Section 2 1 Evolving Theories of Matter The





















- Slides: 48

Section 2. 1 Evolving Theories of Matter The Stone Age (~8000 BC) • Metals had not yet been discovered. • Rock and bone were used to construct tools. • In the Middle East people learned to make and control fire and to change a variety of substances to meet their needs. • They cooked food, hardened mud, brick and tools to make them tougher and eventually made glass and ceramics.

Interest in Metals and Liquids (6000 -1000 BC): • Early chemists investigated only materials thought to be valuable. • Gold – its properties of luster, color, lack of tarnishing and softness made it a valued material used for jewelry and later coins. It was not strong enough, however, to be used for tool or weapons.

• Copper – though brittle when untreated, once heated it becomes useful to make pots, coins, tools and jewelry. It can be rolled into sheets or stretched into wires. Heating copper and tin together lead to the creation of bronze, an alloy of the two metals.

• Iron – the Iron Age began when the Hittites in the Middle East extracted iron from stone to make very strong tools and weapons. Later, iron was mixed with carbon to produce steel and used to make even harder and sharper blades and armor.

• Liquids – the word “chemistry” can be derived from the Greek word khemeia meaning juice of a plant. Many cultures experimented with ways to extract and use juices and oils. The Egyptians preserved the dead by wrapping them in cloths soaked in pigments and resin from the juniper plant.

The Greek Philosophers (2500 years ago): • The Greeks believed all matter was made up of tiny particles. • In about 400 BC, the philosopher Democritus used the word atomos (indivisible) to describe the smallest particle of a substance that could not be broken up any further. • He believed different substances were made up of different types of atomos, giving each substance its own unique properties. • By mixing atomos, you could make new materials with their own unique properties.

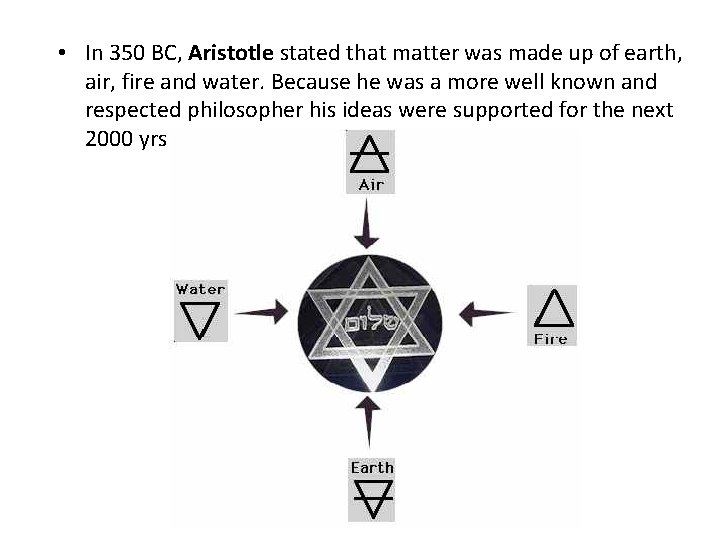
• In 350 BC, Aristotle stated that matter was made up of earth, air, fire and water. Because he was a more well known and respected philosopher his ideas were supported for the next 2000 yrs.

From Alchemy to Chemistry • For the next 2000 years, experiments with matter were done by alchemists who were part magician and part scientist. • They were not interested in understanding the nature of matter, but did perform some of the first chemistry experiments. • They created some of the chemistry tools we use today in labs such as beakers and filters. • They also made some practical discoveries, such as plaster of Paris, used today to hold broken bones in place as they heal.

From the 1500’s: The Modern Scientist • Scientists began to investigate the world around them to gain knowledge about the nature of matter and based their theories on observations and experimentation. • Robert Boyle (in the 1660’s) experimented on gases and became convinced that Democritus was correct, matter was made up of tiny particles. • In the 1770’s, Antoine Laurent Lavoisier and his wife Marie studied chemical interactions and devised a system for naming chemicals so that all chemists could use the same names to describe their observations (eg. hydrogen, carbon, oxygen).

The Beginning of Atomic Theory John Dalton’s Billiard Ball Model (1808) • suggested matter was made up of elements (pure substances that contain no other substances). • Each particle is called an atom. • Every atom of the same element has the same mass, and atoms of different elements have different masses. • His model was called the “billiard ball” model since he thought each atom was a solid sphere.

