Chapter 10 Dilution and Concentration Pharmaceutical Calculations for



















- Slides: 39

Chapter 10 Dilution and Concentration Pharmaceutical Calculations for the Pharmacy Technician Copyright © 2008 Wolters Kluwer Health | Lippincott Williams & Wilkins

Objectives • Upon completion of this chapter, you will be able to: – Describe the relationship of active ingredients and diluents if the amount of active ingredient remains constant and the amount of diluent is increased or decreased – Determine the percent strength and ratio strength of a given product when the active ingredient remains constant and the amount of diluent is increased or decreased Pharmaceutical Calculations for the Pharmacy Technician Copyright © 2008 Wolters Kluwer Health | Lippincott Williams & Wilkins

Objectives (cont. ) – Determine the volume of solution of a desired strength given a specified quantity of any given strength – Determine the volume of a specified stock solution needed to prepare a given solution – Determine the quantity of an active ingredient in a specified amount of solution needed to prepare a given solution – Define the alligation methods of problem solving Pharmaceutical Calculations for the Pharmacy Technician Copyright © 2008 Wolters Kluwer Health | Lippincott Williams & Wilkins

Objectives (cont. ) – Utilize the alligation methods (alligation alternate and alligation medial) to determine the percent strength of alcohol mixtures – Utilize the alligation methods (alligation alternate and alligation medial) to determine relative amounts of components mixed together to make a mixture of a required strength Pharmaceutical Calculations for the Pharmacy Technician Copyright © 2008 Wolters Kluwer Health | Lippincott Williams & Wilkins

Definitions • Diluent: – A substance that is added to a pharmaceutical product to reduce the strength of the product. A diluent most often has no drug substance in it, sterile water and petrolatum for example. • Stock solutions: – Strong solutions from which weaker ones are made Pharmaceutical Calculations for the Pharmacy Technician Copyright © 2008 Wolters Kluwer Health | Lippincott Williams & Wilkins

Rules These two rules, wherever they may be applied, greatly simplify the calculation: • 1. When ratio strengths are given, convert them to them percentage strengths before setting to percentage beforeup a setting up a proportion • 2. Whenever proportional parts enter into a calculation, reduce them to to lowest terms calculation, reduce them lowest terms Pharmaceutical Calculations for the Pharmacy Technician Copyright © 2008 Wolters Kluwer Health | Lippincott Williams & Wilkins

Relationship Between Strength and Quantity • The amount of active ingredient remains constant; any change in the quantity of a solution or mixture of solids is inversely proportional to the percentage or ratio strength. (Or, as the volume increases the strength decreases. ) Pharmaceutical Calculations for the Pharmacy Technician Copyright © 2008 Wolters Kluwer Health | Lippincott Williams & Wilkins

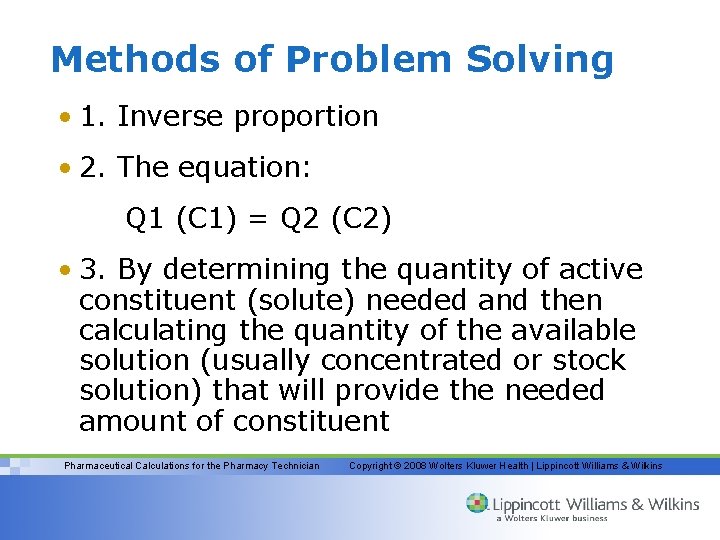
Methods of Problem Solving • 1. Inverse proportion • 2. The equation: Q 1 (C 1) = Q 2 (C 2) • 3. By determining the quantity of active constituent (solute) needed and then calculating the quantity of the available solution (usually concentrated or stock solution) that will provide the needed amount of constituent Pharmaceutical Calculations for the Pharmacy Technician Copyright © 2008 Wolters Kluwer Health | Lippincott Williams & Wilkins

Which one to use? • For most problems, the equation (#2) is the easiest to use • In some situations, when using vials or amps as your stock product, #3 would be the best method Pharmaceutical Calculations for the Pharmacy Technician Copyright © 2008 Wolters Kluwer Health | Lippincott Williams & Wilkins

