Nonsurgical approach to the treatment of liver metastases


























- Slides: 46

Non-surgical approach to the treatment of liver metastases Dr Evan Dyer Austin Health

Liver metastases § 15 -20% of patients at diagnosis § 25% develop metastases following resection of primary § Median survival untreated <12 months § Surgical resection - 25 -50% 5 year survival

Local/regional treatments and Systemic chemotherapy

Local/regional strategies

Local/regional strategies § § § § Radio frequency ablation Cryotherapy Microwave Ablation Laser ablation Hepatic intra-arterial chemotherapy Selective internal radiation therapy High intensity focused ultrasound Percutaneous ethanol/acetic acid injection

Local/regional treatments § Suitable for patients who are not candidates for curative resection § Less morbid alternative to surgical resection in high risk patients § Attempts to cure patients with metastatic disease isolated to the liver in some cases

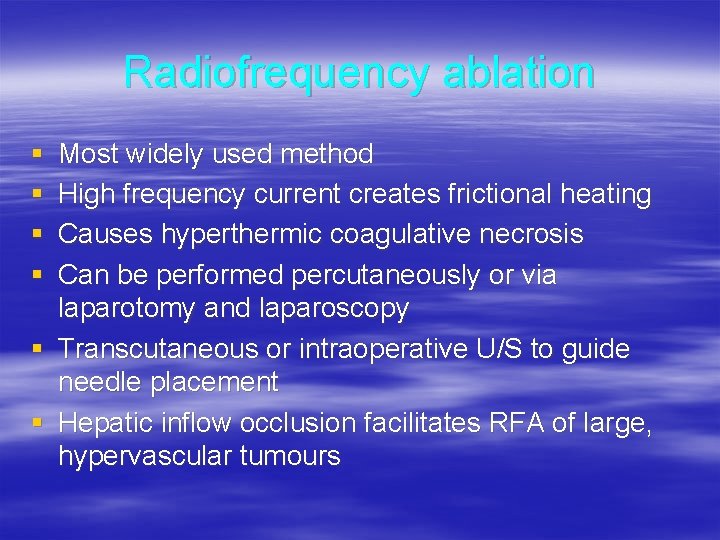
Radiofrequency ablation § § Most widely used method High frequency current creates frictional heating Causes hyperthermic coagulative necrosis Can be performed percutaneously or via laparotomy and laparoscopy § Transcutaneous or intraoperative U/S to guide needle placement § Hepatic inflow occlusion facilitates RFA of large, hypervascular tumours

Radiofrequency ablation § Can be used for metastases close to vessels but not bile ducts § Low complication rate § Widely applied to patients with liver mets § No evidence of extrahepatic disease (except some neuroendocrine tumours)

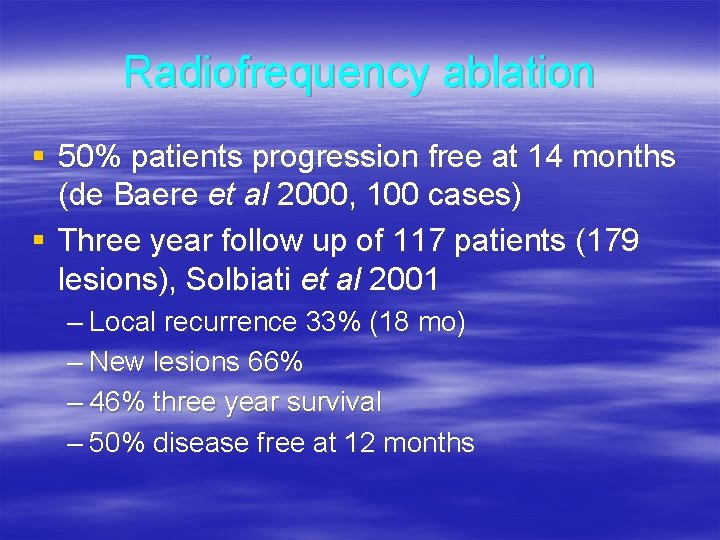
Radiofrequency ablation § 50% patients progression free at 14 months (de Baere et al 2000, 100 cases) § Three year follow up of 117 patients (179 lesions), Solbiati et al 2001 – Local recurrence 33% (18 mo) – New lesions 66% – 46% three year survival – 50% disease free at 12 months

