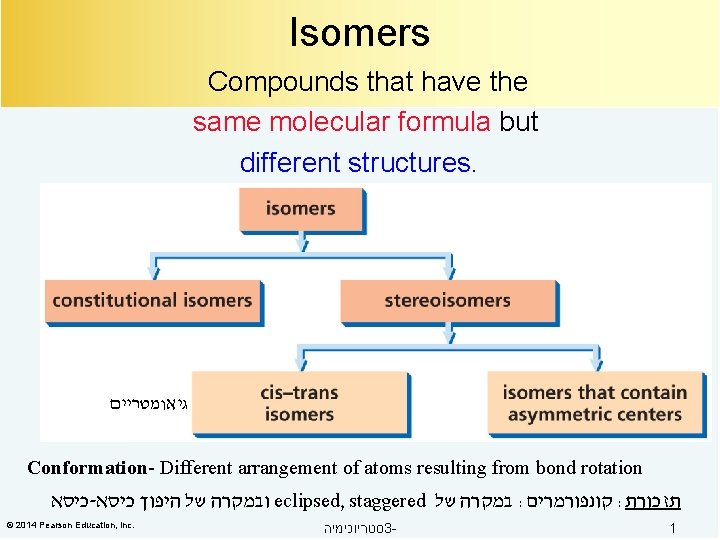
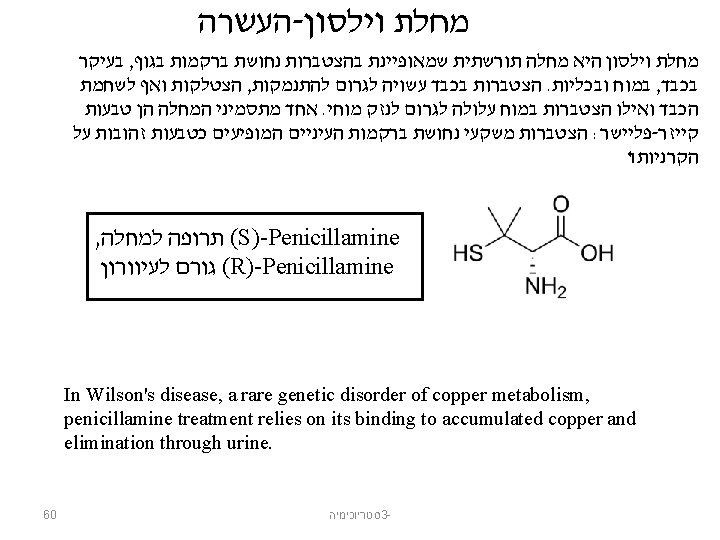
Isomers Compounds that have the same molecular formula



![אלפא ספציפי To have a basis for comparison, define specific rotation, [ ]D אלפא ספציפי To have a basis for comparison, define specific rotation, [ ]D](https://slidetodoc.com/presentation_image_h2/126e7f80e4bb691155a7e102ba9b3642/image-33.jpg)
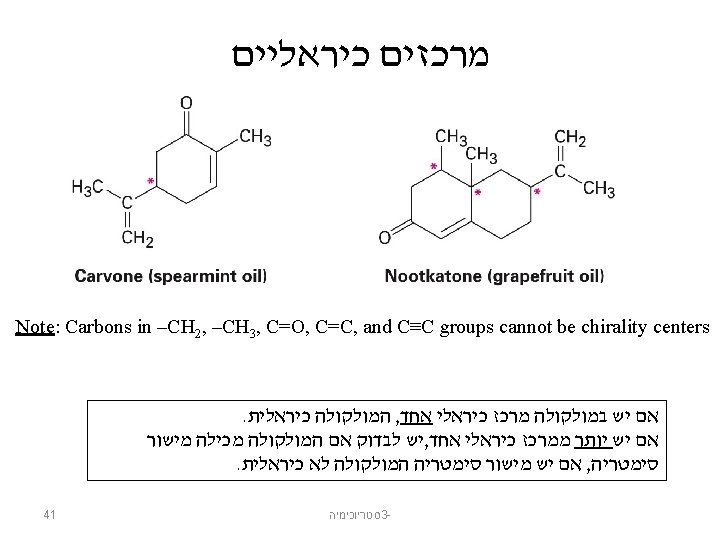


- Slides: 60

Isomers Compounds that have the same molecular formula but different structures. גיאומטריים Conformation- Different arrangement of atoms resulting from bond rotation כיסא - ובמקרה של היפוך כיסא eclipsed, staggered במקרה של : קונפורמרים : תזכורת © 2014 Pearson Education, Inc. סטריוכימיה 3 - 1

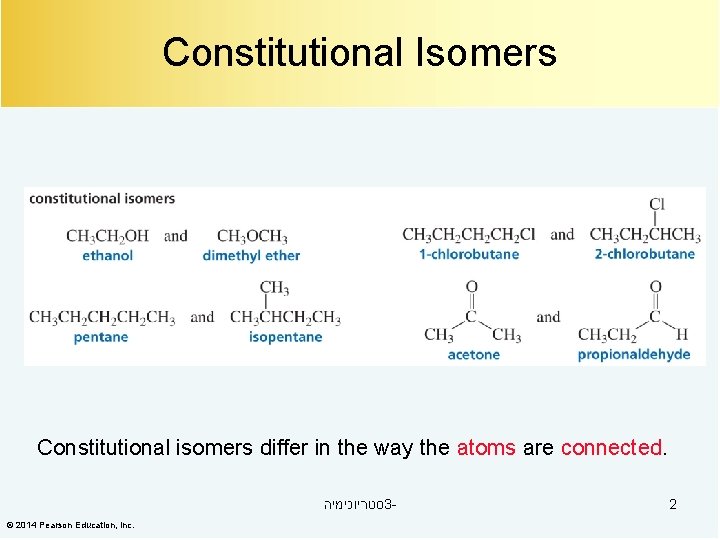
Constitutional Isomers Constitutional isomers differ in the way the atoms are connected. סטריוכימיה 3© 2014 Pearson Education, Inc. 2

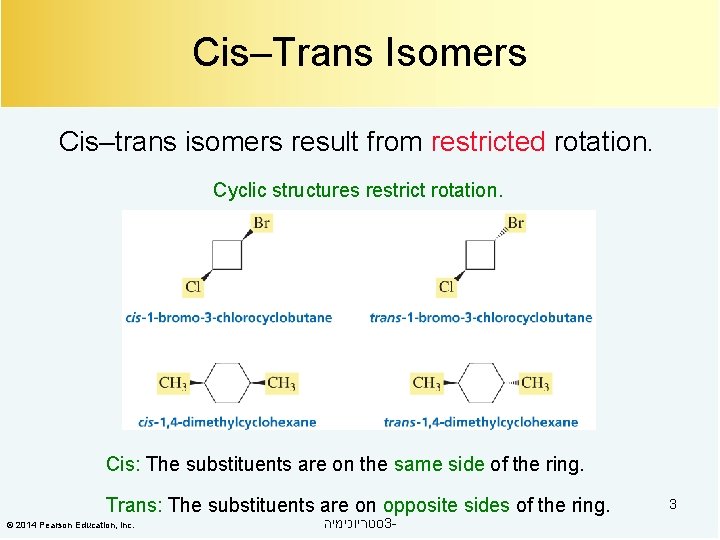
Cis–Trans Isomers Cis–trans isomers result from restricted rotation. Cyclic structures restrict rotation. Cis: The substituents are on the same side of the ring. Trans: The substituents are on opposite sides of the ring. © 2014 Pearson Education, Inc. סטריוכימיה 3 - 3


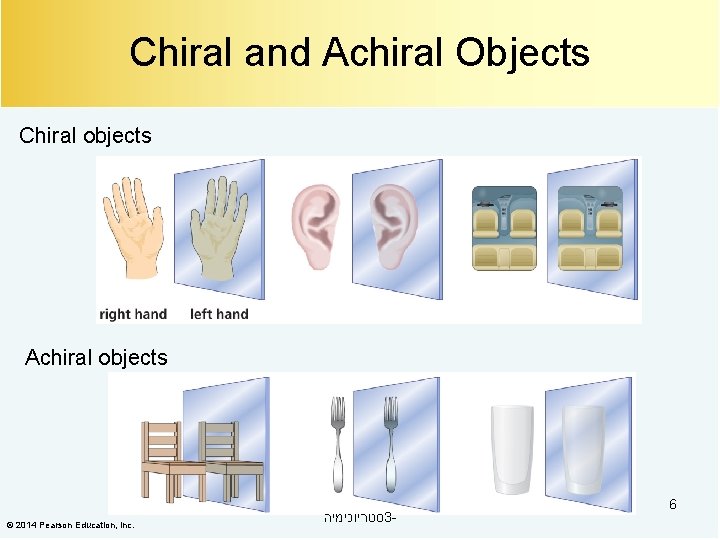
כיראליות Some objects are not the same as their mirror images (technically, they have no plane of symmetry)A right-hand glove is different from a lefthand glove. The property is commonly called “handedness” 5 סטריוכימיה 3 -

Chiral and Achiral Objects Chiral objects Achiral objects © 2014 Pearson Education, Inc. סטריוכימיה 3 - 6