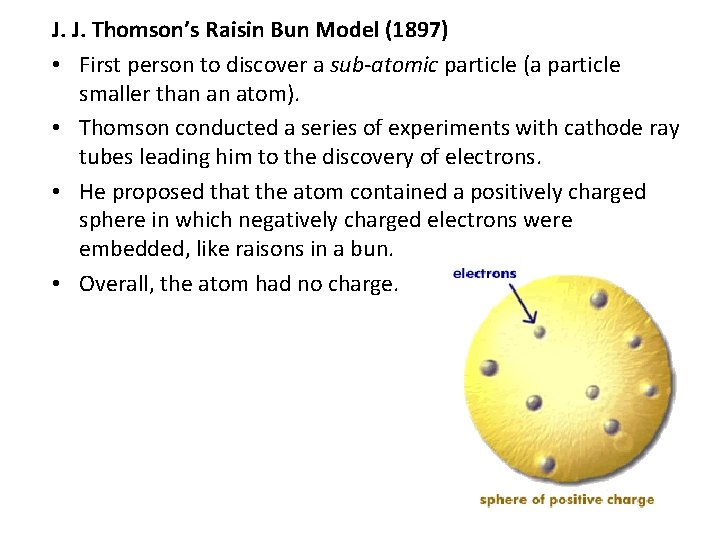
J. J. Thomson’s Raisin Bun Model (1897) • First person to discover a sub-atomic particle (a particle smaller than an atom). • Thomson conducted a series of experiments with cathode ray tubes leading him to the discovery of electrons. • He proposed that the atom contained a positively charged sphere in which negatively charged electrons were embedded, like raisons in a bun. • Overall, the atom had no charge.

• In 1904, Hantaro Nagaoka refined this theory of the atom suggesting that electrons orbited a large central positive mass. See Figure 2. 15 (p. 119).

• Support for this model came from a British scientist at Mc. Gill University in Montreal, Canada named Ernest Rutherford • Introduced the idea of the nucleus.

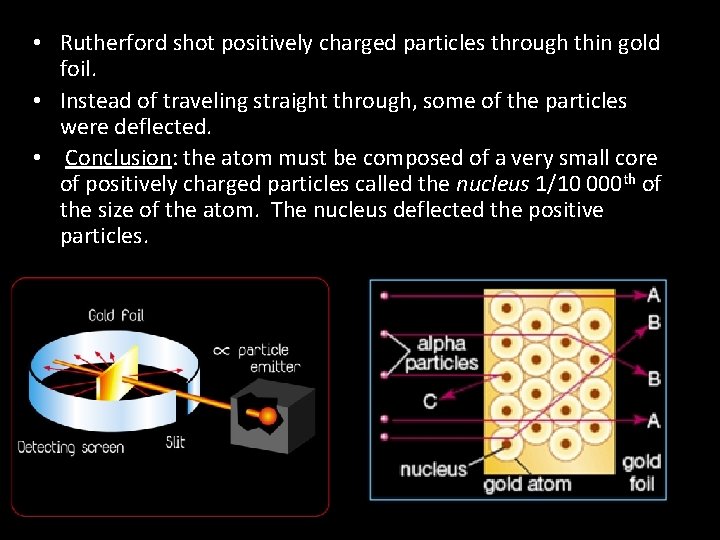
• Rutherford shot positively charged particles through thin gold foil. • Instead of traveling straight through, some of the particles were deflected. • Conclusion: the atom must be composed of a very small core of positively charged particles called the nucleus 1/10 000 th of the size of the atom. The nucleus deflected the positive particles.

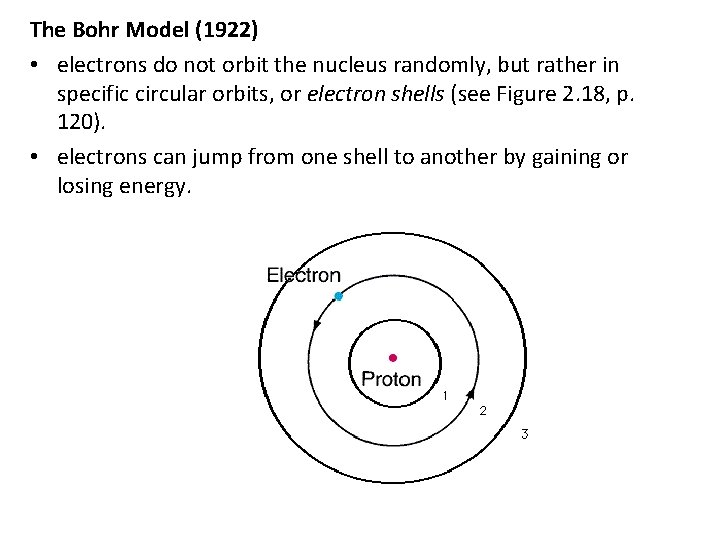
The Bohr Model (1922) • electrons do not orbit the nucleus randomly, but rather in specific circular orbits, or electron shells (see Figure 2. 18, p. 120). • electrons can jump from one shell to another by gaining or losing energy.