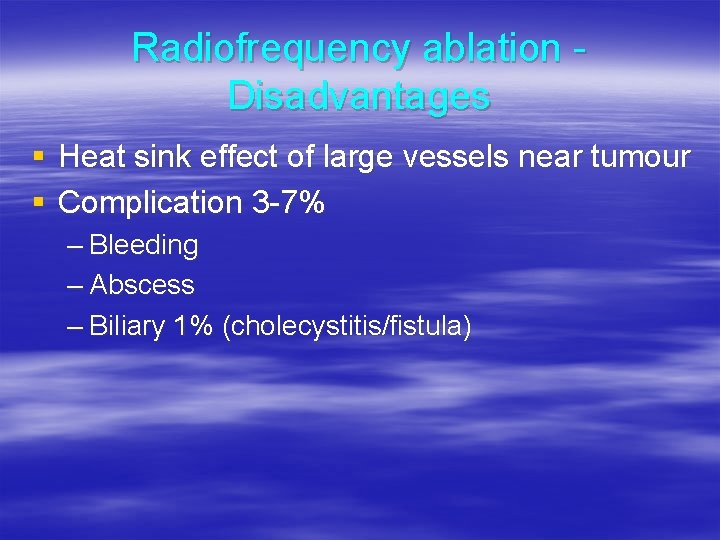
Radiofrequency ablation Disadvantages § Heat sink effect of large vessels near tumour § Complication 3 -7% – Bleeding – Abscess – Biliary 1% (cholecystitis/fistula)

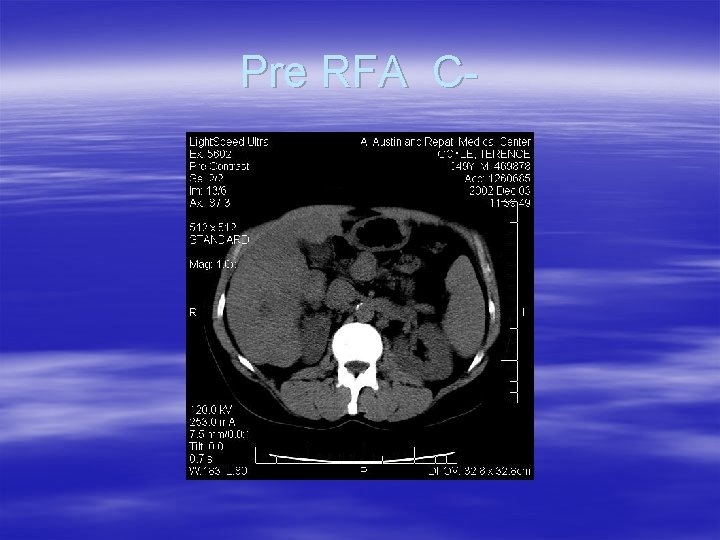
Pre RFA C-

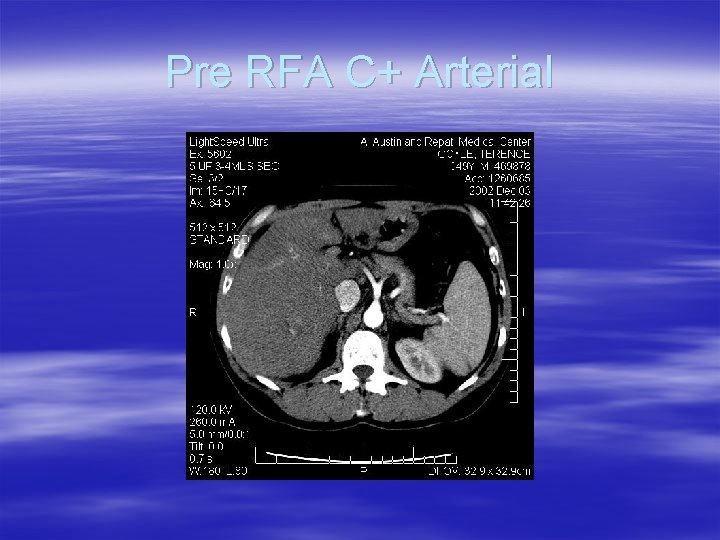
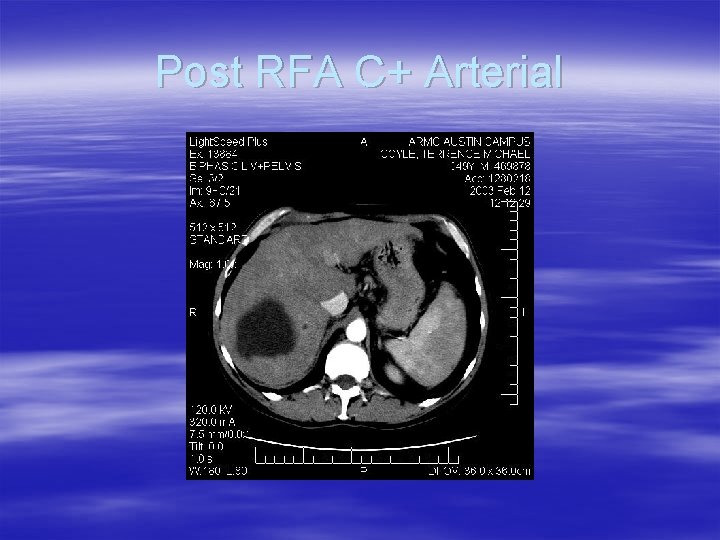
Pre RFA C+ Arterial