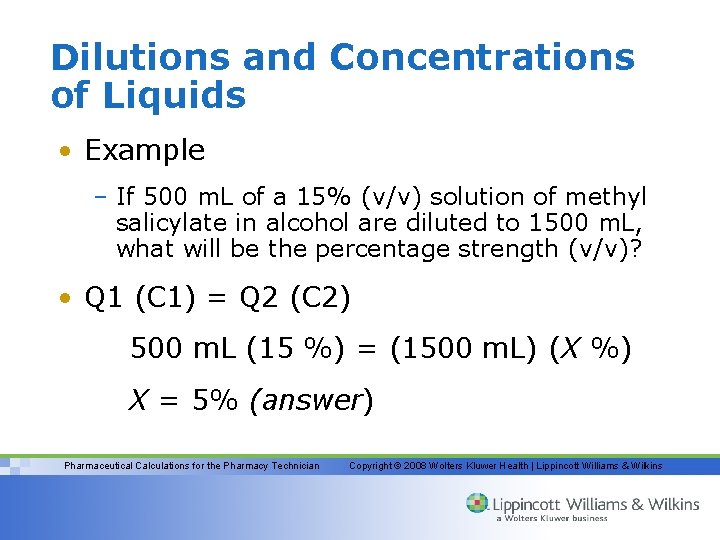
Dilutions and Concentrations of Liquids • Example – If 500 m. L of a 15% (v/v) solution of methyl salicylate in alcohol are diluted to 1500 m. L, what will be the percentage strength (v/v)? • Q 1 (C 1) = Q 2 (C 2) 500 m. L (15 %) = (1500 m. L) (X %) X = 5% (answer) Pharmaceutical Calculations for the Pharmacy Technician Copyright © 2008 Wolters Kluwer Health | Lippincott Williams & Wilkins

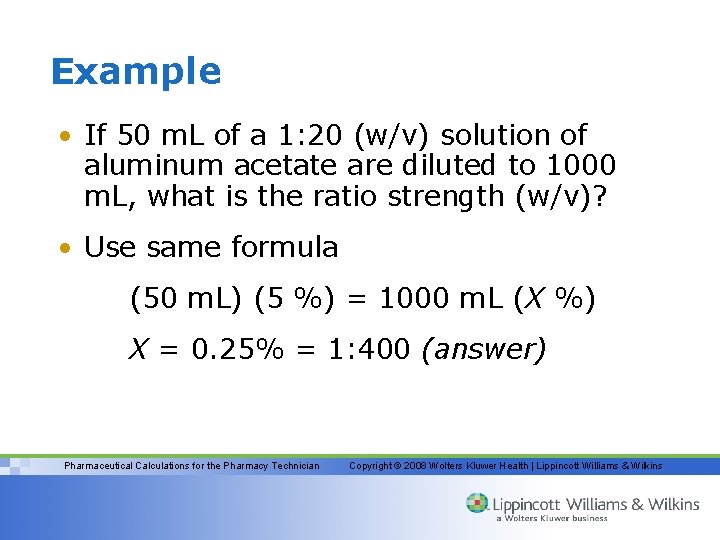
Example • If 50 m. L of a 1: 20 (w/v) solution of aluminum acetate are diluted to 1000 m. L, what is the ratio strength (w/v)? • Use same formula (50 m. L) (5 %) = 1000 m. L (X %) X = 0. 25% = 1: 400 (answer) Pharmaceutical Calculations for the Pharmacy Technician Copyright © 2008 Wolters Kluwer Health | Lippincott Williams & Wilkins

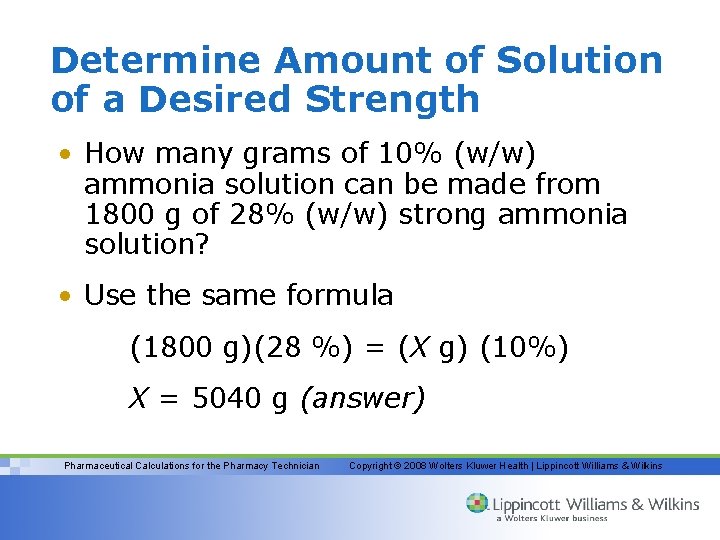
Determine Amount of Solution of a Desired Strength • How many grams of 10% (w/w) ammonia solution can be made from 1800 g of 28% (w/w) strong ammonia solution? • Use the same formula (1800 g)(28 %) = (X g) (10%) X = 5040 g (answer) Pharmaceutical Calculations for the Pharmacy Technician Copyright © 2008 Wolters Kluwer Health | Lippincott Williams & Wilkins

