet Tensile strength and yield strength
Tensile strength and yield strength Half strength darrow's composition
Half strength darrow's composition Difference of ratio and proportion
Difference of ratio and proportion Ratio to percentage
Ratio to percentage Ratio strength formula
Ratio strength formula High strength to weight ratio
High strength to weight ratio Glider horizontal stabilizer
Glider horizontal stabilizer Best bridge design for weight
Best bridge design for weight Acid test ratio and quick ratio
Acid test ratio and quick ratio Phenotypic and genotypic ratio of dihybrid cross
Phenotypic and genotypic ratio of dihybrid cross Current ratio and quick ratio
Current ratio and quick ratio Types of position
Types of position Hazard ratio vs odds ratio
Hazard ratio vs odds ratio Gear ratio vs velocity ratio
Gear ratio vs velocity ratio Primary reinforcer
Primary reinforcer Contoh relative risk
Contoh relative risk Give the definition of reducing and enlarging
Give the definition of reducing and enlarging Pharmaceutical calculation reducing and enlarging formulas
Pharmaceutical calculation reducing and enlarging formulas Pharmaceutical simulation and modeling
Pharmaceutical simulation and modeling Ibn sina university of medical and pharmaceutical sciences
Ibn sina university of medical and pharmaceutical sciences Paten brno
Paten brno University of veterinary and pharmaceutical sciences brno
University of veterinary and pharmaceutical sciences brno Pharmacopedics
Pharmacopedics