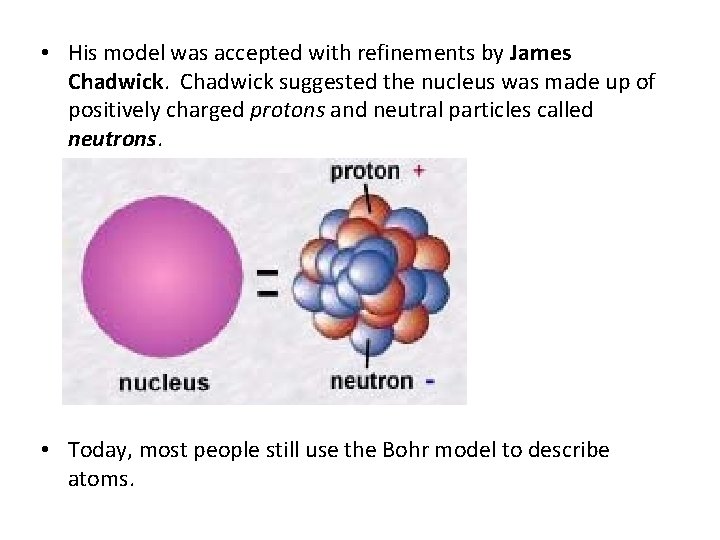
• His model was accepted with refinements by James Chadwick suggested the nucleus was made up of positively charged protons and neutral particles called neutrons. • Today, most people still use the Bohr model to describe atoms.

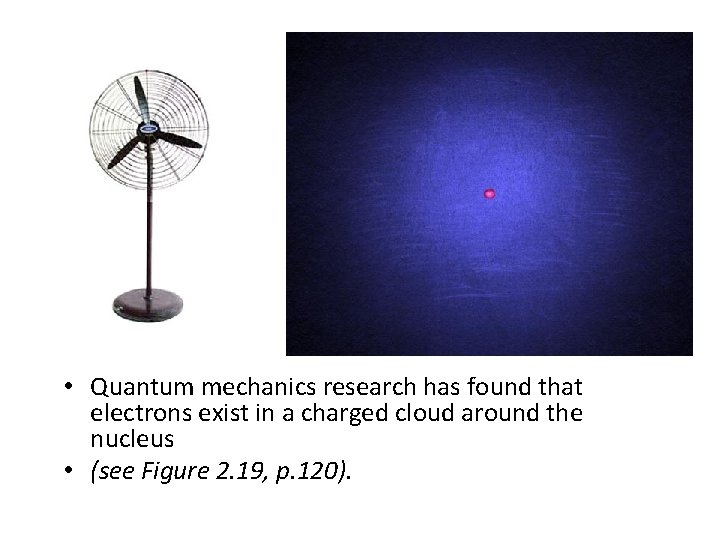
• Quantum mechanics research has found that electrons exist in a charged cloud around the nucleus • (see Figure 2. 19, p. 120).

In Summary • Dalton’s Billiard Ball : Matter is made up of solid spheres called atoms • Thomson’s Raisin Bun: Electrons are embedded in the atom • Rutherford: The nucleus is composed of protons • Bohr: Electrons exist in orbitals • Quantum Mechanics Theory: Most current accepted atomic theory.

Section 2. 2 Organizing the Elements Looking for Patterns • By the late 1700’s, only 7 metallic elements were known and each was represented by a symbol of the Sun or a planet (see Figure 2. 21, p. 123). • By the early 1800’s, more than 30 elements had been discovered and more were identified thereafter. • The need for a common classification system was apparent.

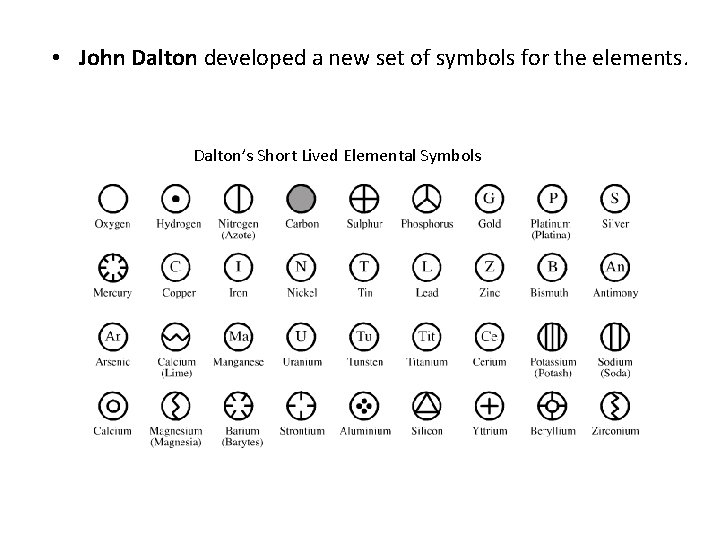
• John Dalton developed a new set of symbols for the elements. Dalton’s Short Lived Elemental Symbols