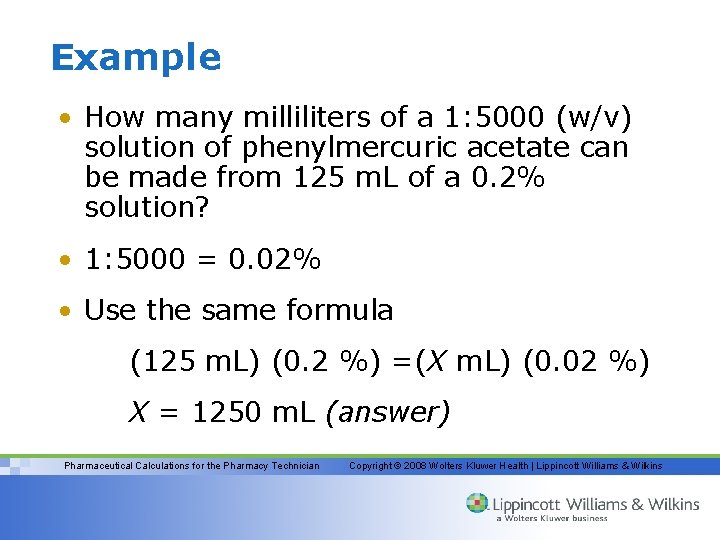
Example • How many milliliters of a 1: 5000 (w/v) solution of phenylmercuric acetate can be made from 125 m. L of a 0. 2% solution? • 1: 5000 = 0. 02% • Use the same formula (125 m. L) (0. 2 %) =(X m. L) (0. 02 %) X = 1250 m. L (answer) Pharmaceutical Calculations for the Pharmacy Technician Copyright © 2008 Wolters Kluwer Health | Lippincott Williams & Wilkins

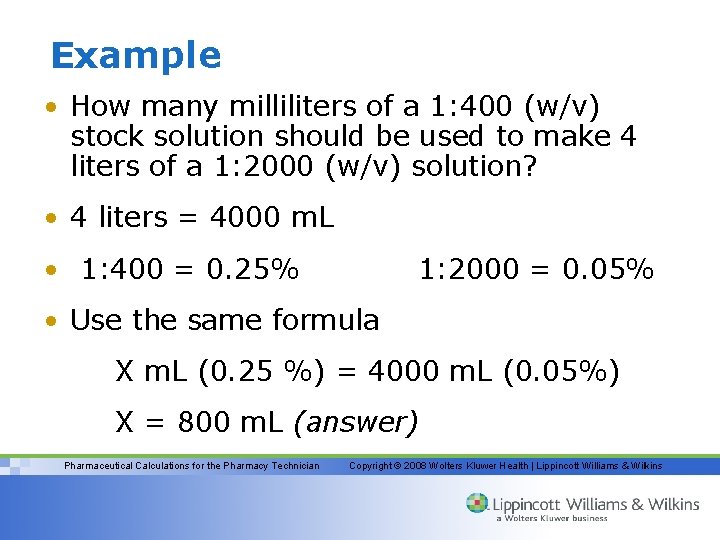
Example • How many milliliters of a 1: 400 (w/v) stock solution should be used to make 4 liters of a 1: 2000 (w/v) solution? • 4 liters = 4000 m. L • 1: 400 = 0. 25% 1: 2000 = 0. 05% • Use the same formula X m. L (0. 25 %) = 4000 m. L (0. 05%) X = 800 m. L (answer) Pharmaceutical Calculations for the Pharmacy Technician Copyright © 2008 Wolters Kluwer Health | Lippincott Williams & Wilkins

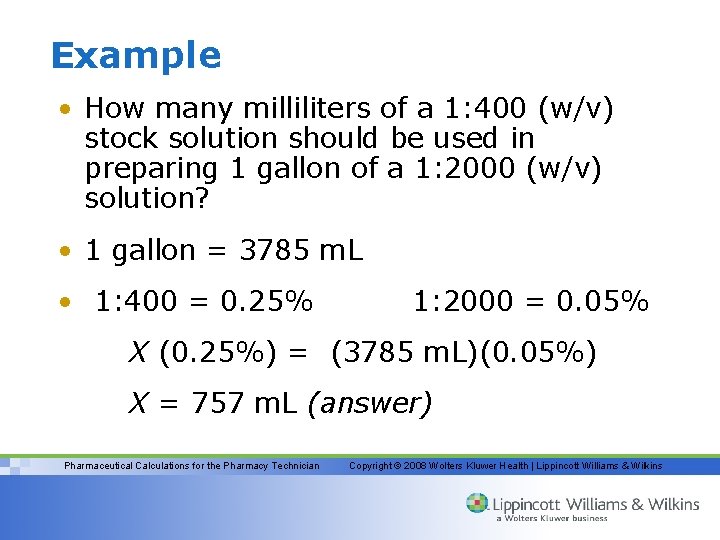
Example • How many milliliters of a 1: 400 (w/v) stock solution should be used in preparing 1 gallon of a 1: 2000 (w/v) solution? • 1 gallon = 3785 m. L • 1: 400 = 0. 25% 1: 2000 = 0. 05% X (0. 25%) = (3785 m. L)(0. 05%) X = 757 m. L (answer) Pharmaceutical Calculations for the Pharmacy Technician Copyright © 2008 Wolters Kluwer Health | Lippincott Williams & Wilkins

