Chapter 2 Matter Section 1 Classifying Matter Section





























- Slides: 37

Chapter 2 Matter Section 1: Classifying Matter Section 2: Properties of Matter Section 3: Changes of Matter

Classifying Matter What is matter? Matter is anything that has mass and takes up space. Air is matter, takes up space and has mass. Light and sound are not matter, they have no mass or volume and they contain no atoms. Chemistry is an important part of your daily life. The items you chose everyday are chosen for their chemical properties.

One important part of chemistry is classification One way this is done is based on their makeup Every sample of matter is either an element, a compound, or a mixture. Examples: gold is an element, water is a compound, and a vegetable salad is a mixture. Elements: An element is a substance that cannot be separated or broken down onto simpler substances by chemical means.

An atom is the smallest unit of an element that maintains the chemical properties of that element. Each element is made up of one kind of atom and every known element is unique. Carbon has multiple forms and these include diamonds

Elements

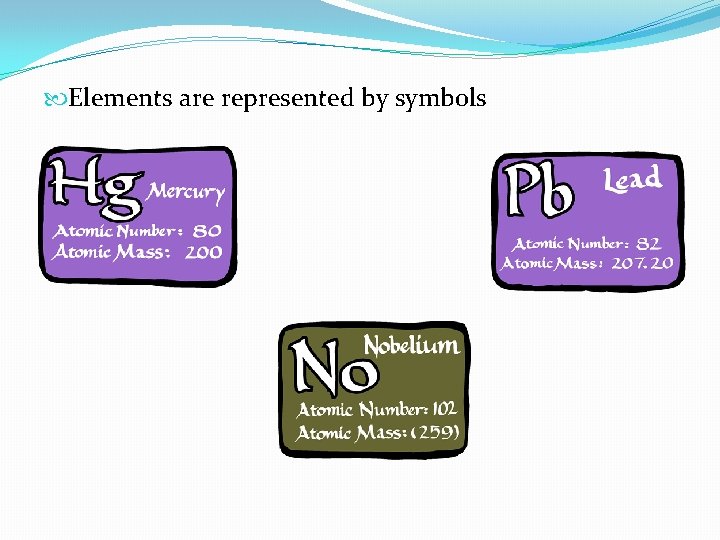
Elements are represented by symbols

Atoms that make up a molecule act as a unit A molecule is a group of atoms that are held together by chemical forces; a molecule is the smallest unit of matter that can assist all by itself and retain all of a substance’s chemical properties Atoms of some elements such as neon are found uncombined in nature. Others such as oxygen form molecules that have more than one atom

Compounds A compound is a substance made up of atoms of two or more different elements joined by chemical bonds They are chemically combined When elements combine to make a certain compound, they always combine in the same proportions Compounds have unique properties Every compound differs from the elements that it contains Example: elements such as hydrogen, oxygen, and nitrogen are colorless gases but when combined with carbon form nylon, a flexible solid

Chemical formulas represent compounds C₁₆H₁₀N₂O₂ this is the chemical formula of a molecule of indigo Pure Substances and Mixtures Pure often means “not mixed with anything” but in chemistry the word pure has a different meaning Pure substance is a sample of matter, either a single element or a single compound, that has definite chemical and physical properties Mixture is a combination of two or more substances that are not chemically combined

Elements and compounds are pure substances, but mixtures are not Mixtures are classified by how well the substances mix A mixture such as a salad is a heterogeneous mixture, the substances in the mixture are not evenly distributed In homogeneous mixture the components are evenly distributed, example would be vinegar which is water and acetic acid molecules. They are evenly distributed in a mixture

Mixtures can be miscible or easily mixed or immiscible or will not mix well Gases can mix with liquids Carbonated drinks are homogeneous mixtures. They contain sugar, flavorings, and carbon dioxide gas, CO₂ and mix well with liquids. The gas does not dissolve in the liquid but forms tiny bubbles in the liquid, this will form bigger bubbles. The bubbles join to form bigger bubbles that escape from foam and cause it to collapse Some foams are stable and last for a long time. Example is whipped egg whites and air, this solid is called meringue

Properties of Matter Physical Properties Shape and mass are examples of physical properties. Some other physical properties are color, volume, and texture Physical properties are characteristics that can be observed without changing the identity of the substance They are usually easily observed, such as color or texture but also not so obvious such as air that is colorless

Physical properties can help identify substances Many physical properties remain constant you can use your observations or measurements of these properties to identify substances Examples: You recognize your friends by their physical properties. You identify water because all samples of water are colorless and liquid

Physical properties can be observed or measured Your senses help you identify basic physical properties such as color, shape, odor, & texture State is another physical property that can be observed: solid, liquid, & gas Other physical properties such as melting point and boiling point can be measured Melting point the temperature and pressure at which a solid becomes a liquid Boiling point the temperature and pressure at which a liquid becomes a gas

Melting point Boiling point

A characteristic of any pure substance is that its boiling point and its melting point are constant if the pressure remains the same At constant pressure, pure water always has the same boiling point and the same melting point. Regardless of the mass or volume of water, the physical properties of the water are the same Other physical properties that can be measured are strength, hardness, and magnetism The ability to conduct electricity or heat is also a physical property

Physical properties help determine uses Physical properties are often used to select substances that may be useful Examples: Copper is used in power and telephone lines because it conducts electricity and aluminum is used in foil because it is lightweight yet durable and flexible

�Density is a physical property �Density is the ratio of the mass of a substance to the volume of the substance �The density of a liquid or a solid is usually expressed in grams per cubic centimeters (g/cm³) �Density is different from weight �A substance that has a low density is “light” in comparison with something else of the same volume �Why do we fill balloons with helium?