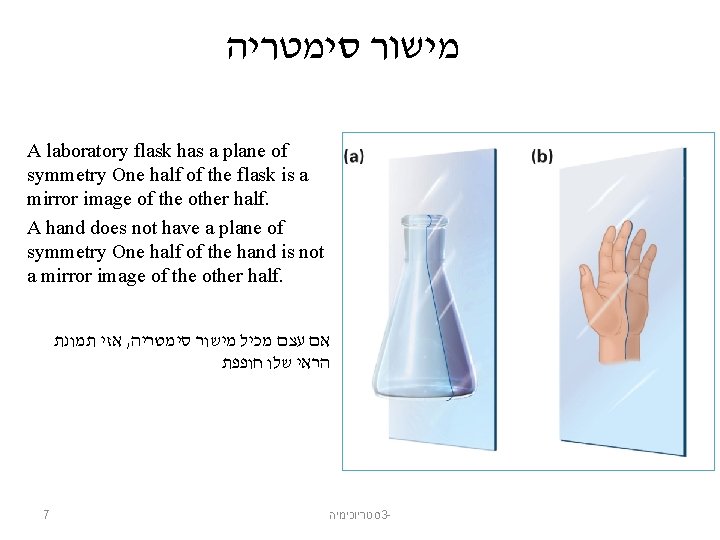
מישור סימטריה A laboratory flask has a plane of symmetry One half of the flask is a mirror image of the other half. A hand does not have a plane of symmetry One half of the hand is not a mirror image of the other half. אזי תמונת , אם עצם מכיל מישור סימטריה הראי שלו חופפת 7 סטריוכימיה 3 -


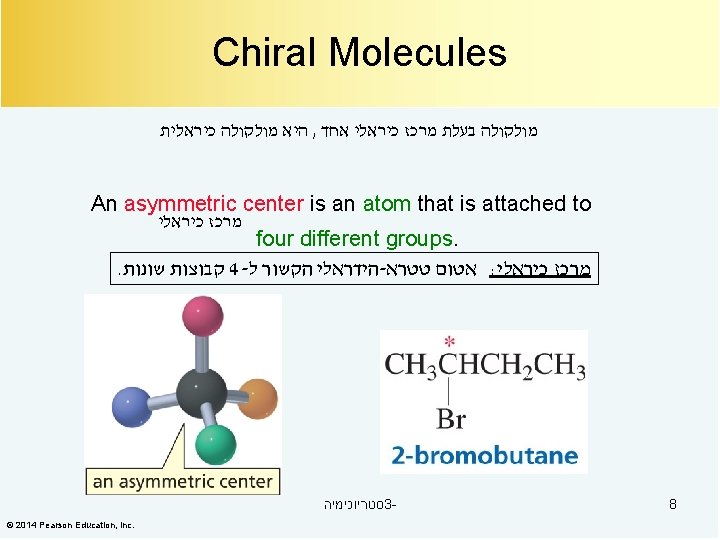
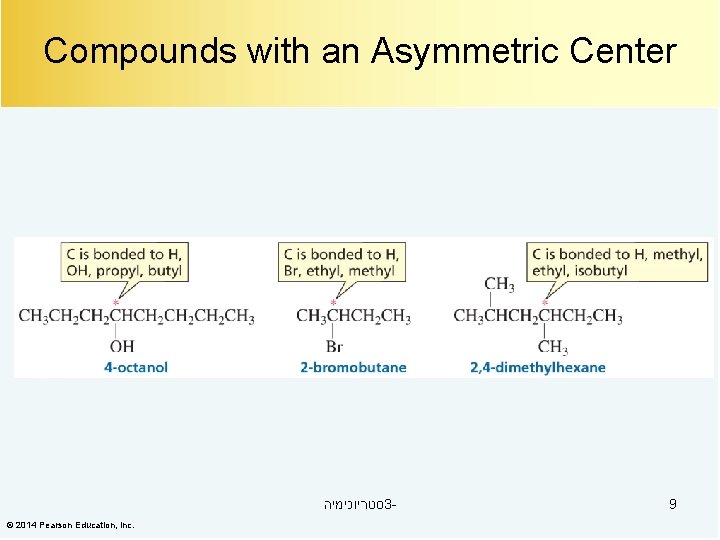
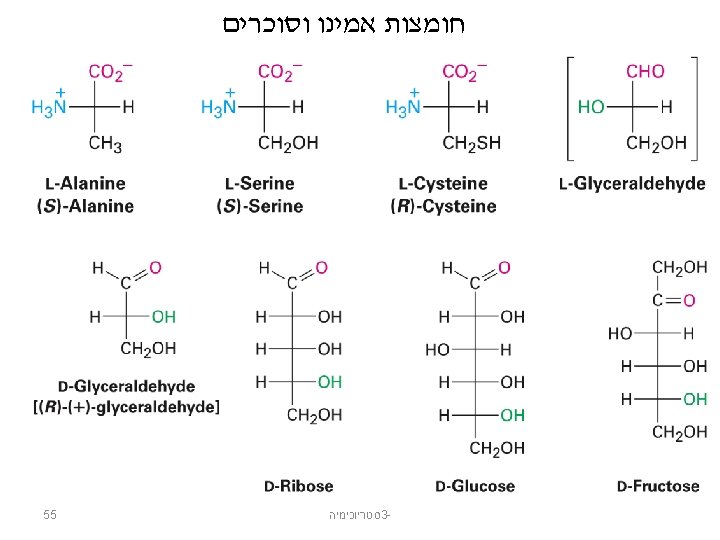
Compounds with an Asymmetric Center סטריוכימיה 3© 2014 Pearson Education, Inc. 9

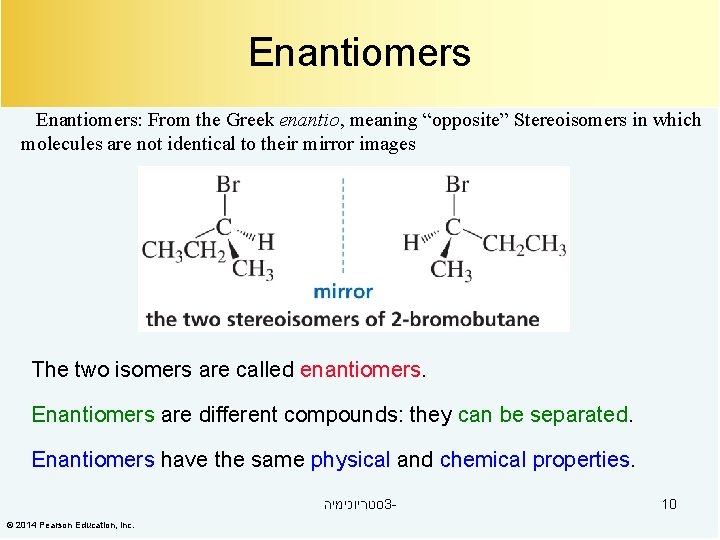
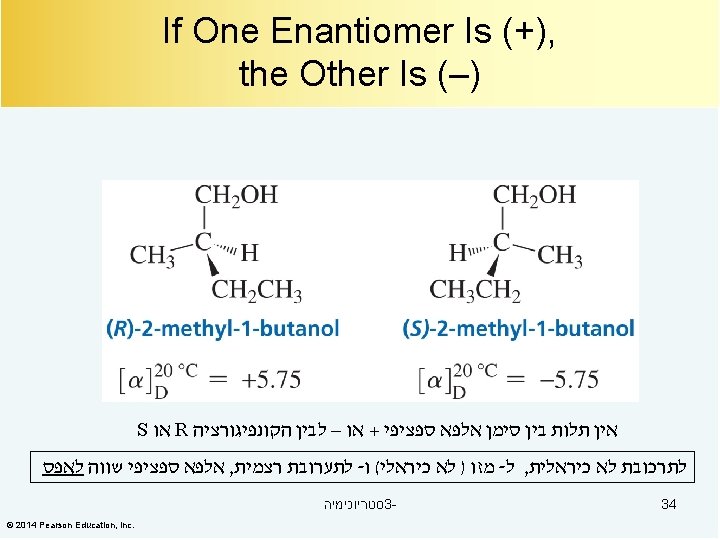
Enantiomers: From the Greek enantio, meaning “opposite” Stereoisomers in which molecules are not identical to their mirror images The two isomers are called enantiomers. Enantiomers are different compounds: they can be separated. Enantiomers have the same physical and chemical properties. סטריוכימיה 3© 2014 Pearson Education, Inc. 10

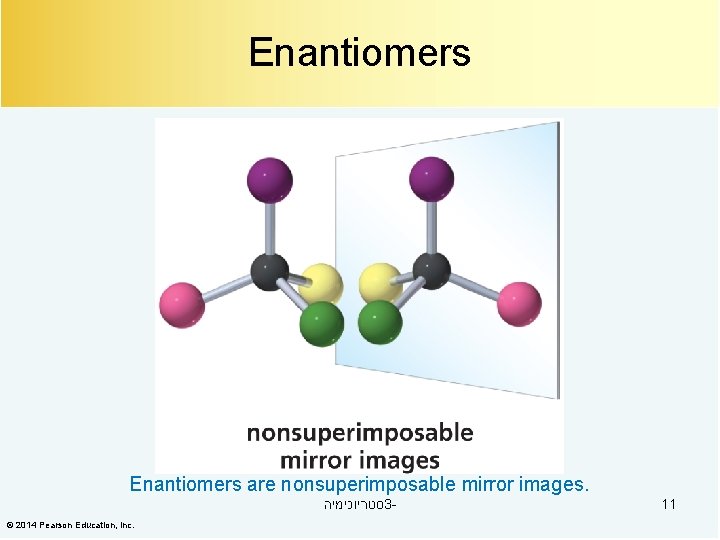
Enantiomers are nonsuperimposable mirror images. סטריוכימיה 3© 2014 Pearson Education, Inc. 11