Using a Vial or Amp Stock • We are to prepare 30 m. L of a 5 mg/m. L oral phenobarbital solution using 1 m. L vials with a concentration of 65 mg/m. L. How much stock solution will be required? Pharmaceutical Calculations for the Pharmacy Technician Copyright © 2008 Wolters Kluwer Health | Lippincott Williams & Wilkins

Step 1: • Calculate the amount of phenobarbital needed – 30 ml x 5 mg/m. L tells us we need 150 mg of phenobarbital for the solution Pharmaceutical Calculations for the Pharmacy Technician Copyright © 2008 Wolters Kluwer Health | Lippincott Williams & Wilkins

Step 2: • Calculate the volume of available stock needed. Our supply is 65 mg/m. L, so 65 mg 1 m. L = 150 mg X X = 2. 3 m. L of phenobarbital will have to be drawn up Pharmaceutical Calculations for the Pharmacy Technician Copyright © 2008 Wolters Kluwer Health | Lippincott Williams & Wilkins

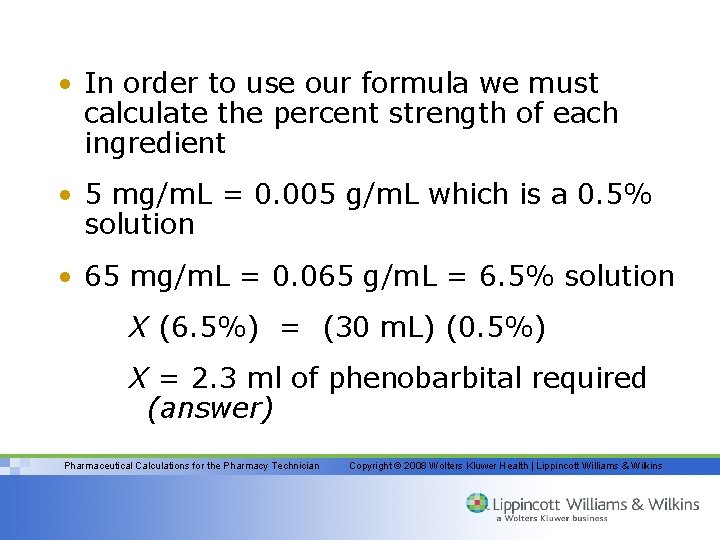
In touse formula we • Inorder to ourour formula we must calculate thestrength percent calculate the percent of each ingredient strength 5 • 5 mg/m. L = 0. 005 g/m. L which is a 0. 5% solution 65 mg/m. L = 0. 065 g/m. L = • 65 mg/m. L = 0. 065 g/m. L = 6. 5% solution X (6. 5%) (30 m. L) (0. 5%) X (6. 5%) = =(30 (0. 5%) X = 2. 3 ml of phenobarbital required (answer) Pharmaceutical Calculations for the Pharmacy Technician Copyright © 2008 Wolters Kluwer Health | Lippincott Williams & Wilkins

Determining Quantity of Active Ingredient in Specified Amount of Solution Given Strength of Diluted Portion Pharmaceutical Calculations for the Pharmacy Technician Copyright © 2008 Wolters Kluwer Health | Lippincott Williams & Wilkins

Example • How much silver nitrate should be used in preparing 50 m. L of a solution such that 5 m. L diluted to 500 m. L will yield a 1: 1000 solution? 1000 m. L 500 m. L = 1 g Xg – X = 0. 5 g of silver nitrate in 500 m. L of diluted solution (1: 1000), which is also the amount in 5 m. L of the stronger (stock) solution, since the 50 m. L and the 5 m. L are the same strength Pharmaceutical Calculations for the Pharmacy Technician Copyright © 2008 Wolters Kluwer Health | Lippincott Williams & Wilkins

Example (cont. ) 5 m. L = 50 m. L 0. 5 g Xg X = 5 g (answer) Pharmaceutical Calculations for the Pharmacy Technician Copyright © 2008 Wolters Kluwer Health | Lippincott Williams & Wilkins

Amount of Diluent Needed for Preparing Solution of Specified Lower Strength Pharmaceutical Calculations for the Pharmacy Technician Copyright © 2008 Wolters Kluwer Health | Lippincott Williams & Wilkins