• His system was soon displaced by a system of symbols using letters of the alphabet developed by Jons Jacob Berzelius in 1814. Now chemists had a common “language”. • Atomic mass was used to arrange the elements in a pattern. • Atomic mass refers to the mass of one atom of an element and it is measured by atomic mass units or AMU. • Chemist John Newlands first recognized a pattern in 1864. • when the elements were arranged in increasing atomic mass it seemed as though the properties of the elements repeated at regular intervals, a pattern he called the “law of octaves”.

• Dmitiri Mendeleev organized the elements in a way that reflected these common properties. • He wrote all the properties of each element known at the time on a card and laid them out on a table to find a pattern. • He came upon a layout that followed an increasing atomic mass and seemed to group elements with similar properties (see Figure 2. 23, p 124). • There were gaps in his chart of elements. He predicted these gaps were elements that had not yet been discovered. • Other scientists were skeptical, but within 16 years many of those gaps were filled.

Now here’s a little song about the elements. "The Elements". A Flash animation • Enjoy!

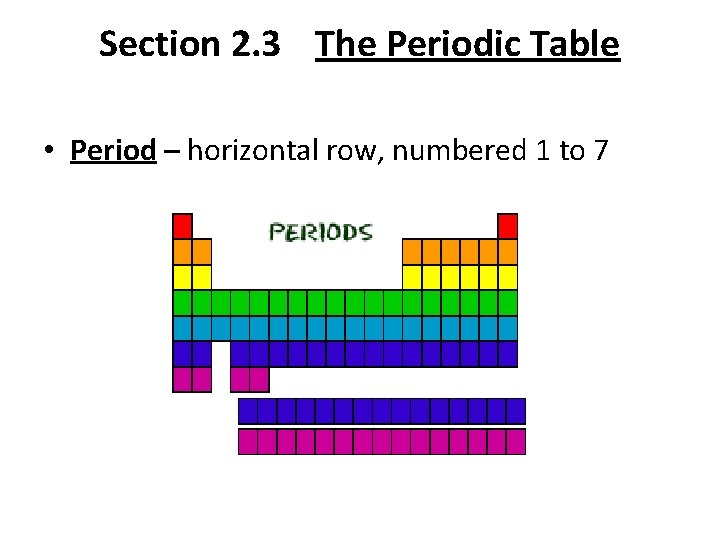
Section 2. 3 The Periodic Table • Period – horizontal row, numbered 1 to 7

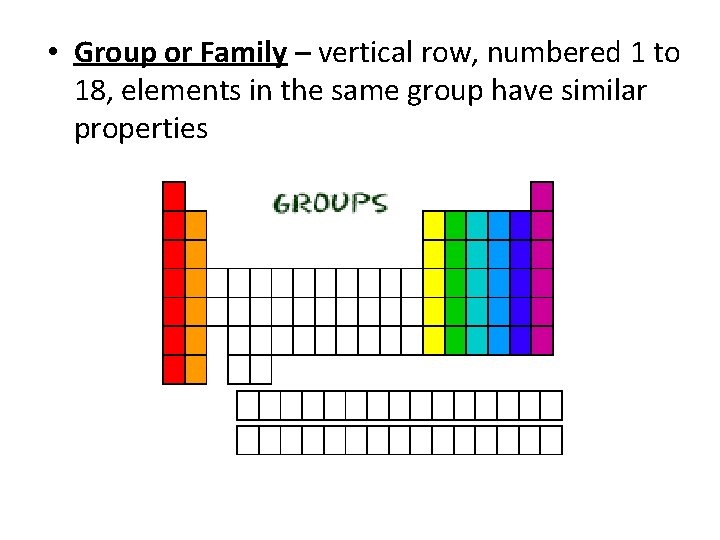
• Group or Family – vertical row, numbered 1 to 18, elements in the same group have similar properties