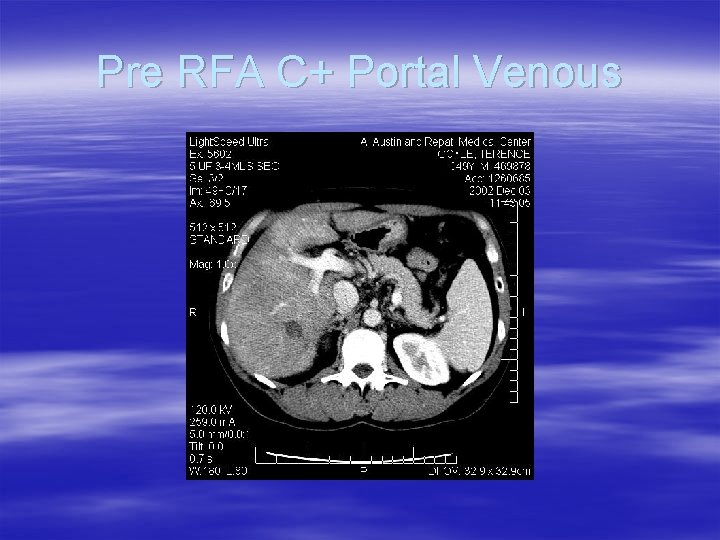
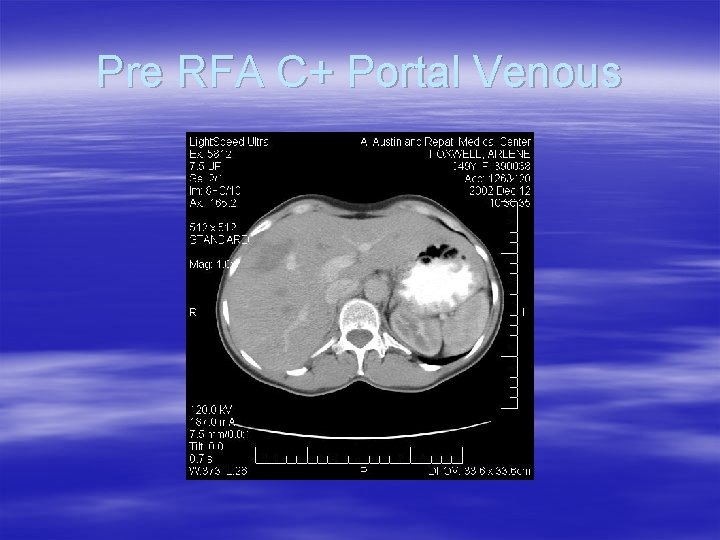
Pre RFA C+ Portal Venous