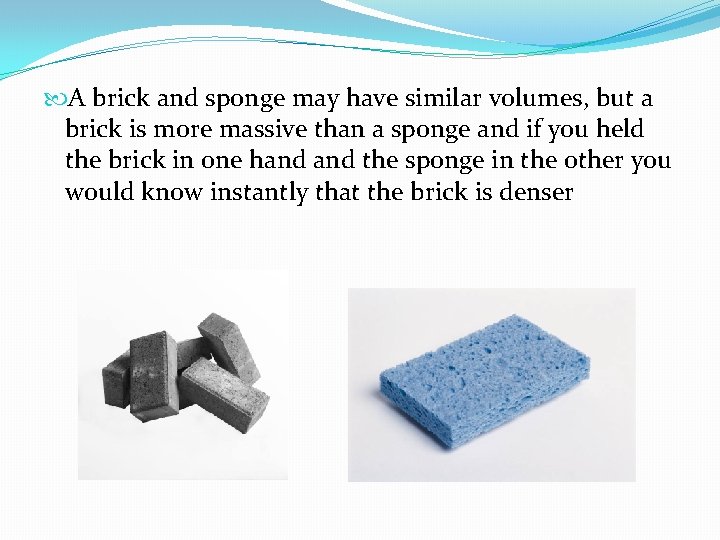
A brick and sponge may have similar volumes, but a brick is more massive than a sponge and if you held the brick in one hand the sponge in the other you would know instantly that the brick is denser

Remember weight and density are different, items can have the same volume but different densities Now compare two objects that have different volumes. Two pounds of feathers are heavier than one pound of steel, but…. . the feathers are less dense than the steel. So the two pounds of feathers have a greater volume that one pound of steel does

Chemical properties Some elements react very easily with other elements Reactive elements are usually found as compounds in nature and elements that are less reactive are often found uncombined. A chemical property describes how a substance changes into a new substance, either by combining with other elements or by breaking apart into new substances Chemical properties are generally not easy to observe as physical properties

Flammability is a chemical property It is the ability to burn. An example would be wood when burned it forms a new substance with new properties A substance always has its chemical properties even when you cannot observe them A substance that does not burn has a chemical property of nonflammability

Reactivity is a chemical property Another chemical property is the reactivity of elements or compounds with oxygen, water, or other substances Reactivity is the capacity of a substance to combine chemically with another substance Example: Iron is reactive with oxygen forming iron (III) oxide or rust

Physical and chemical properties are different You can observe physical properties without changing the identity of the substance You can observe chemical properties only in a situations in which the identity of the substance changes Example: Wood and rubbing alcohol both have chemical properties of being flammable but they have very different physical properties

Changes of Matter Physical Changes: A melting substance, getting your hair cut, and crushing a can A physical change is a change of matter from one form to another without a change in chemical properties

Physical changes do not change a substances identity During a physical change energy is absorbed or released. After a physical change, a substance may look different, but the arrangement of atoms that make up a substance is not changed Dissolving is a physical change When you stir sugar into water, the sugar dissolves and seems to disappear. So what happens to the sugar?

When sugar dissolves, the sugar molecules become spread out between the water molecules

Chemical Changes Some materials are useful because of their ability to change and combine to form new substance A chemical change is one that occurs when one or more substances change into entirely new substances with different properties The burning of the compounds is a chemical change What’s the difference between a chemical and physical change?

Chemical changes happen everywhere Examples: when a battery dies, it can no longer supply energy, when fruit and vegetables ripen, and when you breath in oxygen a series of reactions occur Also changing leaves, effervescent tablets and water, and the Statue of Liberty

Chemical changes form new substances So when combining the ingredients for bread, then heating it in the oven an interaction of the ingredients and the heat takes place causing a chemical change. A new substance is formed The properties are different than the original properties.

Chemical changes can be detected Clues often suggest a chemical change has happened. A change in odor or color is a good indication that a substance is changing chemically. Chemical changes cause things like color changes, fizzing or foaming, or the production of sound, heat, light, or odor.

Chemical changes cannot be reversed by physical changes However, under the right conditions, some chemical changes can be reversed by other chemical changes

Breaking down mixtures and compounds Remember a mixture is a combination of substances that are not chemically combined and a compound is made of atoms that are chemically combined. A mixture can be separated by a physical changes, but a compound must be broken down by chemical changes

Mixtures can be physically separated Because mixtures are not chemically combined, each part of the mixture has the same chemical makeup as it had before the mixture was formed Some mixtures are easy to separate like salad or pizza, others slightly harder to separate like seawater and blood Ways to separate mixtures is through boiling and through the use of a centrifuge which spins a mixture rapidly causing the separation

Some compounds can be broken down through chemical changes This can be done through heating such as in mercury (II) oxide breaking it down to mercury and oxygen Electric currents can be used to separate compounds such as passing a current through melted table salt this causes the elements sodium and chlorine to be produced

Other compounds undergo chemical changes to form simpler compounds. Example: opening a bottle of soda, compounds in the soda break down into carbon dioxide and water, and it is the reason for the soda bubbles when you open it

Are changes in states a physical or chemical change? How to we classify matter? It can be an Element, compound or mixture