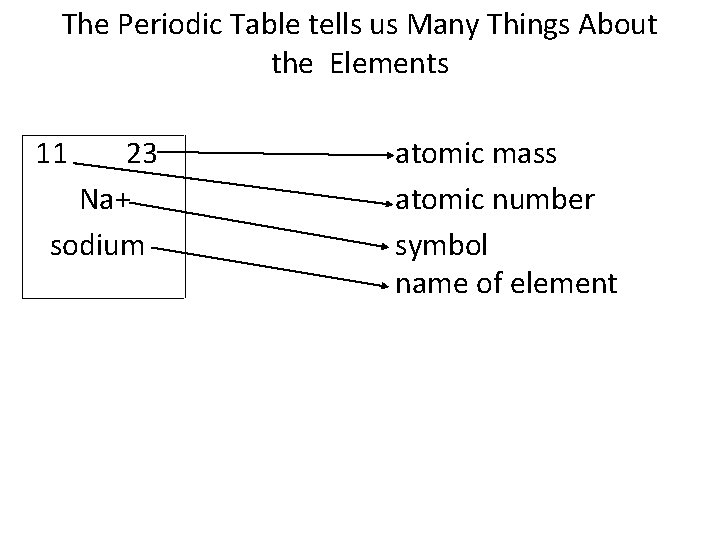
The Periodic Table tells us Many Things About the Elements 11 23 Na+ sodium • atomic mass atomic number symbol name of element

Element Symbol and Name • a capital letter followed by a lower case letter, usually an abbreviation of the element’s name. • Some elements have a Latin name (potassium’s Latin name is kalium, so its symbol is K). • Other elements are named after the location in which they were discovered (califorium) • Others are named after the discoverer (einsteinium). • See the Table on page 128.

Atomic Number • # of protons in the nucleus of an atom (ie. oxygen always has 8 protons in its nucleus). • Also indicates the # of electrons in the atom, since all atoms are neutral in charge. • (Note: the atomic number increases by 1 from left to right across the periodic table. )

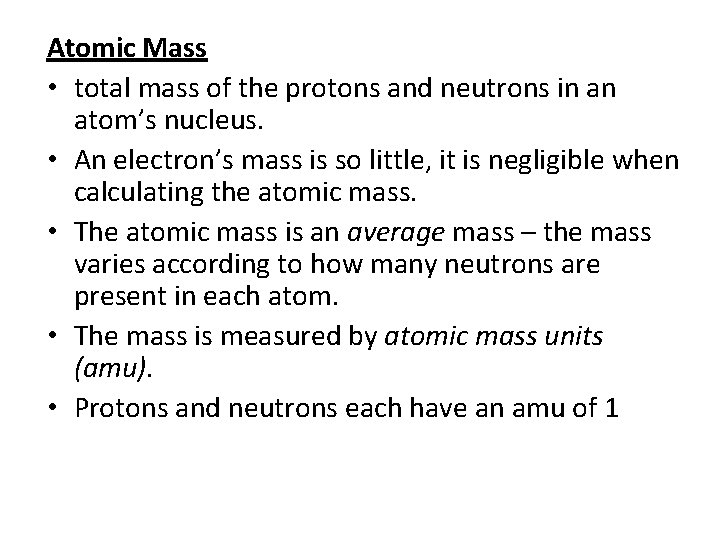
Atomic Mass • total mass of the protons and neutrons in an atom’s nucleus. • An electron’s mass is so little, it is negligible when calculating the atomic mass. • The atomic mass is an average mass – the mass varies according to how many neutrons are present in each atom. • The mass is measured by atomic mass units (amu). • Protons and neutrons each have an amu of 1

Mass Number • # protons + # neutrons in an atom • mass number can be used to discover how many neutrons are in an atom: mass # – atomic # = # of neutrons

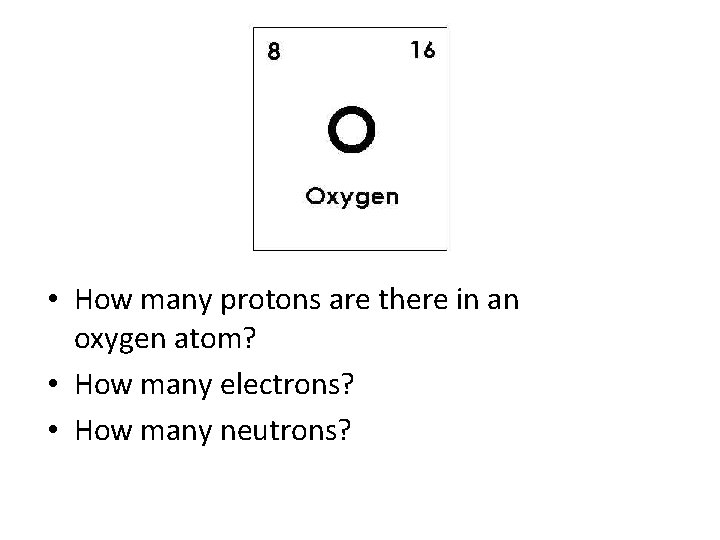
• How many protons are there in an oxygen atom? • How many electrons? • How many neutrons?