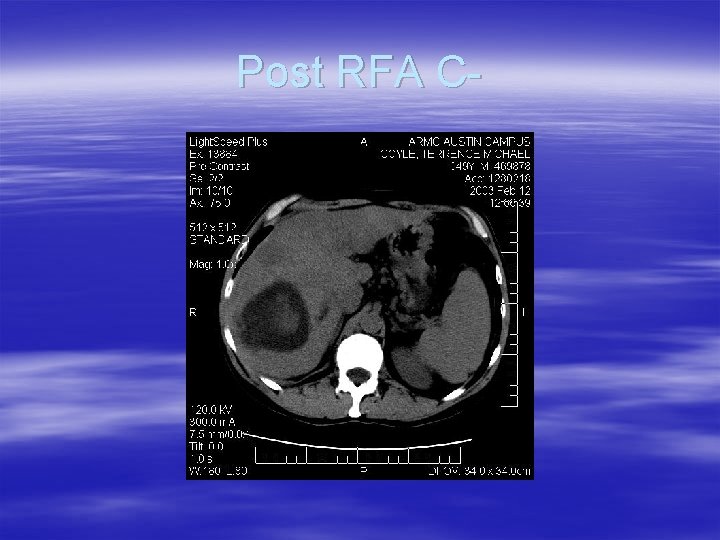
Post RFA C-

Post RFA C+ Arterial

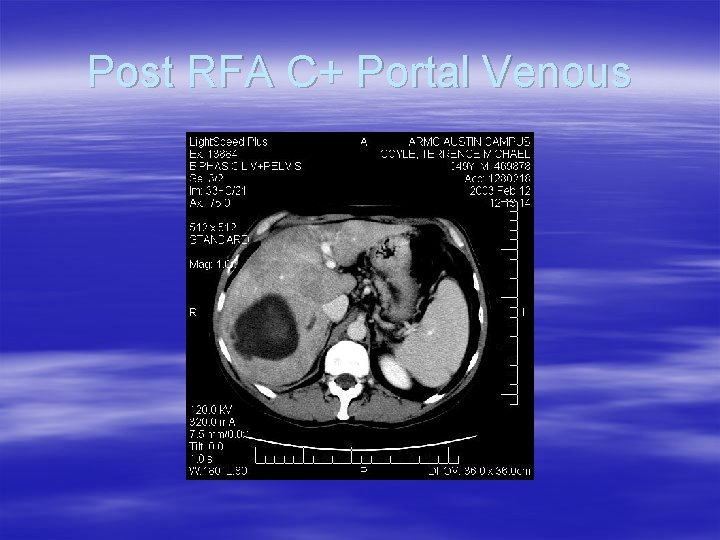
Post RFA C+ Portal Venous

Pre RFA C+ Portal Venous

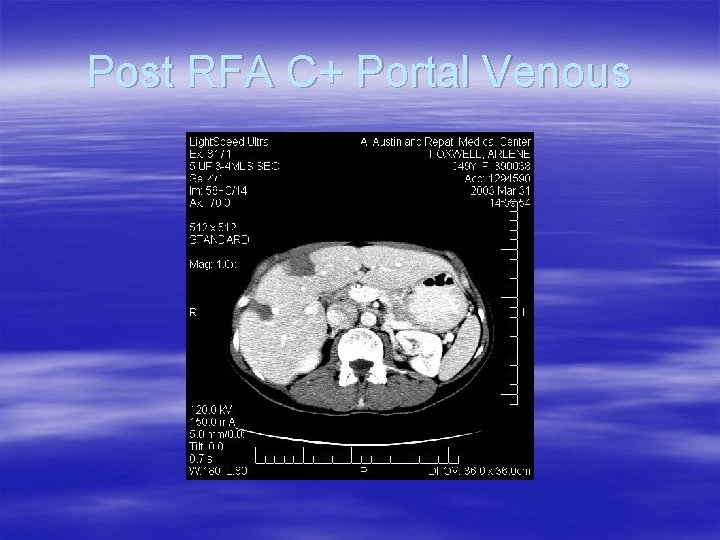
Post RFA C+ Portal Venous

Cryotherapy § Use of low temperature for tumour destruction § Suitable for tumours < 5 cm and multiple tumours § Zone of necrosis 3 -5 cm § May be useful with incomplete resection

Cryotherapy § § Performed at open surgery with U/S guide Freeze thaw cycles Follow up with CT Results – – 2 year survival to 62% (BJS Review) Median survival 24 -30 months § Ravikumar 1991 – – – 24 Patients 29% disease free at 2 years <10% recurrence at treatment site