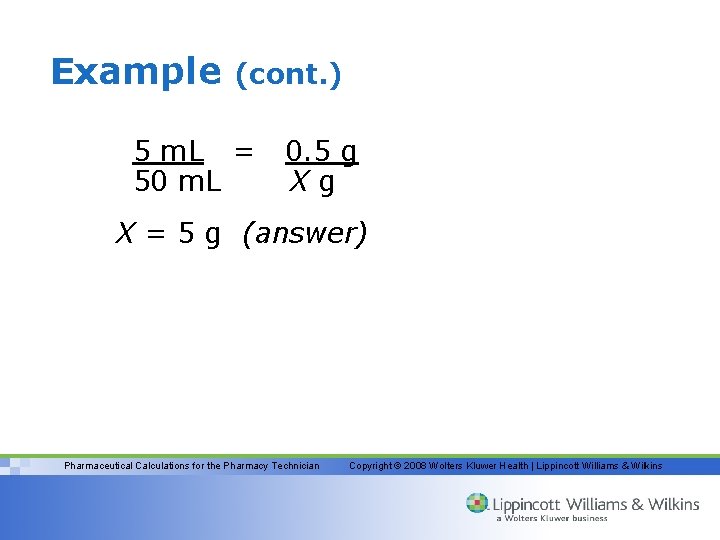
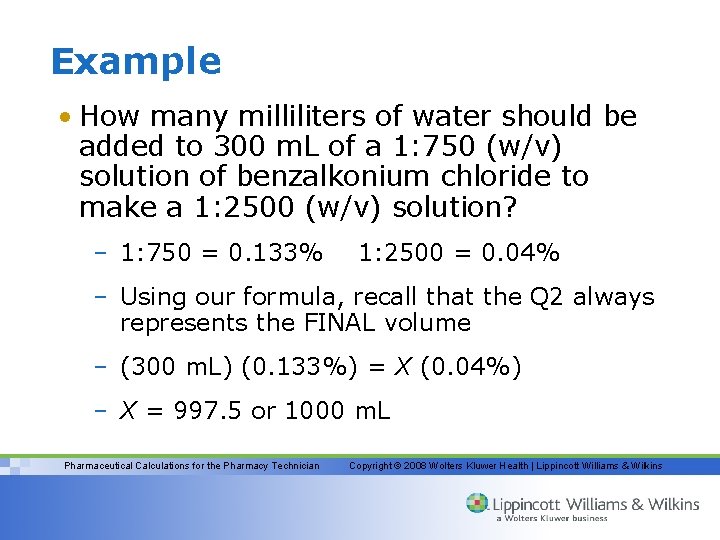
Example • How many milliliters of water should be added to 300 m. L of a 1: 750 (w/v) solution of benzalkonium chloride to make a 1: 2500 (w/v) solution? – 1: 750 = 0. 133% 1: 2500 = 0. 04% – Using our formula, recall that the Q 2 always represents the FINAL volume – (300 m. L) (0. 133%) = X (0. 04%) – X = 997. 5 or 1000 m. L Pharmaceutical Calculations for the Pharmacy Technician Copyright © 2008 Wolters Kluwer Health | Lippincott Williams & Wilkins

Example (cont. ) – This is the volume of our 0. 04% solution; therefore, we must subtract the volume of the 0. 133% solution (the amount we started with) from this final volume to determine how much water or diluent was added. 1000 m. L – 300 m. L = 700 m. L (answer) Pharmaceutical Calculations for the Pharmacy Technician Copyright © 2008 Wolters Kluwer Health | Lippincott Williams & Wilkins

Alligation • Arithmetical method of solving problems that involves mixing of solutions or solids that have different percentage strengths Pharmaceutical Calculations for the Pharmacy Technician Copyright © 2008 Wolters Kluwer Health | Lippincott Williams & Wilkins

Alligation Medial • Uses the weighted average • Used when the percent strength of each component is KNOWN • Express the percent strength by a decimal fraction • Multiply the decimal by the corresponding quantity • Add the products and divide by the total quantity of the mixture Pharmaceutical Calculations for the Pharmacy Technician Copyright © 2008 Wolters Kluwer Health | Lippincott Williams & Wilkins

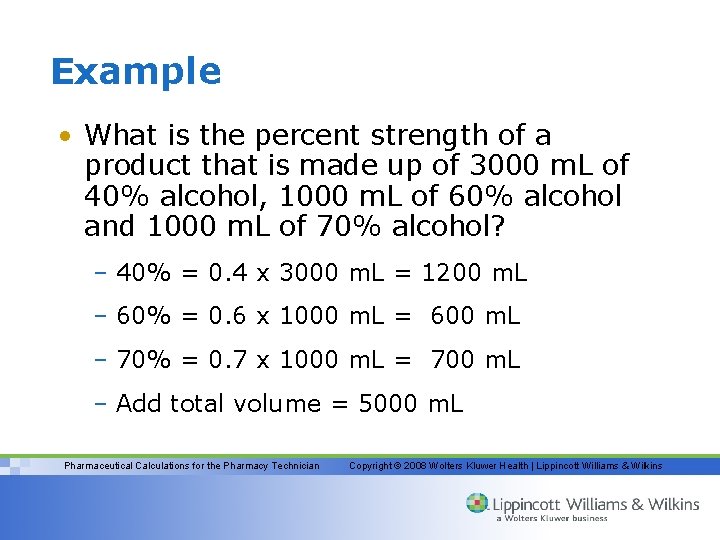
Example • What is the percent strength of a product that is made up of 3000 m. L of 40% alcohol, 1000 m. L of 60% alcohol and 1000 m. L of 70% alcohol? – 40% = 0. 4 x 3000 m. L = 1200 m. L – 60% = 0. 6 x 1000 m. L = 600 m. L – 70% = 0. 7 x 1000 m. L = 700 m. L – Add total volume = 5000 m. L Pharmaceutical Calculations for the Pharmacy Technician Copyright © 2008 Wolters Kluwer Health | Lippincott Williams & Wilkins