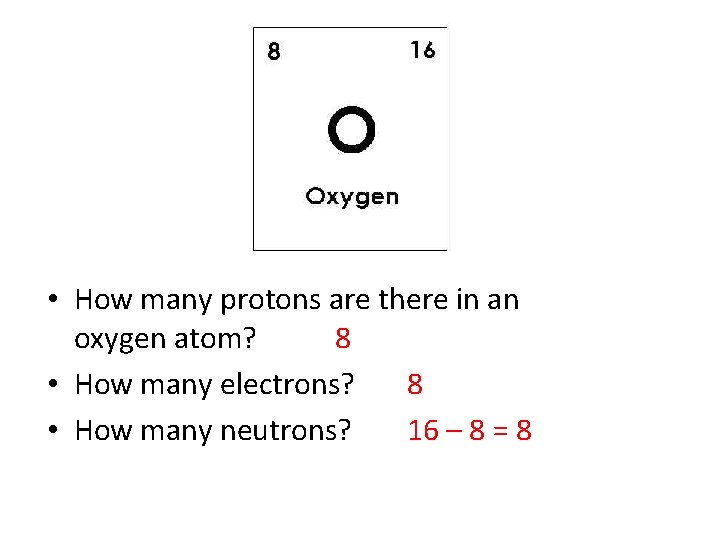
• How many protons are there in an oxygen atom? 8 • How many electrons? 8 • How many neutrons? 16 – 8 = 8

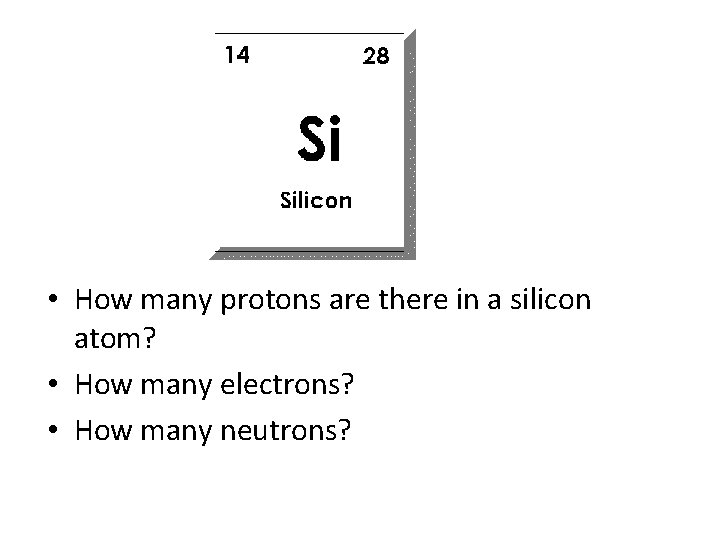
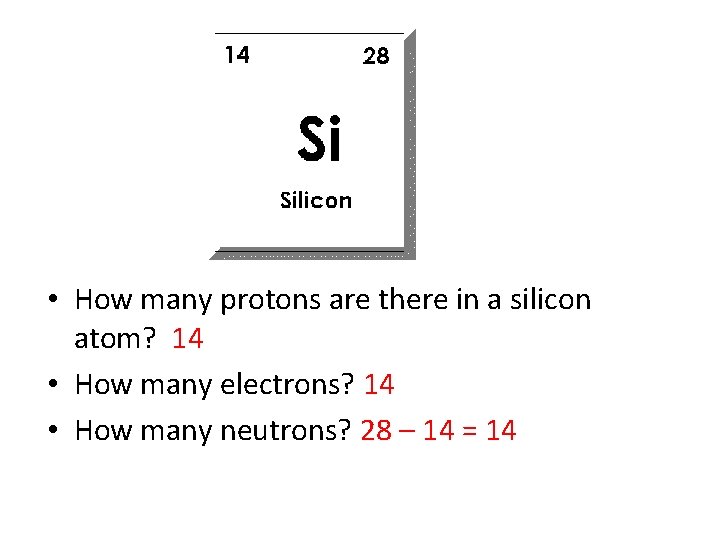
• How many protons are there in a silicon atom? • How many electrons? • How many neutrons?