Cryotherapy - disadvantages § § Non specific tissue damage ? True margin of tumour Requires laparotomy Complications – Bleeding – Thrombocytopaenia – Cold injury to normal tissues/hypothermia – Cryoshock

Microwave coagulation § Coagulates liver by agitation of water molecules causing frictional heating § Needle placed under ultrasound control § Survival rates similar to resection § Principally used in HCC § Small trials show disease free survival of 13 months and median survival 24 mo (Shibata et al 2000)

Laser ablation § § § Placement of laser fibres into tumour Causes lethal thermal injury to tumour cells Interaction between photons and chromophores (eg Hb, cytochrome pigments) § Unable to achieve large volumes of tumour necrosis

Laser ablation § Local control 40 – 75% § 30% 5 year survival

Hepatic intra-arterial chemotherapy § Tumours not suited to resection or local ablation § Adjuvant treatment post resection § Principles – High levels of rapidly metabolised drug – Macrometastases derive blood supply from hepatic artery – Percutaneous catheter or implantable pump

Hepatic intra-arterial chemotherapy § Technique – Placement of catheter to allow bilobar perfusion without chemotherapy to stomach/bowel – Angiogram pre-op – Laparotomy – Cannulation of gastroduodenal artery – Perfusion checked with Fluorescein/Woods lamp – Post op Tc-99 m scan

Hepatic intra-arterial chemotherapy § Studies show superior response rates but no survival advantage in colorectal cancer § Kemeny et al, Increased 2 year survival (86 v’s 72%) and freedom from disease (90 v’s 60%) § Explanation – Systemic disease – Toxicity leading to cessation of therapy – Surgical complications

Hepatic intra-arterial chemotherapy Complications § § § Arterial injury/thrombosis Incomplete or misperfusion Inflammation/ulceration stomach or duodenum § Biliary toxicity/sclerosis § Infection

Selective internal radiation therapy § § Labelled biocompatible microspheres 20 -40µm diameter Used in conjunction with chemotherapy Labelled 111 In-Octreotide used for neuroendocrine tumours

Selective internal radiation therapy § Results controlled trial 74 patients (Gray et al 2001) – Better response rate – Increased median time to progression – ? survival benefit

Selective internal radiation therapy § Drawbacks – Requires two angiograms – Nuclear medicine – Significant coordination between oncology/nuclear medicine/radiology

High intensity focused ultrasound § Use of ultrasound waves to produce heat § “Magnifying Glass” principle to focus in on lesion § Requires monitoring by MRI § Small tumours only

Percutaneous injection § Typically used for small hepatocellular tumours § Poor response with other histologies § Not an appropriate therapy for colorectal metastases

Systemic Chemotherapy § § § § 5 -Fluorouracil Leucovorin Capecitabine Irinotecan Oxaliplatin Combinations Other agents Future directions

Systemic chemotherapy § 30 -40% have locally advanced or metastatic disease at time of diagnosis § Treatment is palliative § Most effective single agents are 5 -FU, Irinotecan and oxaliplatin § Increases median and disease free survival

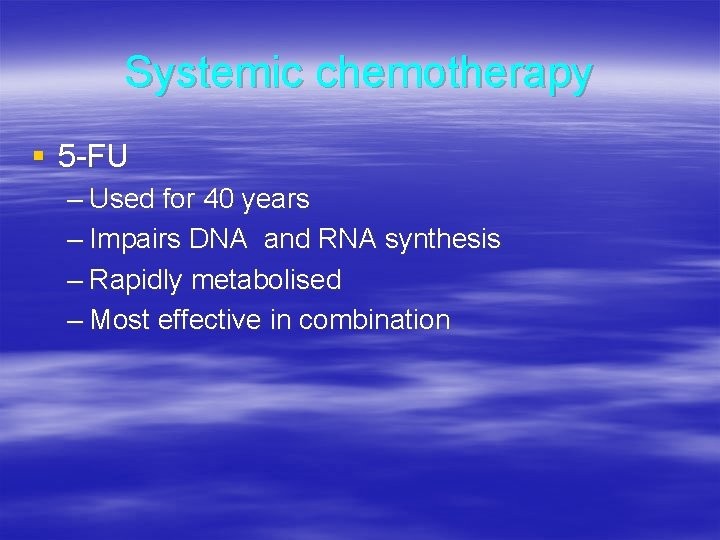
Systemic chemotherapy § 5 -FU – Used for 40 years – Impairs DNA and RNA synthesis – Rapidly metabolised – Most effective in combination