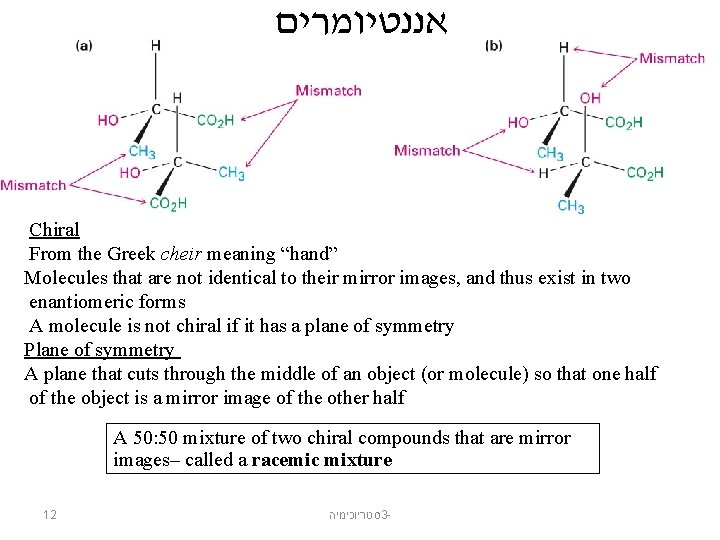
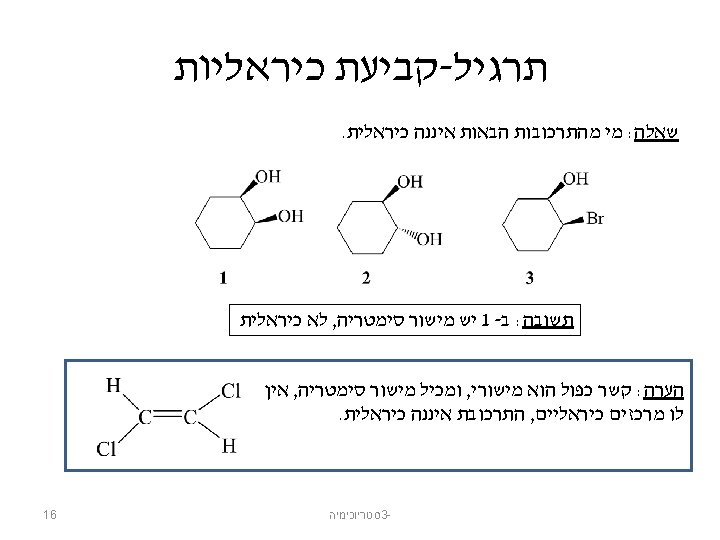
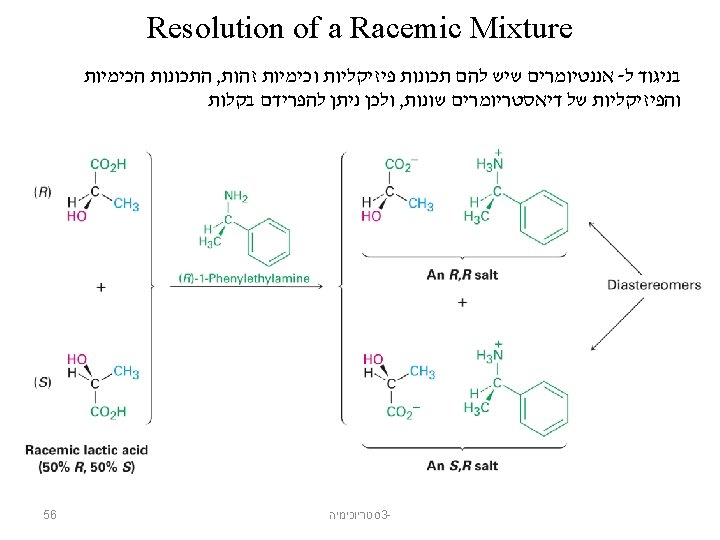
אננטיומרים Chiral From the Greek cheir meaning “hand” Molecules that are not identical to their mirror images, and thus exist in two enantiomeric forms A molecule is not chiral if it has a plane of symmetry Plane of symmetry A plane that cuts through the middle of an object (or molecule) so that one half of the object is a mirror image of the other half A 50: 50 mixture of two chiral compounds that are mirror images– called a racemic mixture 12 סטריוכימיה 3 -

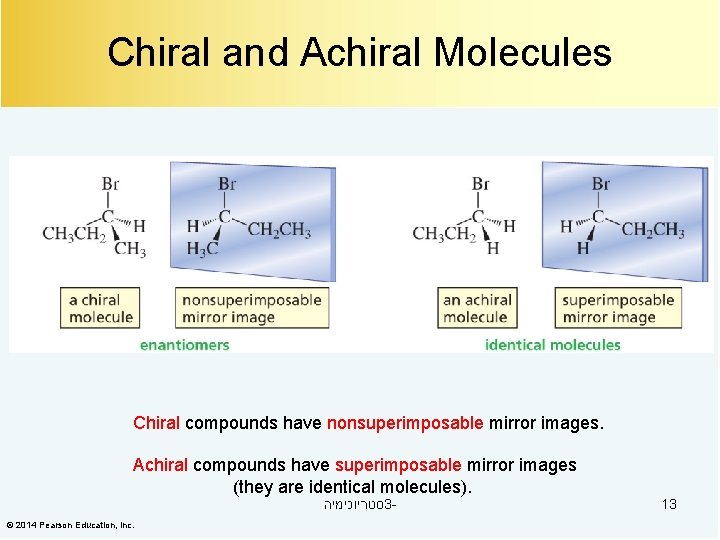
Chiral and Achiral Molecules Chiral compounds have nonsuperimposable mirror images. Achiral compounds have superimposable mirror images (they are identical molecules). סטריוכימיה 3 - © 2014 Pearson Education, Inc. 13

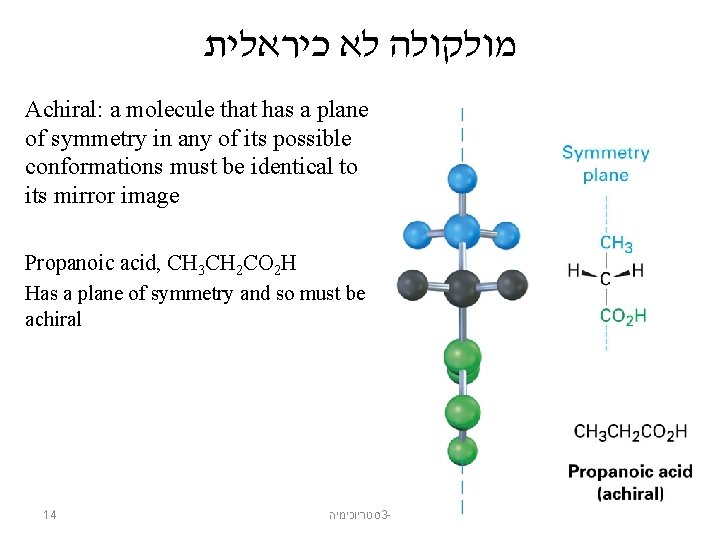
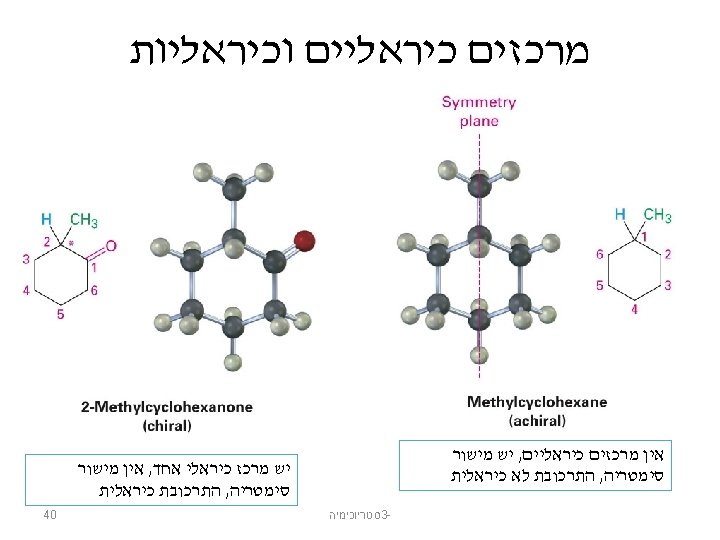
מולקולה לא כיראלית Achiral: a molecule that has a plane of symmetry in any of its possible conformations must be identical to its mirror image Propanoic acid, CH 3 CH 2 CO 2 H Has a plane of symmetry and so must be achiral 14 סטריוכימיה 3 -

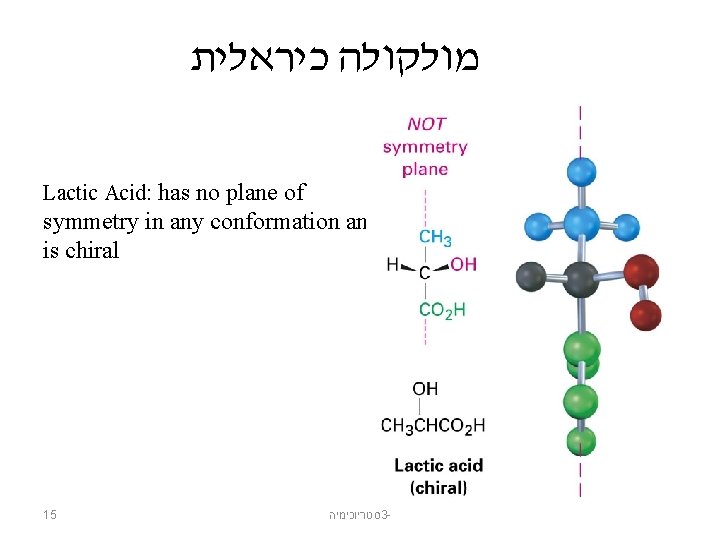
מולקולה כיראלית Lactic Acid: has no plane of symmetry in any conformation and is chiral 15 סטריוכימיה 3 -