• How many protons are there in a silicon atom? 14 • How many electrons? 14 • How many neutrons? 28 – 14 = 14

• How many protons are there in a nitrogen atom? • How many electrons? • How many neutrons?

• How many protons are there in a nitrogen atom? 7 • How many electrons? 7 • How many neutrons? 14 – 7 = 7

• Do Skill Practice P. 129 – Organize your answers into a chart. Use the atomic mass on your periodic tables for all calculations rounded to nearest whole number. Element Vanadium Nickel Phosphorous Bromine Beryllium Argon Magnesium Uranium Silicon Chromium Titanium Protons Electrons Neutrons

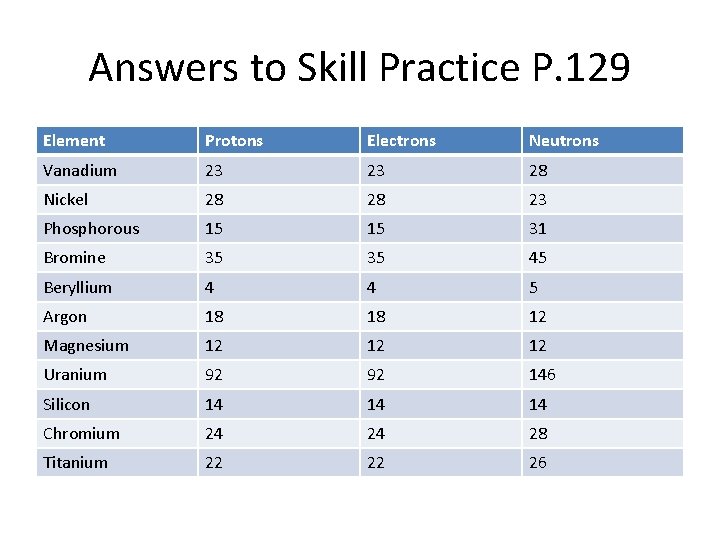
Answers to Skill Practice P. 129 Element Protons Electrons Neutrons Vanadium 23 23 28 Nickel 28 28 23 Phosphorous 15 15 31 Bromine 35 35 45 Beryllium 4 4 5 Argon 18 18 12 Magnesium 12 12 12 Uranium 92 92 146 Silicon 14 14 14 Chromium 24 24 28 Titanium 22 22 26

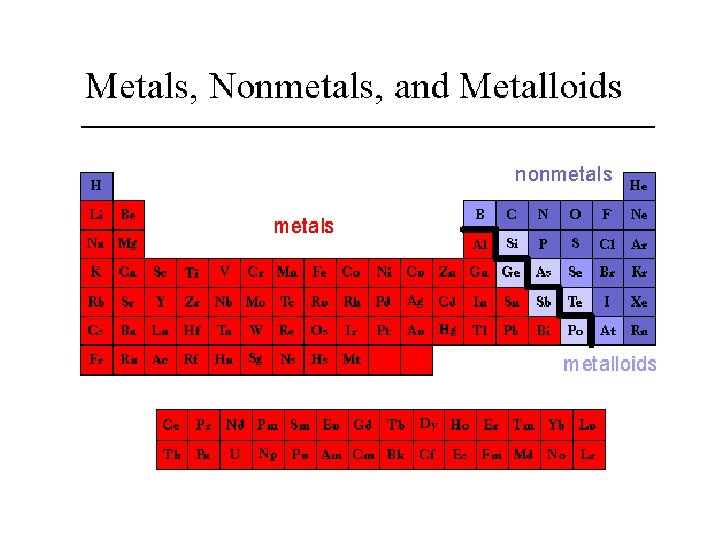
Patterns in the Periodic Table • metals: • To the left the staircase line. • shiny, malleable, ductile, and conduct electricity. • All are solids at room temp. except Hg • non-metals: • To the right of the staircase line. • Can be solids, liquids or gases at room temp. • dull, brittle and do not conduct electricity. • Surrounding the staircase are the metalloids: these elements display both metal and non-metal properties.


Periods • The period number tells you how many energy levels you have. • Periods 6 and 7 have 14 additional elements that are listed at the bottom of the periodic table so it is easier to print the table on a standard page. • Properties change in 2 ways as you move from left to right across a period: – The elements change from metal to non-metal – The elements become less reactive.

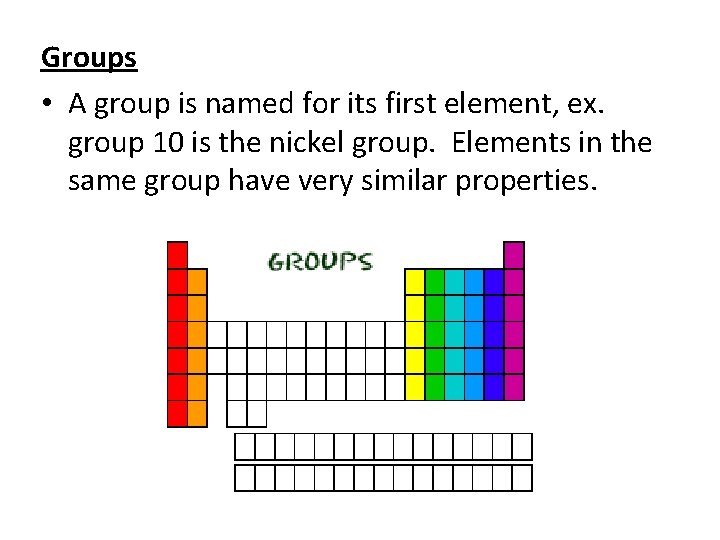
Groups • A group is named for its first element, ex. group 10 is the nickel group. Elements in the same group have very similar properties.

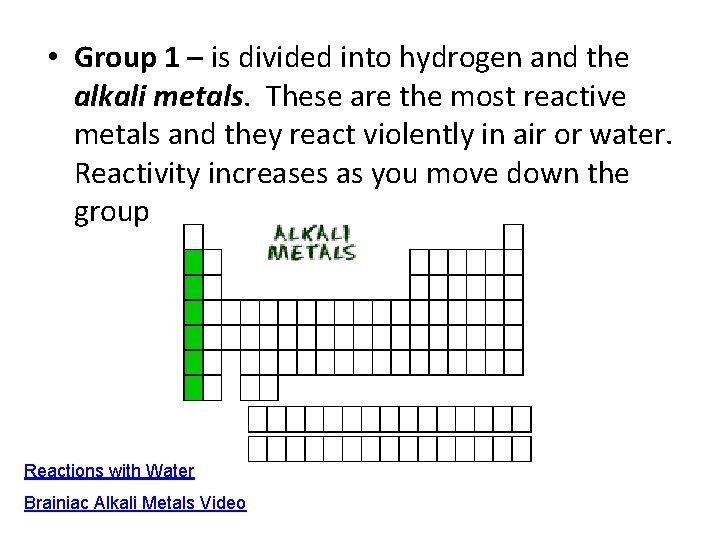
• Group 1 – is divided into hydrogen and the alkali metals. These are the most reactive metals and they react violently in air or water. Reactivity increases as you move down the group Reactions with Water Brainiac Alkali Metals Video

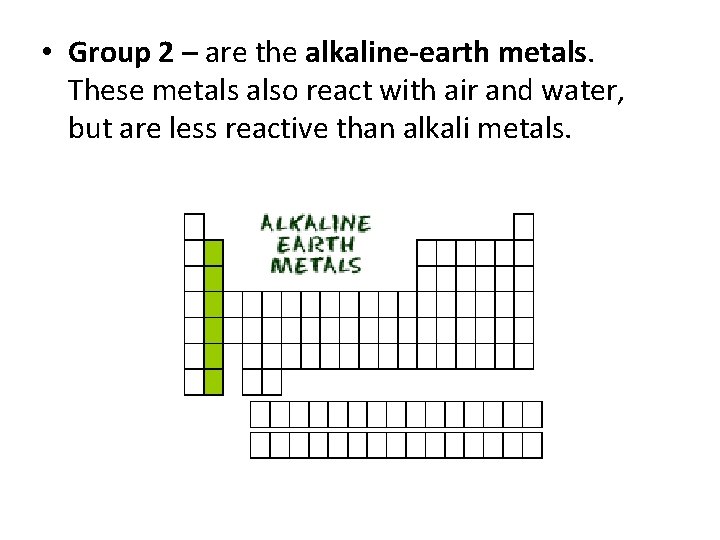
• Group 2 – are the alkaline-earth metals. These metals also react with air and water, but are less reactive than alkali metals.

• Group 17 – are the halogens and are the most reactive of the non-metals. They tend to combine with other elements to make compounds. Halogen reactions video

• Group 18 – are the noble gases and are the most stable and unreactive of the elements.

• Do Check and Reflect P. 134 # 1 -5, 7 -9 • Do Section Review P. 136 # 2, 3, 4, 6, 8, 10

Web Elements. com