Example (cont. ) – Add the products 1200 m. L + 600 m. L + 700 m. L = 2500 m. L – Divide 2500 m. L by 5000 m. L = 0. 5 (change this to a percent) x 100 = 50% – Therefore by mixing these products the final product is 5000 m. L of a 50% alcohol product Pharmaceutical Calculations for the Pharmacy Technician Copyright © 2008 Wolters Kluwer Health | Lippincott Williams & Wilkins

Alligation Alternate • This method calculates the number of PARTS of two or more components of a certain strength. The final proportion allows us to use proportional parts to make any amount of the product we wish. • Strength of the final product must be in -between the strengths of the components we are mixing Pharmaceutical Calculations for the Pharmacy Technician Copyright © 2008 Wolters Kluwer Health | Lippincott Williams & Wilkins

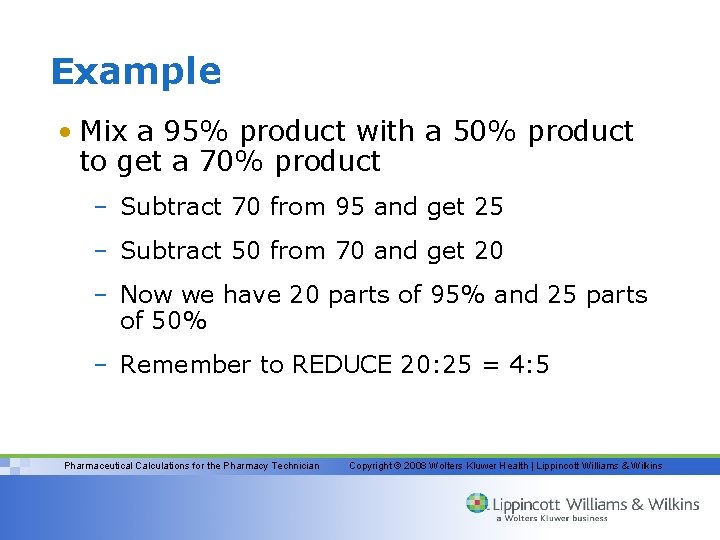
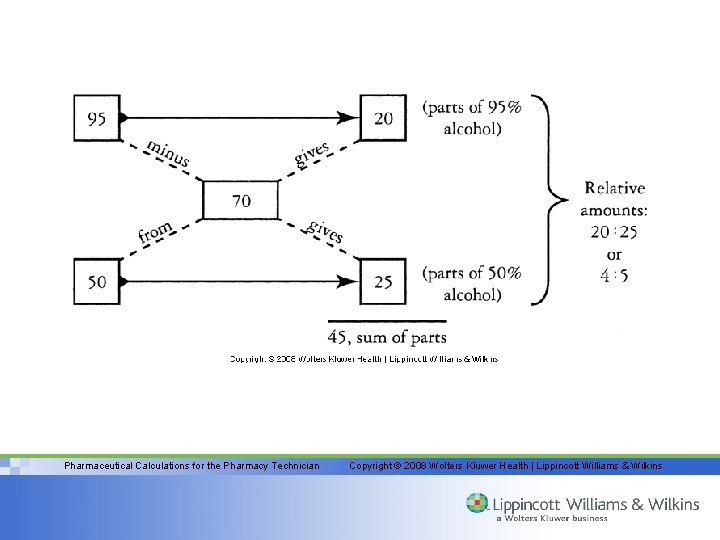
Example • Mix a 95% product with a 50% product to get a 70% product – Subtract 70 from 95 and get 25 – Subtract 50 from 70 and get 20 – Now we have 20 parts of 95% and 25 parts of 50% – Remember to REDUCE 20: 25 = 4: 5 Pharmaceutical Calculations for the Pharmacy Technician Copyright © 2008 Wolters Kluwer Health | Lippincott Williams & Wilkins

Pharmaceutical Calculations for the Pharmacy Technician Copyright © 2008 Wolters Kluwer Health | Lippincott Williams & Wilkins

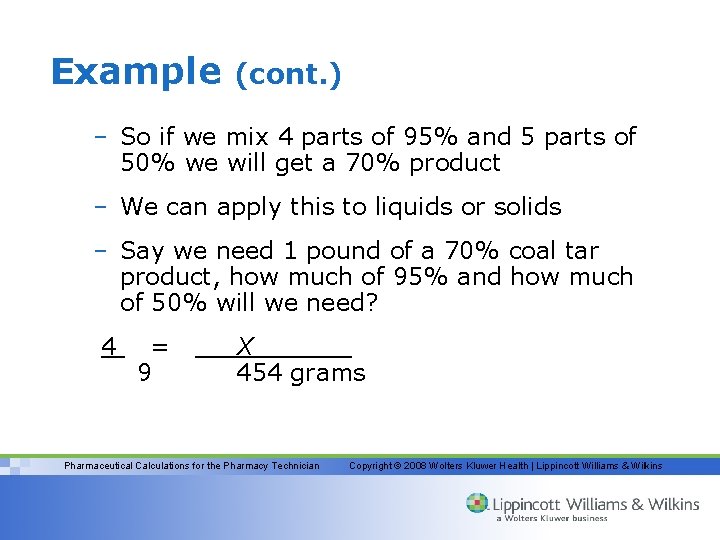
Example (cont. ) – So if we mix 4 parts of 95% and 5 parts of 50% we will get a 70% product – We can apply this to liquids or solids – Say we need 1 pound of a 70% coal tar product, how much of 95% and how much of 50% will we need? 4 = 9 X 454 grams Pharmaceutical Calculations for the Pharmacy Technician Copyright © 2008 Wolters Kluwer Health | Lippincott Williams & Wilkins

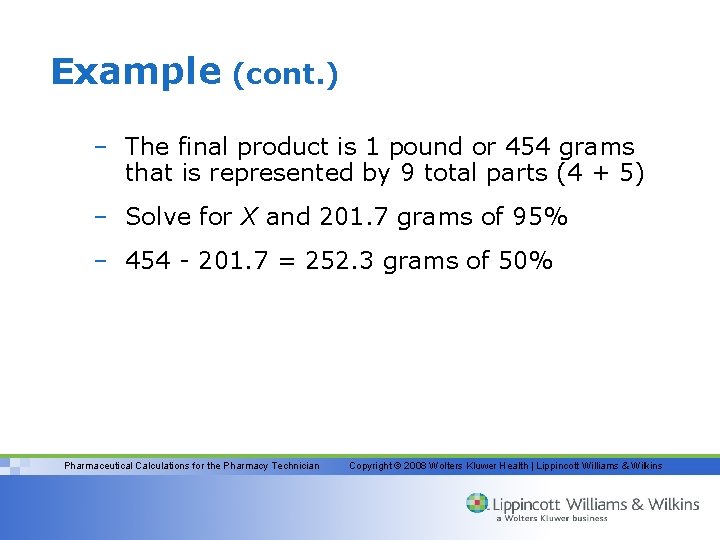
Example (cont. ) – The final product is 1 pound or 454 grams that is represented by 9 total parts (4 + 5) – Solve for X and 201. 7 grams of 95% – 454 - 201. 7 = 252. 3 grams of 50% Pharmaceutical Calculations for the Pharmacy Technician Copyright © 2008 Wolters Kluwer Health | Lippincott Williams & Wilkins

Using Alligation Alternate When the Quantity of One Ingredient Is Known Pharmaceutical Calculations for the Pharmacy Technician Copyright © 2008 Wolters Kluwer Health | Lippincott Williams & Wilkins

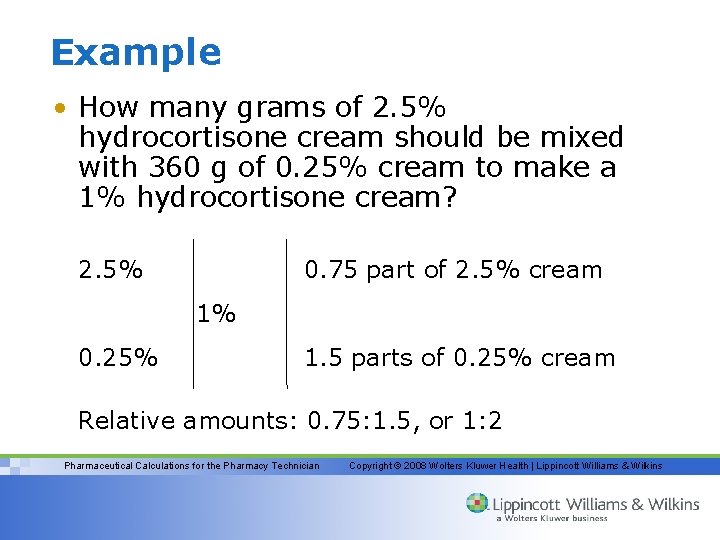
Example • How many grams of 2. 5% hydrocortisone cream should be mixed with 360 g of 0. 25% cream to make a 1% hydrocortisone cream? 2. 5% 0. 75 part of 2. 5% cream 1% 0. 25% 1. 5 parts of 0. 25% cream Relative amounts: 0. 75: 1. 5, or 1: 2 Pharmaceutical Calculations for the Pharmacy Technician Copyright © 2008 Wolters Kluwer Health | Lippincott Williams & Wilkins