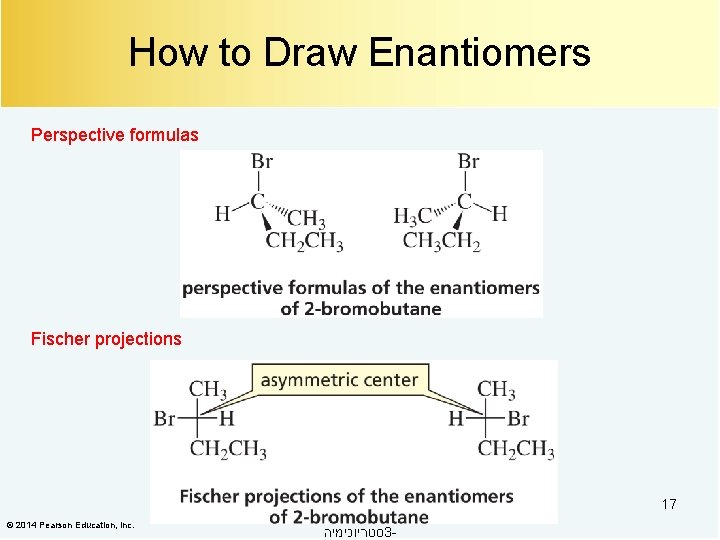
How to Draw Enantiomers Perspective formulas Fischer projections 17 © 2014 Pearson Education, Inc. סטריוכימיה 3 -

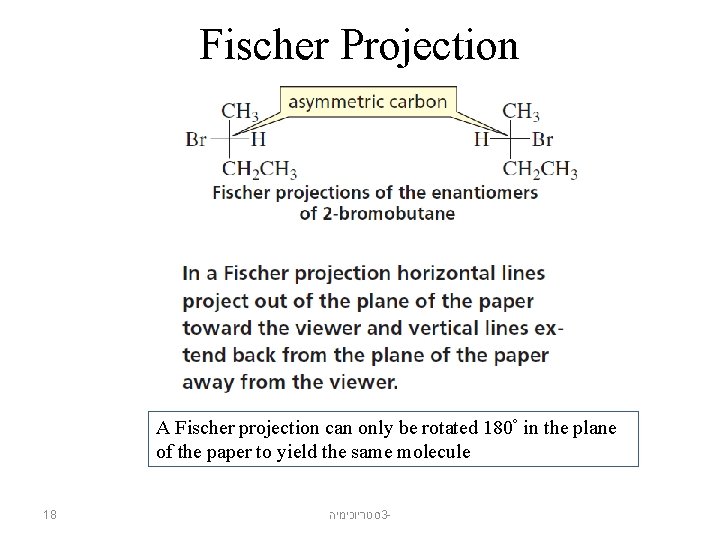
Fischer Projection A Fischer projection can only be rotated 180° in the plane of the paper to yield the same molecule 18 סטריוכימיה 3 -

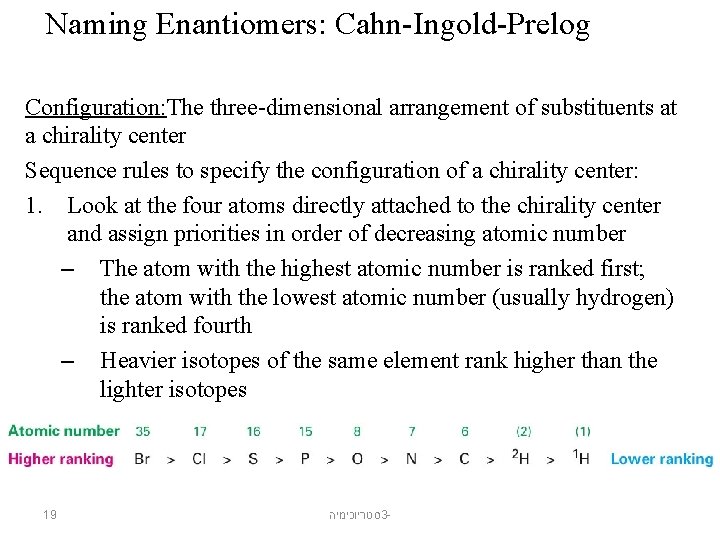
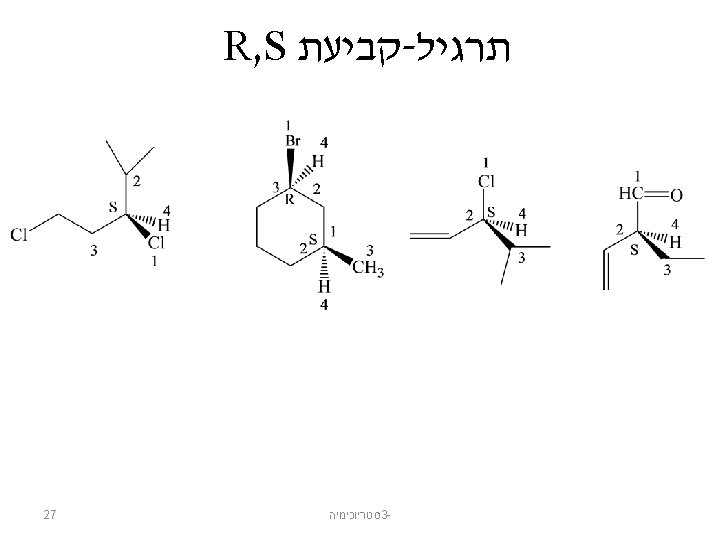
Naming Enantiomers: Cahn-Ingold-Prelog Configuration: The three-dimensional arrangement of substituents at a chirality center Sequence rules to specify the configuration of a chirality center: 1. Look at the four atoms directly attached to the chirality center and assign priorities in order of decreasing atomic number – The atom with the highest atomic number is ranked first; the atom with the lowest atomic number (usually hydrogen) is ranked fourth – Heavier isotopes of the same element rank higher than the lighter isotopes 19 סטריוכימיה 3 -

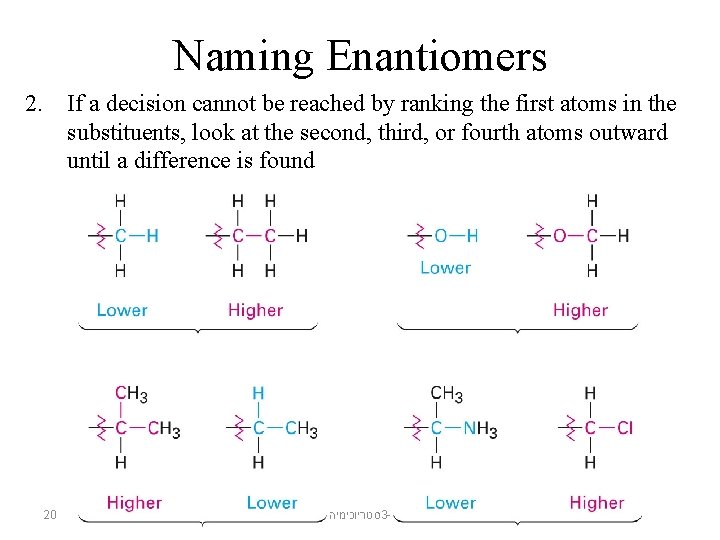
Naming Enantiomers 2. If a decision cannot be reached by ranking the first atoms in the substituents, look at the second, third, or fourth atoms outward until a difference is found 20 סטריוכימיה 3 -

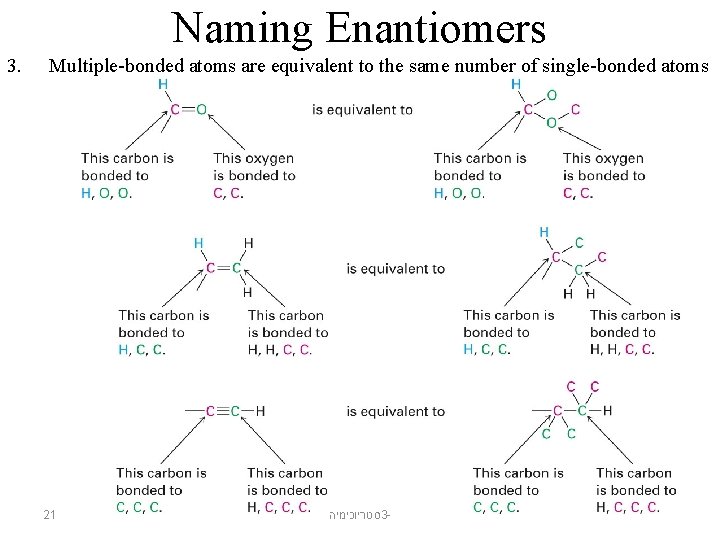
Naming Enantiomers 3. Multiple-bonded atoms are equivalent to the same number of single-bonded atoms 21 סטריוכימיה 3 -

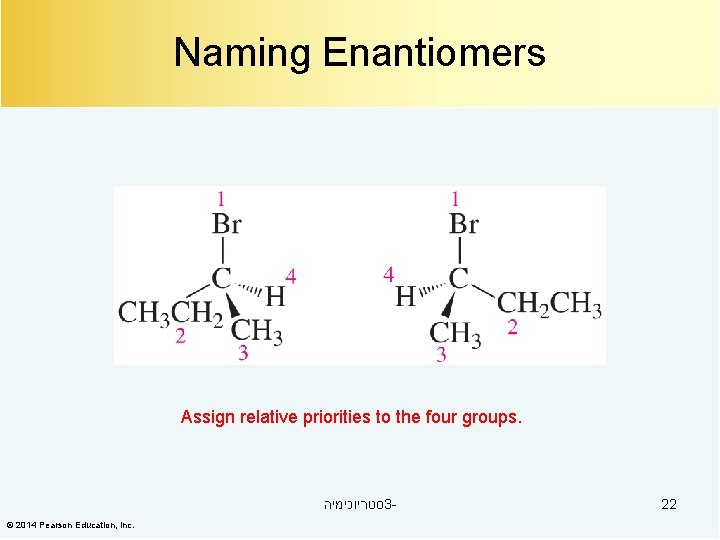
Naming Enantiomers Assign relative priorities to the four groups. סטריוכימיה 3© 2014 Pearson Education, Inc. 22

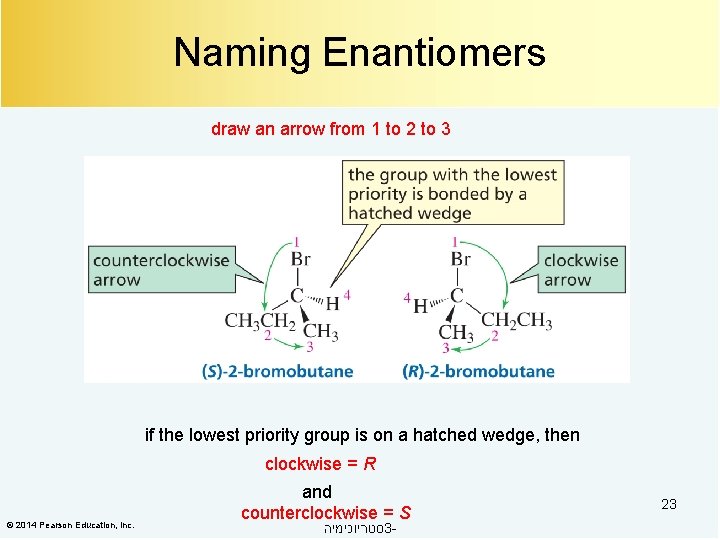
Naming Enantiomers draw an arrow from 1 to 2 to 3 if the lowest priority group is on a hatched wedge, then clockwise = R © 2014 Pearson Education, Inc. and counterclockwise = S סטריוכימיה 3 - 23

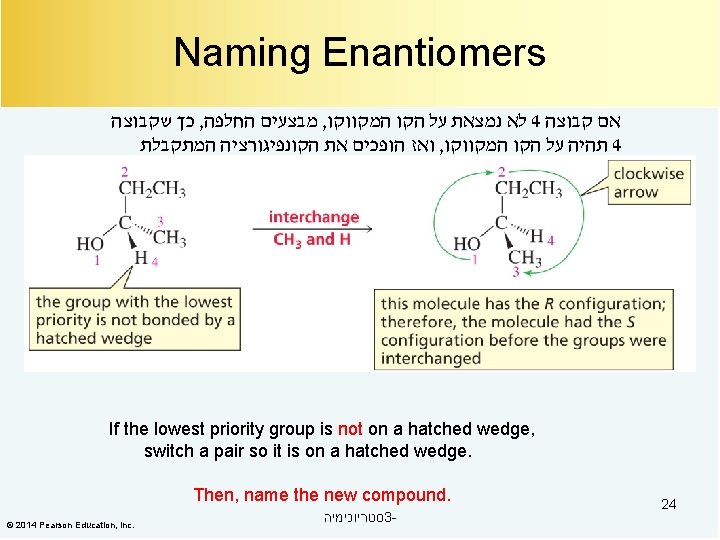
Naming Enantiomers כך שקבוצה , מבצעים החלפה , לא נמצאת על הקו המקווקו 4 אם קבוצה ואז הופכים את הקונפיגורציה המתקבלת , תהיה על הקו המקווקו 4 If the lowest priority group is not on a hatched wedge, switch a pair so it is on a hatched wedge. Then, name the new compound. © 2014 Pearson Education, Inc. סטריוכימיה 3 - 24

Naming Enantiomers if the lowest priority group is on a vertical bond, then clockwise = R and counterclockwise = S סטריוכימיה 3© 2014 Pearson Education, Inc. 25

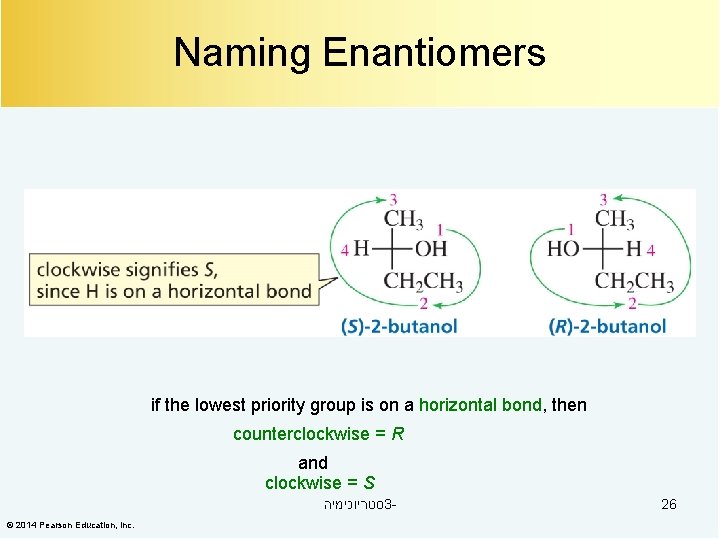
Naming Enantiomers if the lowest priority group is on a horizontal bond, then counterclockwise = R and clockwise = S סטריוכימיה 3© 2014 Pearson Education, Inc. 26


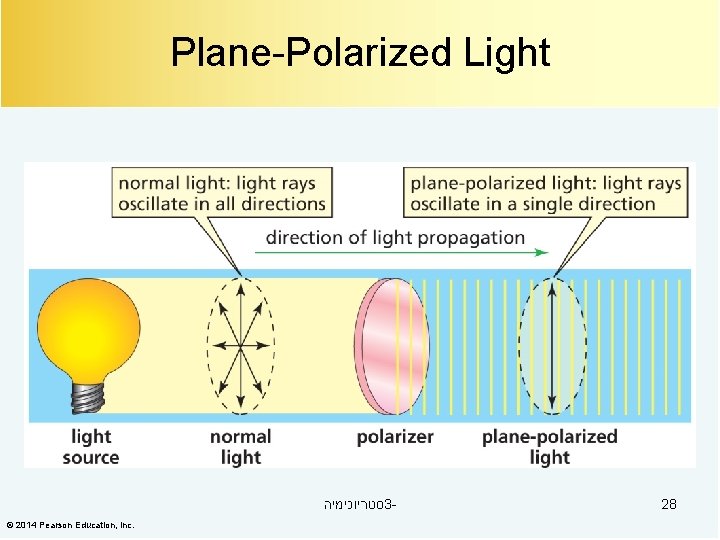
Plane-Polarized Light סטריוכימיה 3© 2014 Pearson Education, Inc. 28

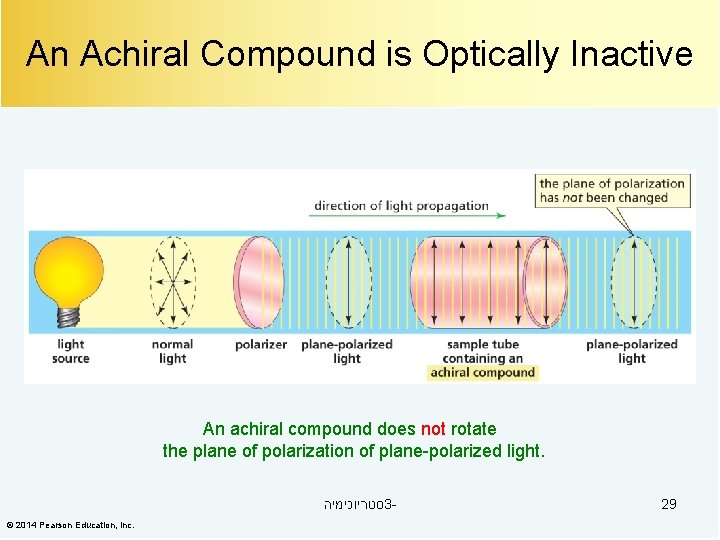
An Achiral Compound is Optically Inactive An achiral compound does not rotate the plane of polarization of plane-polarized light. סטריוכימיה 3© 2014 Pearson Education, Inc. 29

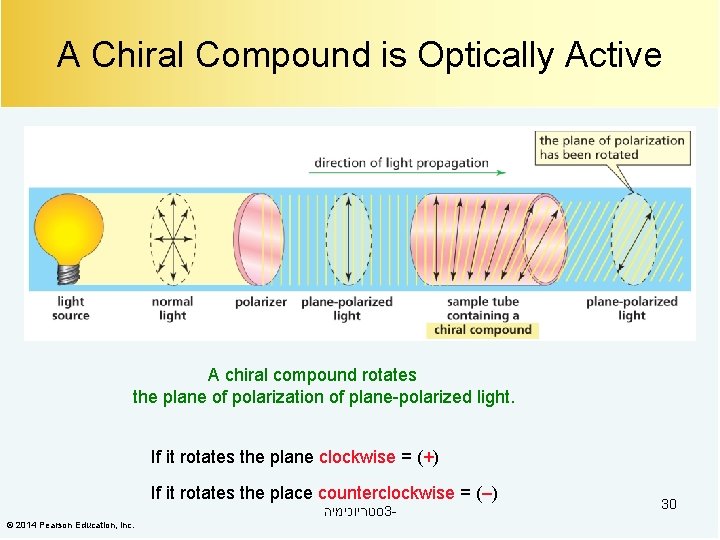
A Chiral Compound is Optically Active A chiral compound rotates the plane of polarization of plane-polarized light. If it rotates the plane clockwise = (+) If it rotates the place counterclockwise = (–) סטריוכימיה 3© 2014 Pearson Education, Inc. 30

R and S Versus (+) and (–) Some R enantiomers are (+) and some are (–). Some S enantiomers are (+) and some are (–). סטריוכימיה 3© 2014 Pearson Education, Inc. 31