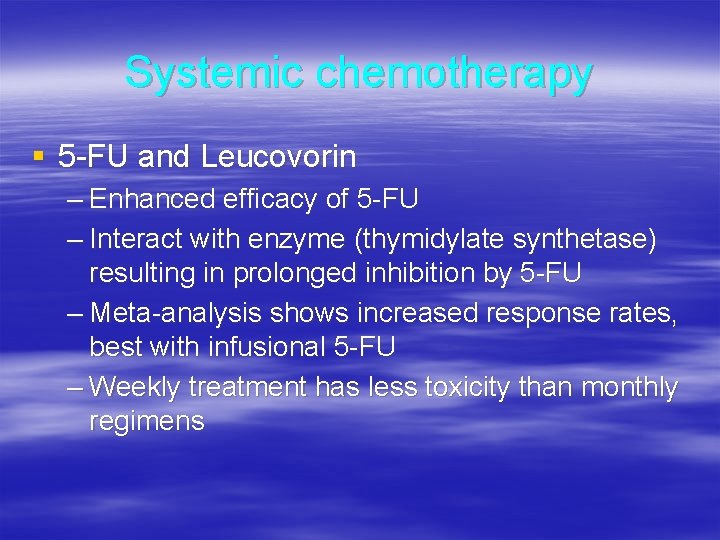
Systemic chemotherapy § 5 -FU and Leucovorin – Enhanced efficacy of 5 -FU – Interact with enzyme (thymidylate synthetase) resulting in prolonged inhibition by 5 -FU – Meta-analysis shows increased response rates, best with infusional 5 -FU – Weekly treatment has less toxicity than monthly regimens

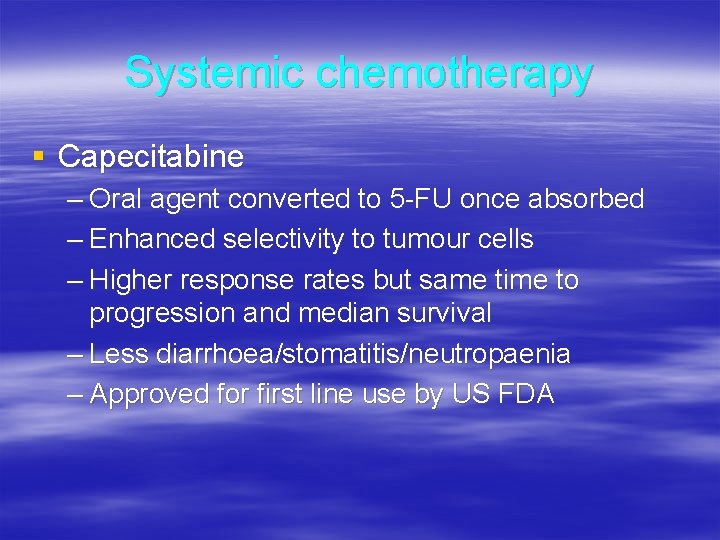
Systemic chemotherapy § Capecitabine – Oral agent converted to 5 -FU once absorbed – Enhanced selectivity to tumour cells – Higher response rates but same time to progression and median survival – Less diarrhoea/stomatitis/neutropaenia – Approved for first line use by US FDA

Systemic chemotherapy § Irinotecan – Topoisomerase I inhibitor – Clinical benefit after 5 -FU failure – Increased one year survival compared to best supportive care – Diarrhoea is dose limiting side effect

Systemic chemotherapy § Irinotecan + 5 -FU + Leucovorin – 49% response rate v’s 31% for dual therapy – Increased time to progression and survival – Results seen in two trials