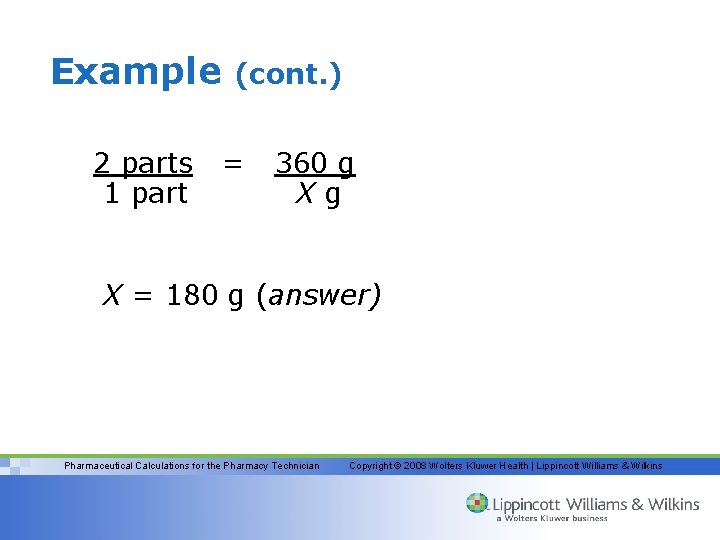
Example 2 parts 1 part (cont. ) = 360 g Xg X = 180 g (answer) Pharmaceutical Calculations for the Pharmacy Technician Copyright © 2008 Wolters Kluwer Health | Lippincott Williams & Wilkins

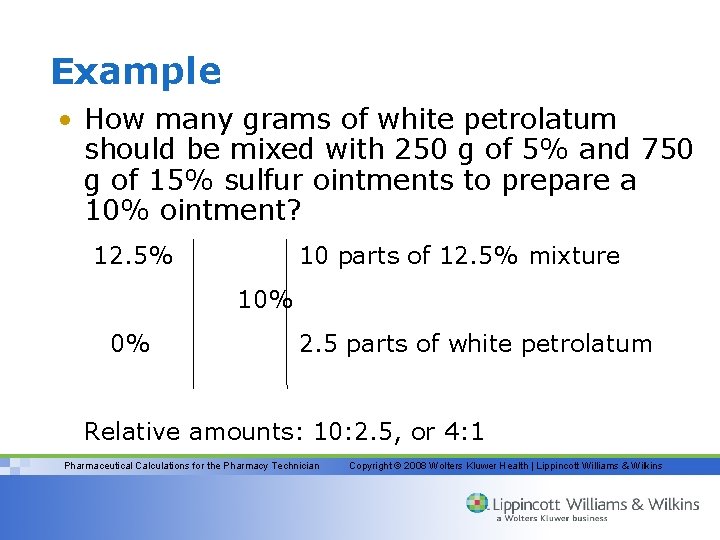
Example • How many grams of white petrolatum should be mixed with 250 g of 5% and 750 g of 15% sulfur ointments to prepare a 10% ointment? 12. 5% 10 parts of 12. 5% mixture 10% 0% 2. 5 parts of white petrolatum Relative amounts: 10: 2. 5, or 4: 1 Pharmaceutical Calculations for the Pharmacy Technician Copyright © 2008 Wolters Kluwer Health | Lippincott Williams & Wilkins

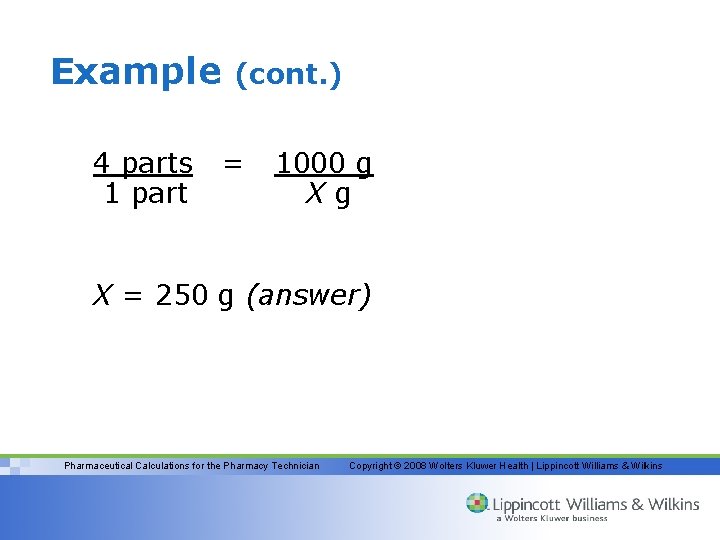
Example 4 parts 1 part (cont. ) = 1000 g Xg X = 250 g (answer) Pharmaceutical Calculations for the Pharmacy Technician Copyright © 2008 Wolters Kluwer Health | Lippincott Williams & Wilkins