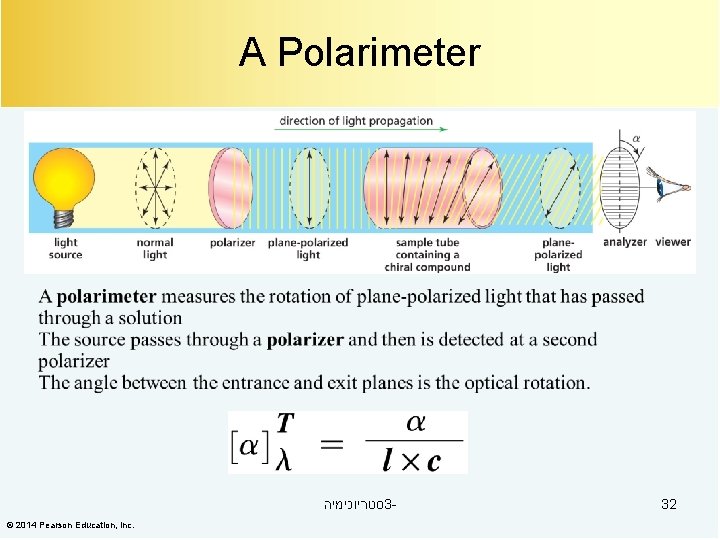
A Polarimeter סטריוכימיה 3© 2014 Pearson Education, Inc. 32
![אלפא ספציפי To have a basis for comparison define specific rotation D אלפא ספציפי To have a basis for comparison, define specific rotation, [ ]D](https://slidetodoc.com/presentation_image_h2/126e7f80e4bb691155a7e102ba9b3642/image-33.jpg)
אלפא ספציפי To have a basis for comparison, define specific rotation, [ ]D for an optically active compound The observed rotation (α) T is the temp in °C is the wavelength is the measured rotation in degrees l is the path length in decimeters c is the concentration in grams per m. L 33 סטריוכימיה 3 - Specific rotation is that observed for 1 g/m. L in solution in cell with a 10 cm path using light from sodium metal vapor (589 nm, D-line)


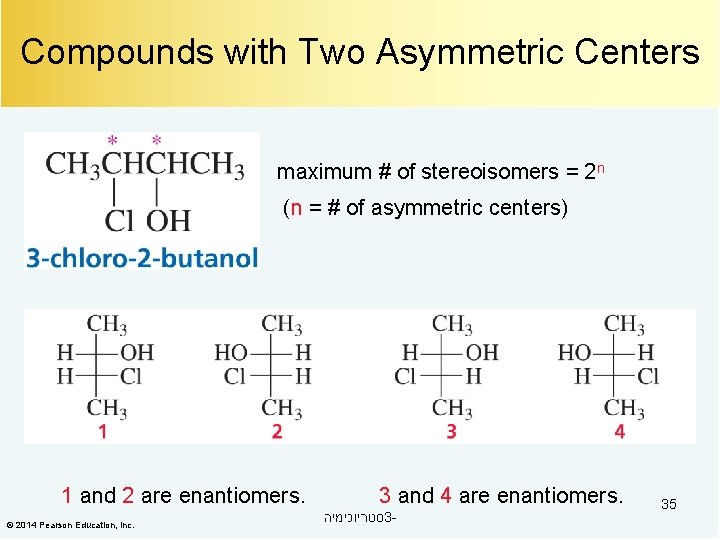
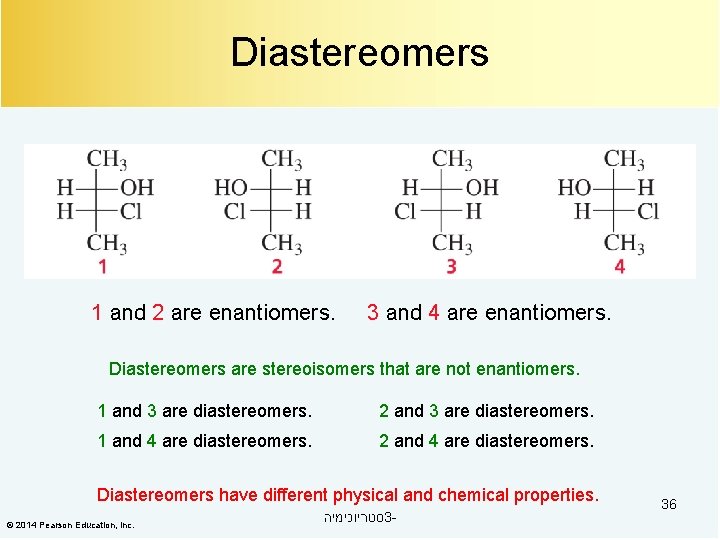
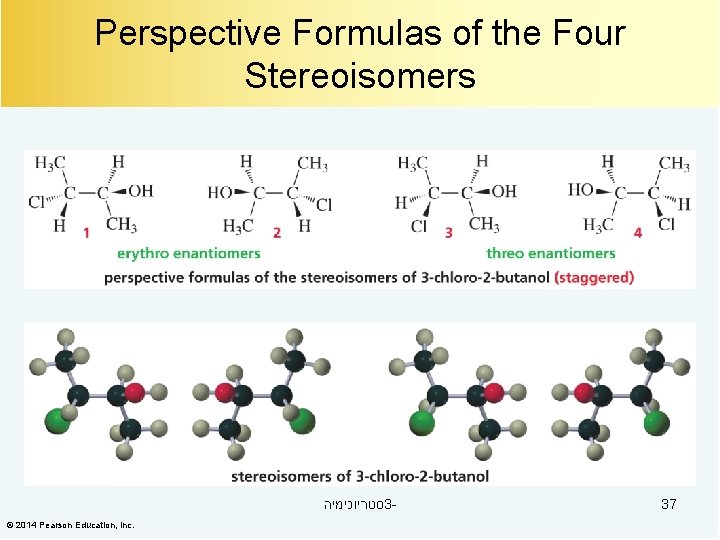
Compounds with Two Asymmetric Centers maximum # of stereoisomers = 2 n (n = # of asymmetric centers) 1 and 2 are enantiomers. © 2014 Pearson Education, Inc. 3 and 4 are enantiomers. סטריוכימיה 3 - 35

Diastereomers 1 and 2 are enantiomers. 3 and 4 are enantiomers. Diastereomers are stereoisomers that are not enantiomers. 1 and 3 are diastereomers. 2 and 3 are diastereomers. 1 and 4 are diastereomers. 2 and 4 are diastereomers. Diastereomers have different physical and chemical properties. © 2014 Pearson Education, Inc. סטריוכימיה 3 - 36

Perspective Formulas of the Four Stereoisomers סטריוכימיה 3© 2014 Pearson Education, Inc. 37

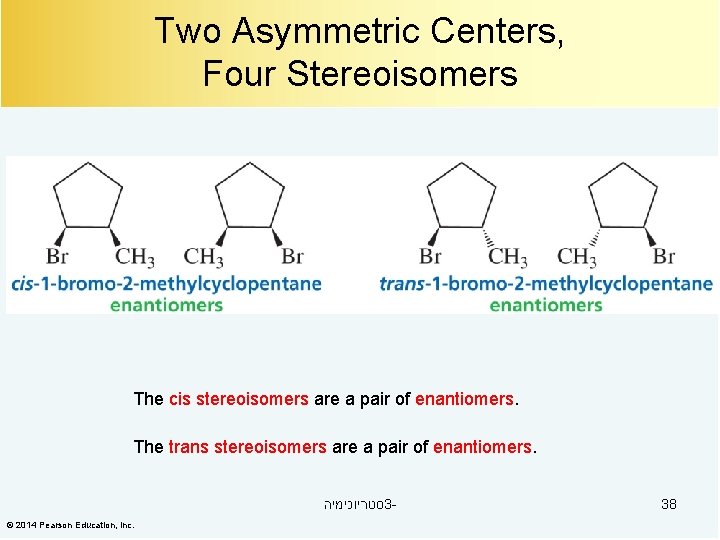
Two Asymmetric Centers, Four Stereoisomers The cis stereoisomers are a pair of enantiomers. The trans stereoisomers are a pair of enantiomers. סטריוכימיה 3© 2014 Pearson Education, Inc. 38

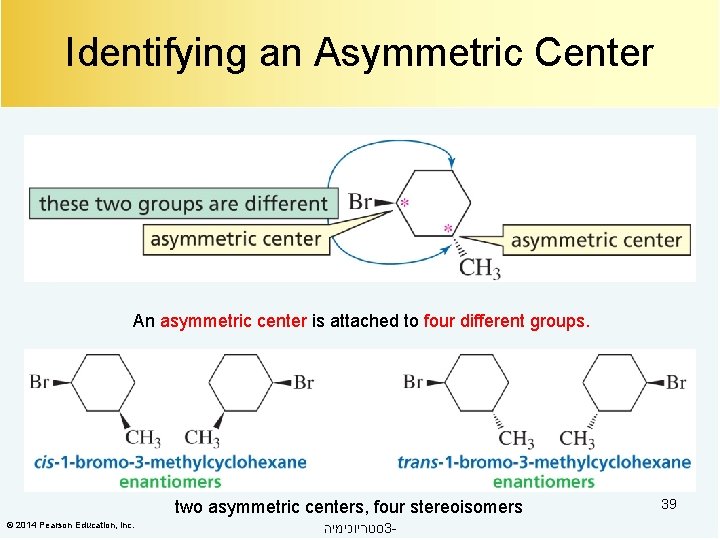
Identifying an Asymmetric Center An asymmetric center is attached to four different groups. two asymmetric centers, four stereoisomers © 2014 Pearson Education, Inc. סטריוכימיה 3 - 39



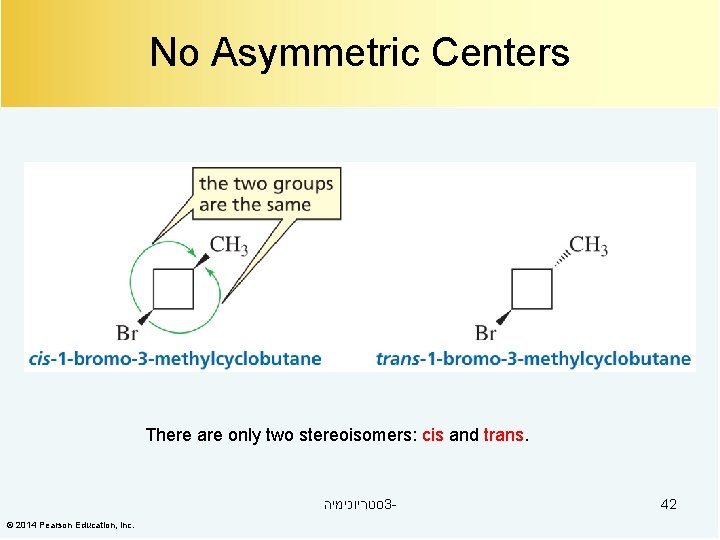
No Asymmetric Centers There are only two stereoisomers: cis and trans. סטריוכימיה 3© 2014 Pearson Education, Inc. 42

No Asymmetric Centers There are only two stereoisomers: cis and trans. סטריוכימיה 3© 2014 Pearson Education, Inc. 43

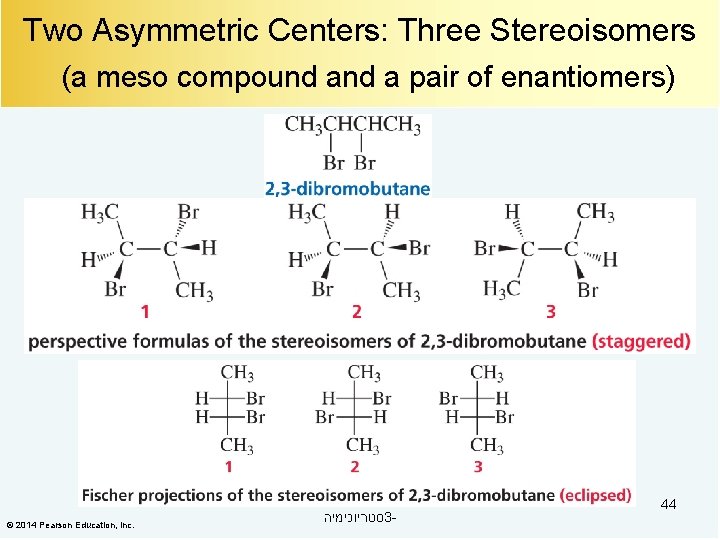
Two Asymmetric Centers: Three Stereoisomers (a meso compound a pair of enantiomers) © 2014 Pearson Education, Inc. סטריוכימיה 3 - 44

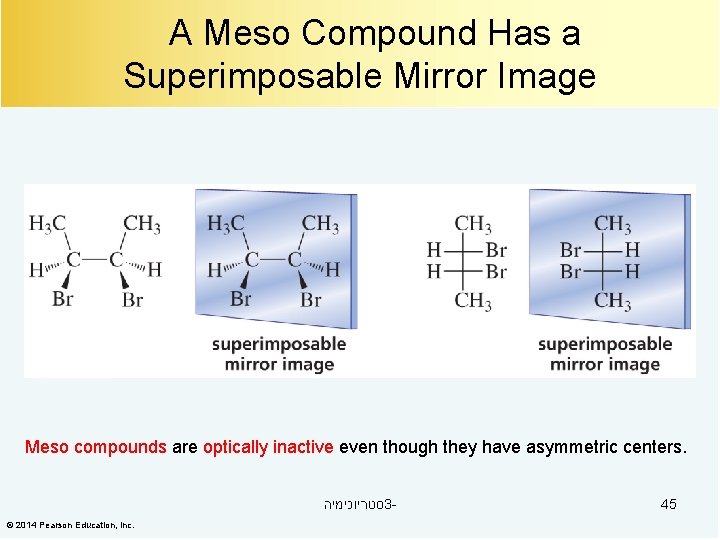
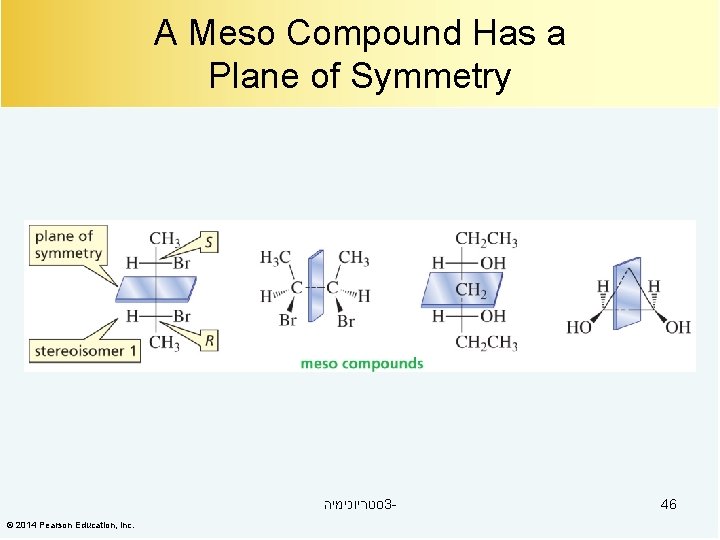
A Meso Compound Has a Superimposable Mirror Image Meso compounds are optically inactive even though they have asymmetric centers. סטריוכימיה 3© 2014 Pearson Education, Inc. 45

A Meso Compound Has a Plane of Symmetry סטריוכימיה 3© 2014 Pearson Education, Inc. 46

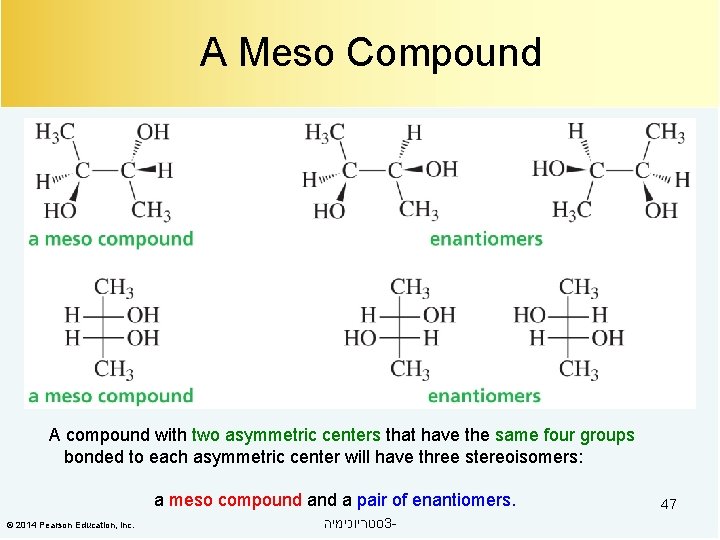
A Meso Compound A compound with two asymmetric centers that have the same four groups bonded to each asymmetric center will have three stereoisomers: a meso compound a pair of enantiomers. © 2014 Pearson Education, Inc. סטריוכימיה 3 - 47

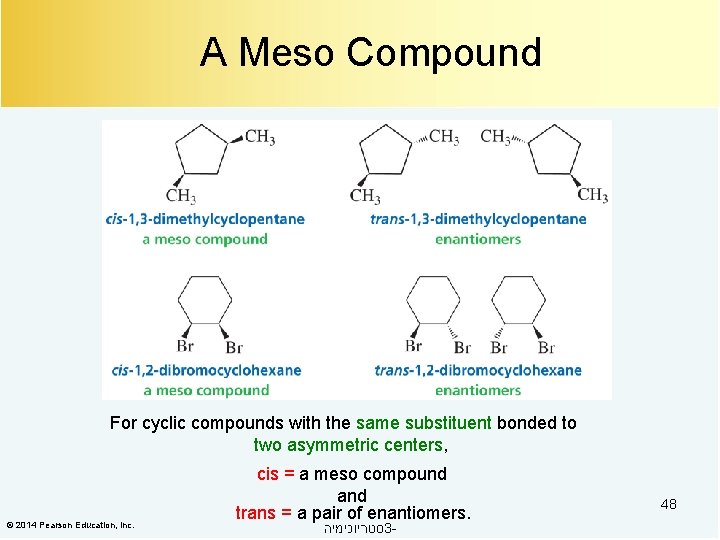
A Meso Compound For cyclic compounds with the same substituent bonded to two asymmetric centers, © 2014 Pearson Education, Inc. cis = a meso compound and trans = a pair of enantiomers. סטריוכימיה 3 - 48

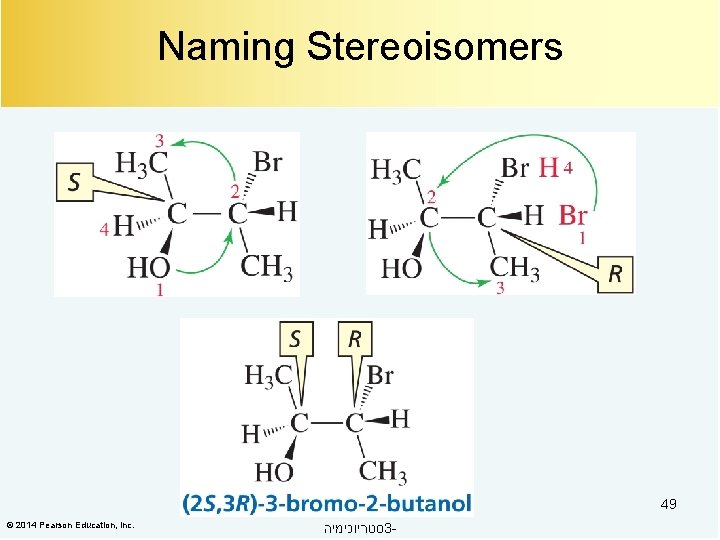
Naming Stereoisomers 49 © 2014 Pearson Education, Inc. סטריוכימיה 3 -

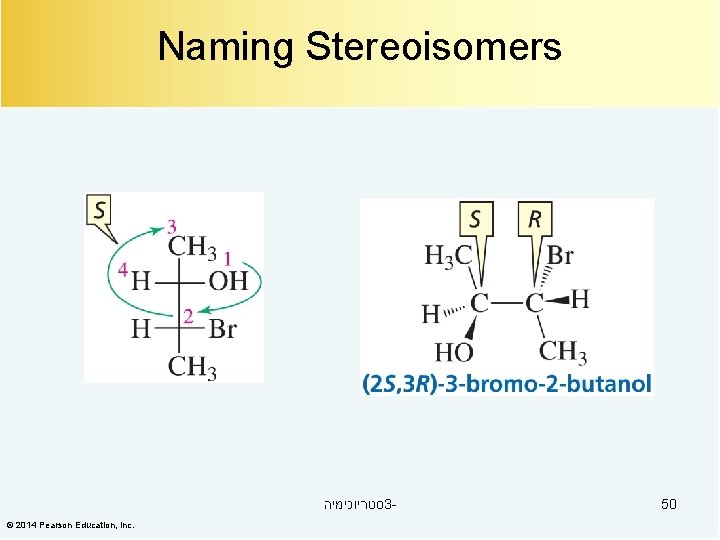
Naming Stereoisomers סטריוכימיה 3© 2014 Pearson Education, Inc. 50

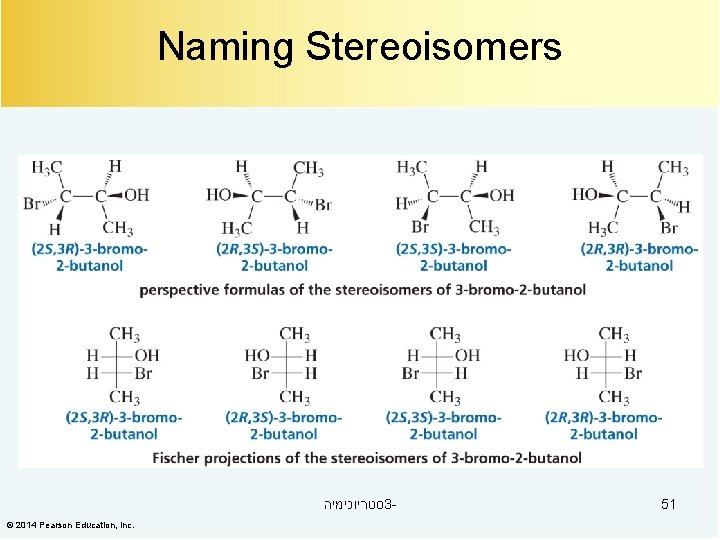
Naming Stereoisomers סטריוכימיה 3© 2014 Pearson Education, Inc. 51

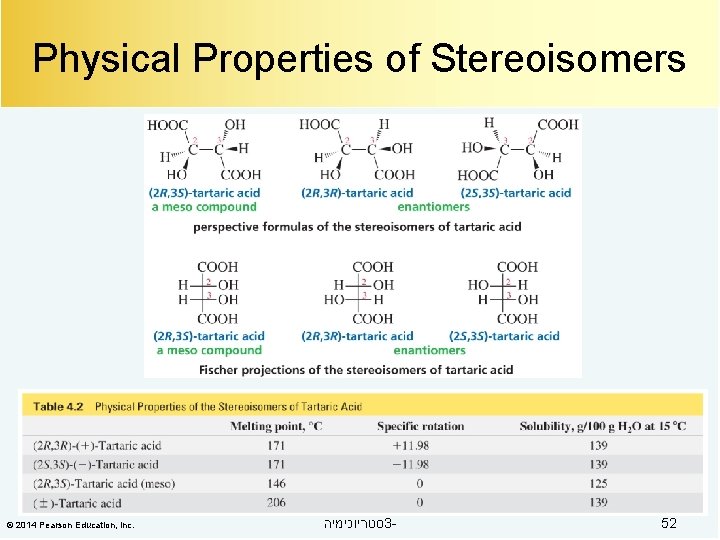
Physical Properties of Stereoisomers © 2014 Pearson Education, Inc. סטריוכימיה 3 - 52

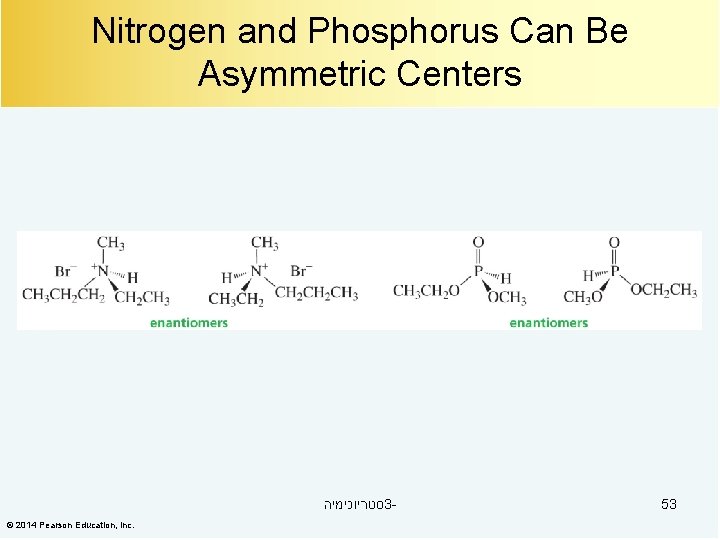
Nitrogen and Phosphorus Can Be Asymmetric Centers סטריוכימיה 3© 2014 Pearson Education, Inc. 53

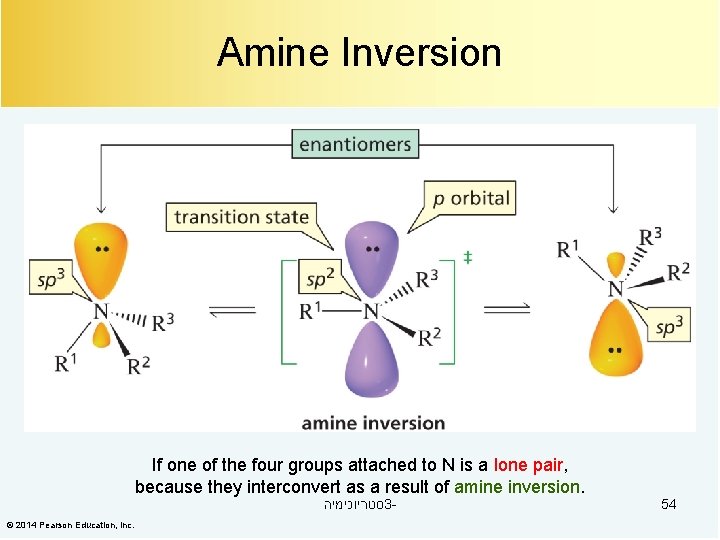
Amine Inversion If one of the four groups attached to N is a lone pair, because they interconvert as a result of amine inversion. סטריוכימיה 3 - © 2014 Pearson Education, Inc. 54



The Stereospecificity of an Enzyme-Catalyzed Reaction Allows Enantiomers to Be Separated © 2014 Pearson Education, Inc. סטריוכימיה 3 - 57

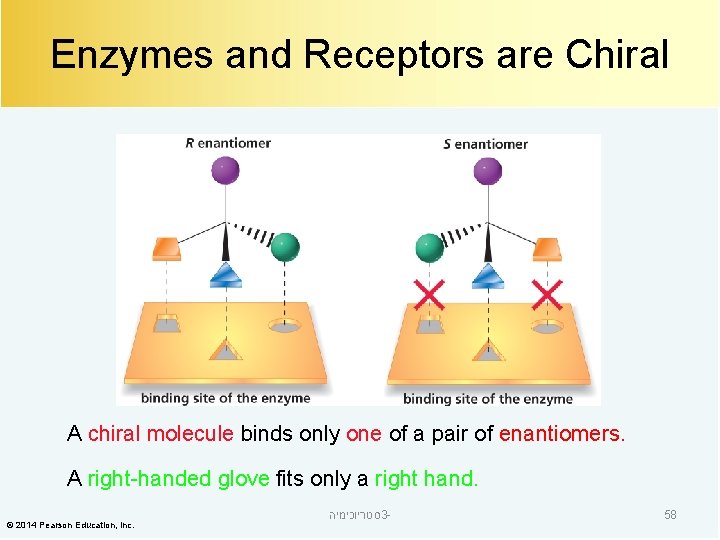
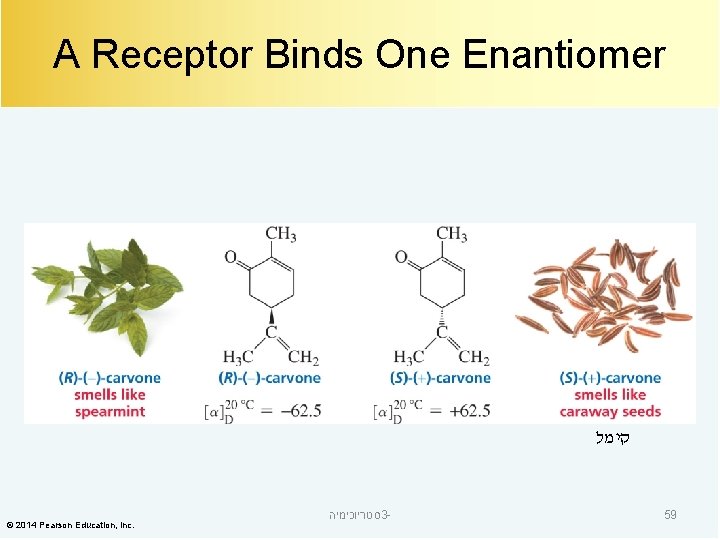
Enzymes and Receptors are Chiral A chiral molecule binds only one of a pair of enantiomers. A right-handed glove fits only a right hand. © 2014 Pearson Education, Inc. סטריוכימיה 3 - 58

A Receptor Binds One Enantiomer קימל © 2014 Pearson Education, Inc. סטריוכימיה 3 - 59