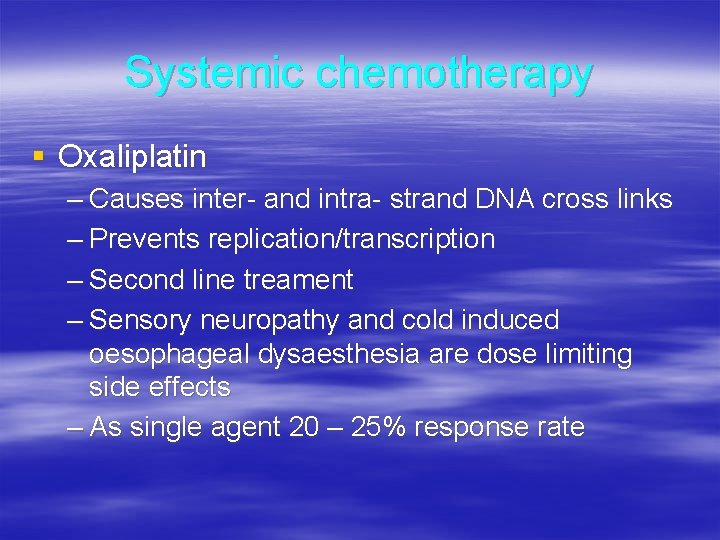
Systemic chemotherapy § Oxaliplatin – Causes inter- and intra- strand DNA cross links – Prevents replication/transcription – Second line treament – Sensory neuropathy and cold induced oesophageal dysaesthesia are dose limiting side effects – As single agent 20 – 25% response rate

Systemic chemotherapy § Oxaliplatin + 5 -FU + leucovorin – Preliminary results suggest superior efficacy other triple regimens – 1 st or 2 nd line therapy – No significant proven benefit as 2 nd line treatment – Unexpectedly high rates of toxicity (diarrhoea 20%, neutropaenia 39% and infection 13%. US Intergroup study) – Chronomodulation may improve response

Systemic chemotherapy § Trials underway (EORTC) comparing triple therapy pre +/- post surgery and surgery alone § Recruiting 330 patients 2000 -3 § Median follow up five years

Systemic chemotherapy § Other agents – Raltitrexed: Folate analogue, poorer profile than 5 -FU – Nitrosoureas, mitomycin C: Minimally active – Methotrexate, lomustine, vincristine – partial response

Systemic chemotherapy § Future directions – Targeted monoclonal therapies – Investigation of p 53, ras mutations, growth factors and their role in colorectal cancer – Vaccines – Signalling pathways

Summary § Long term prognosis remains poor § Short term prolongation of survival with palliative treatments § Interstitial ablative techniques show promise, are feasible and reasonably safe § Triple therapy most effective
 What is a research
What is a research Country attractiveness matrix
Country attractiveness matrix Approach to system development
Approach to system development Avoidance
Avoidance Deep learning approach and surface learning approach
Deep learning approach and surface learning approach A switch in a datagram network uses
A switch in a datagram network uses Cognitive approach vs behavioral approach
Cognitive approach vs behavioral approach Theoretical models of counseling
Theoretical models of counseling Chó sói
Chó sói Thẻ vin
Thẻ vin Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Diễn thế sinh thái là
Diễn thế sinh thái là Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Slidetodoc
Slidetodoc Thứ tự các dấu thăng giáng ở hóa biểu
Thứ tự các dấu thăng giáng ở hóa biểu Làm thế nào để 102-1=99
Làm thế nào để 102-1=99 Lời thề hippocrates
Lời thề hippocrates Sự nuôi và dạy con của hươu
Sự nuôi và dạy con của hươu Chụp tư thế worms-breton
Chụp tư thế worms-breton đại từ thay thế
đại từ thay thế Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Cong thức tính động năng
Cong thức tính động năng Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Dạng đột biến một nhiễm là
Dạng đột biến một nhiễm là Bổ thể
Bổ thể Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Biện pháp chống mỏi cơ
Biện pháp chống mỏi cơ Phản ứng thế ankan
Phản ứng thế ankan Môn thể thao bắt đầu bằng từ chạy
Môn thể thao bắt đầu bằng từ chạy Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan Chúa yêu trần thế alleluia
Chúa yêu trần thế alleluia điện thế nghỉ
điện thế nghỉ Một số thể thơ truyền thống
Một số thể thơ truyền thống Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Thế nào là số nguyên tố
Thế nào là số nguyên tố Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Sơ đồ cơ thể người
Sơ đồ cơ thể người ưu thế lai là gì
ưu thế lai là gì Tư thế ngồi viết
Tư thế ngồi viết Cái miệng bé xinh thế chỉ nói điều hay thôi
Cái miệng bé xinh thế chỉ nói điều hay thôi Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể