Are different compounds with the same molecular formula

- Slides: 20

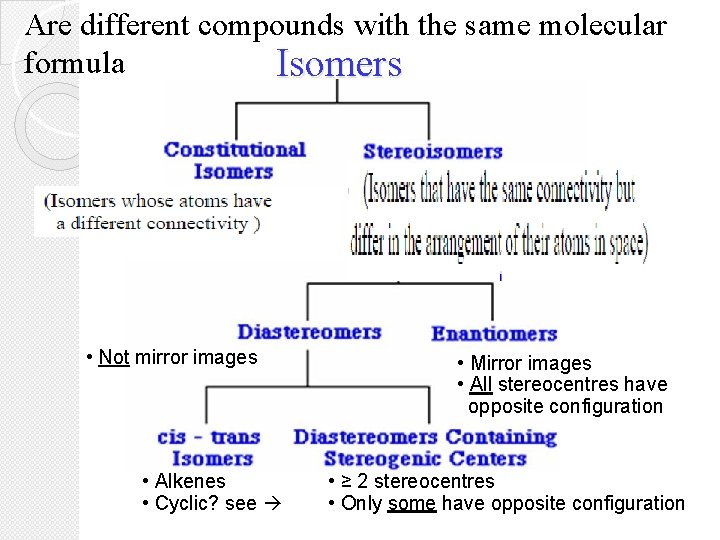
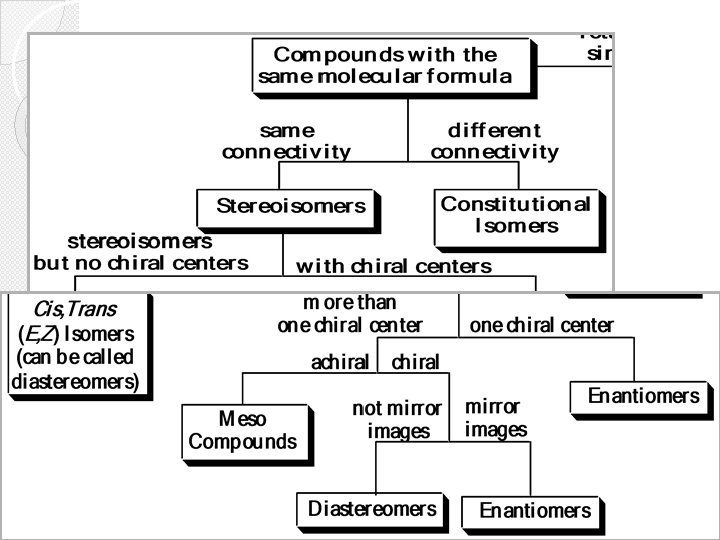
Are different compounds with the same molecular formula Isomers • Not mirror images • Alkenes • Cyclic? see • Mirror images • All stereocentres have opposite configuration • ≥ 2 stereocentres • Only some have opposite configuration

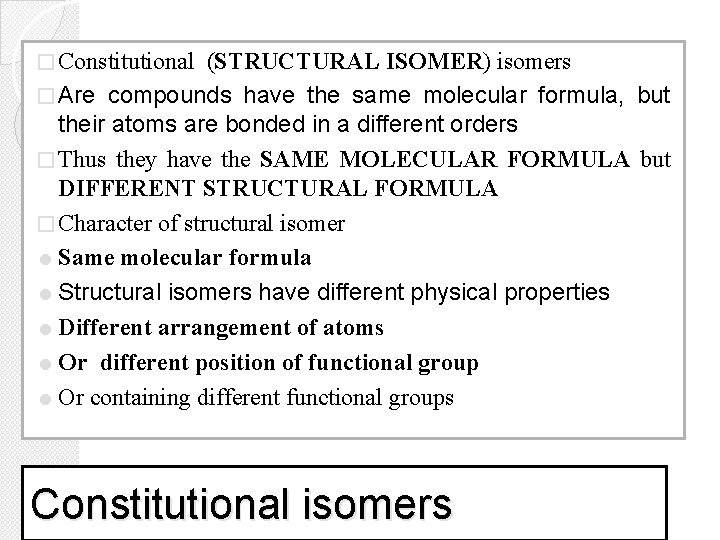
� Constitutional (STRUCTURAL ISOMER) isomers � Are compounds have the same molecular formula, but their atoms are bonded in a different orders � Thus they have the SAME MOLECULAR FORMULA but DIFFERENT STRUCTURAL FORMULA � Character of structural isomer = Same molecular formula = Structural isomers have different physical properties = Different arrangement of atoms = Or different position of functional group = Or containing different functional groups Constitutional isomers

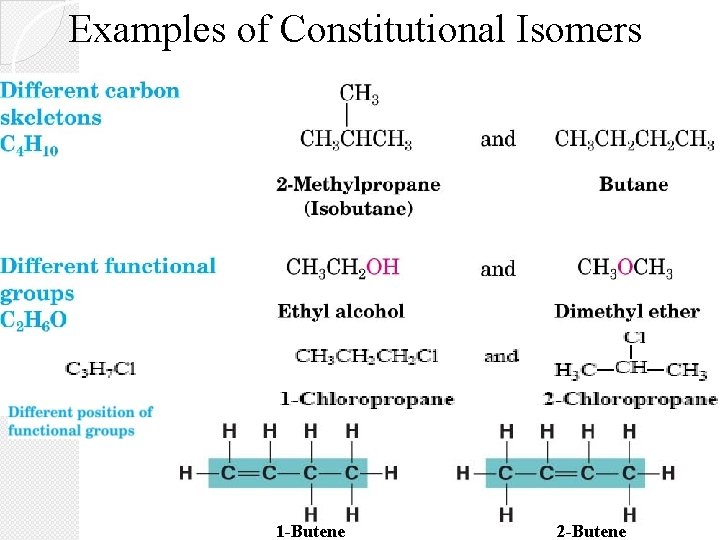
Examples of Constitutional Isomers 1 -Butene 2 -Butene

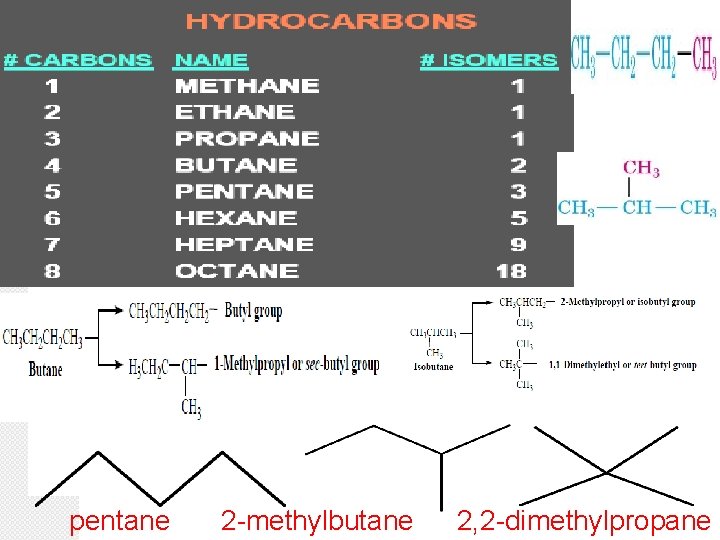
pentane 2 -methylbutane 2, 2 -dimethylpropane

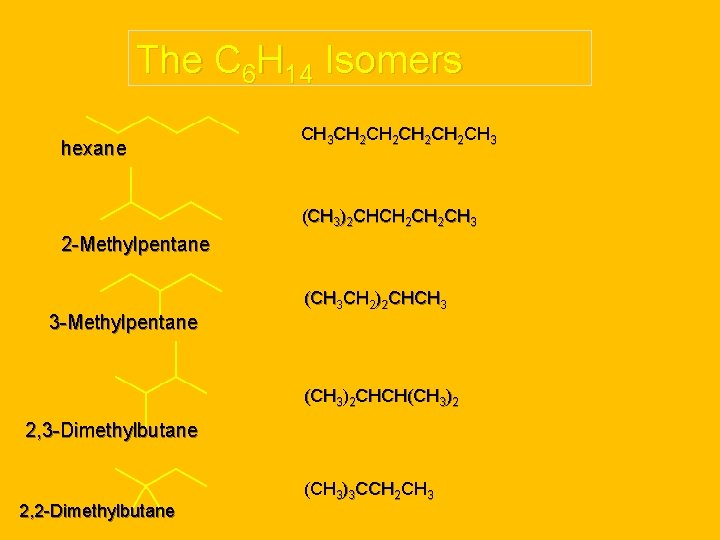
The C 6 H 14 Isomers hexane CH 3 CH 2 CH 2 CH 3 (CH 3)2 CHCH 2 CH 3 2 -Methylpentane 3 -Methylpentane (CH 3 CH 2)2 CHCH 3 (CH 3)2 CHCH(CH 3)2 2, 3 -Dimethylbutane 2, 2 -Dimethylbutane (CH 3)3 CCH 2 CH 3

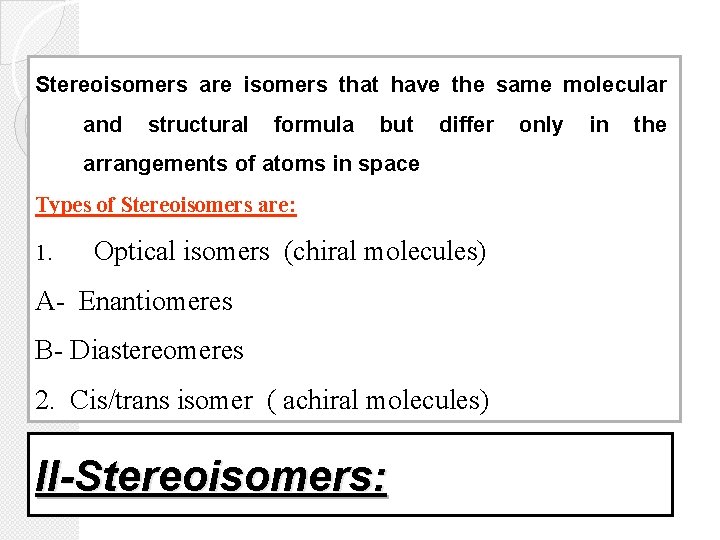
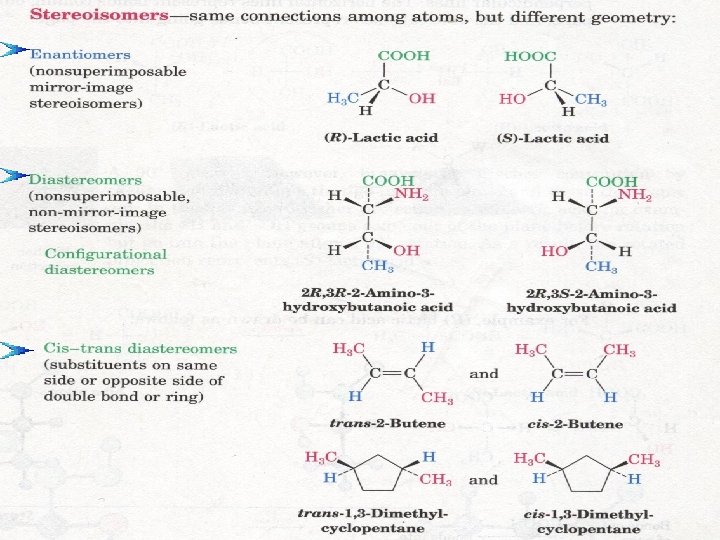
Stereoisomers are isomers that have the same molecular and structural formula but differ arrangements of atoms in space Types of Stereoisomers are: 1. Optical isomers (chiral molecules) A- Enantiomeres B- Diastereomeres 2. Cis/trans isomer ( achiral molecules) II-Stereoisomers: only in the

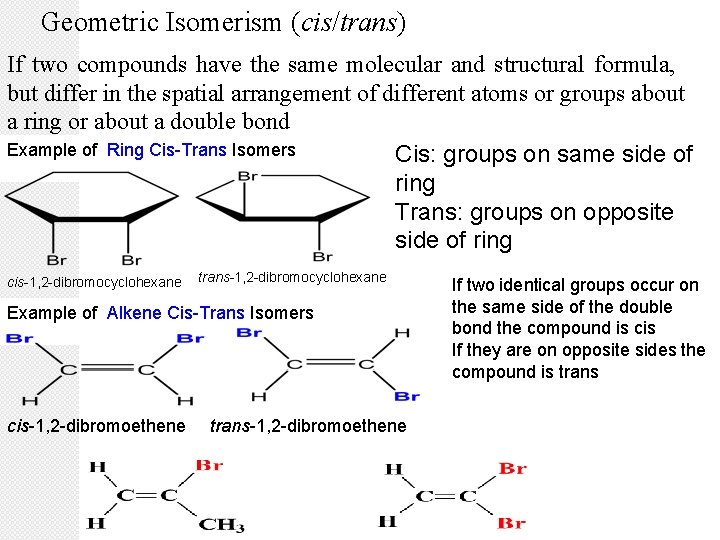
Geometric Isomerism (cis/trans) If two compounds have the same molecular and structural formula, but differ in the spatial arrangement of different atoms or groups about a ring or about a double bond Example of Ring Cis-Trans Isomers Cis: groups on same side of ring Trans: groups on opposite side of ring cis-1, 2 -dibromocyclohexane trans-1, 2 -dibromocyclohexane Example of Alkene Cis-Trans Isomers cis-1, 2 -dibromoethene trans-1, 2 -dibromoethene If two identical groups occur on the same side of the double bond the compound is cis If they are on opposite sides the compound is trans

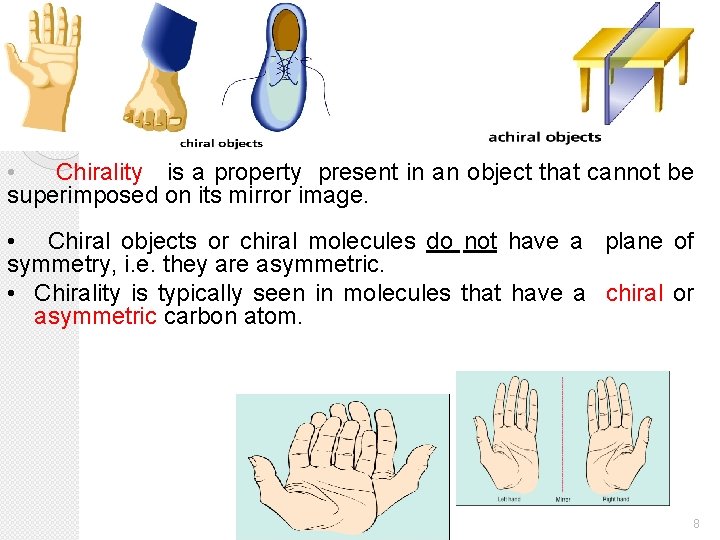
• Chirality is a property present in an object that cannot be superimposed on its mirror image. • Chiral objects or chiral molecules do not have a plane of symmetry, i. e. they are asymmetric. • Chirality is typically seen in molecules that have a chiral or asymmetric carbon atom. 8

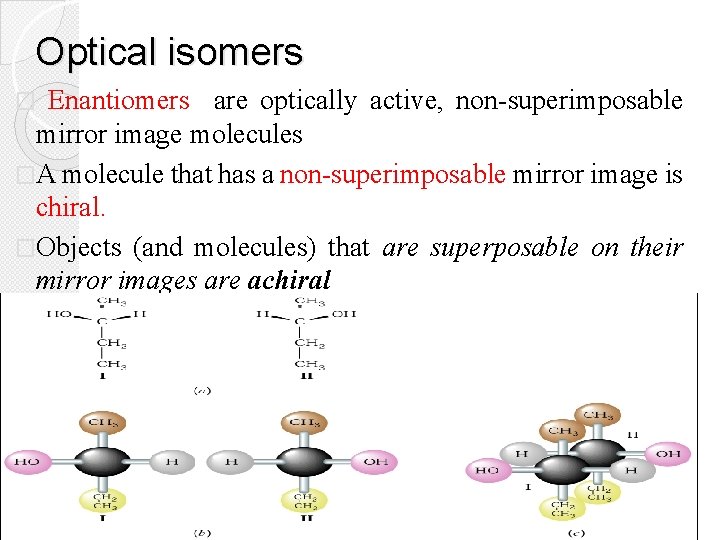
Optical isomers Enantiomers are optically active, non-superimposable mirror image molecules �A molecule that has a non-superimposable mirror image is chiral. �Objects (and molecules) that are superposable on their mirror images are achiral �

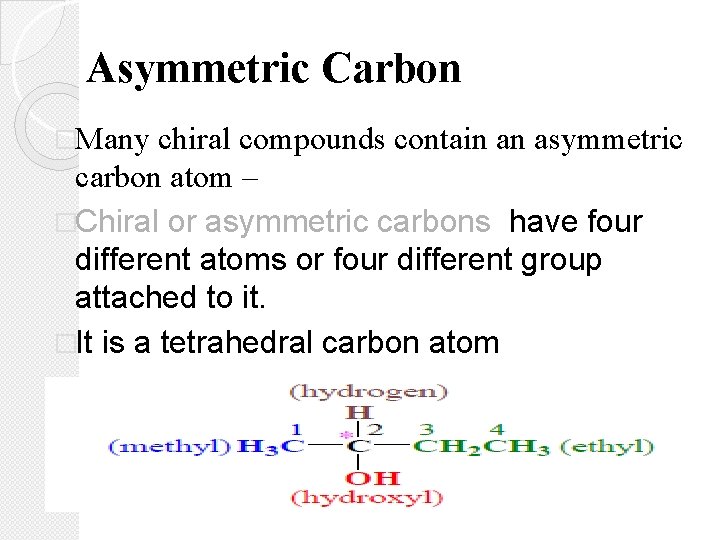
Asymmetric Carbon �Many chiral compounds contain an asymmetric carbon atom – �Chiral or asymmetric carbons have four different atoms or four different group attached to it. �It is a tetrahedral carbon atom

Optical activity 1. Enantiomers are optically active; that is, they rotate plane-polarized light. 2. One isomer of an enantiomeric pair rotates polarized light to the left (counterclockwise). The other isomer rotates polarized light to the right (clockwise). The degree of rotation is the same but in opposite directions. 3. Rotation of polarized light to the right is indicated by a (+) dextrorotatory 4. Rotation of polarized light to the left is indiated by a (-) levorotatory. 11

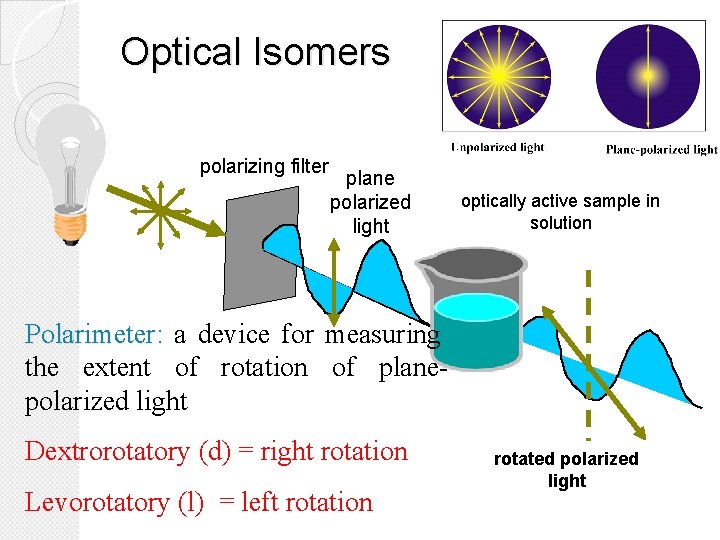
Optical Isomers polarizing filter plane polarized light optically active sample in solution Polarimeter: a device for measuring the extent of rotation of planepolarized light Dextrorotatory (d) = right rotation Levorotatory (l) = left rotation rotated polarized light

�RACEMIC MIXTURE = equal amounts of two enantiomers; These mixtures are inactive. optically �Because two enantiomers rotate plane-polarized light to an equal extent but in opposite directions, the rotations cancel, and no rotation is observed. �RESOLUTION: Separation of a racemic mixture into its optically active forms (pair of enantiomers)

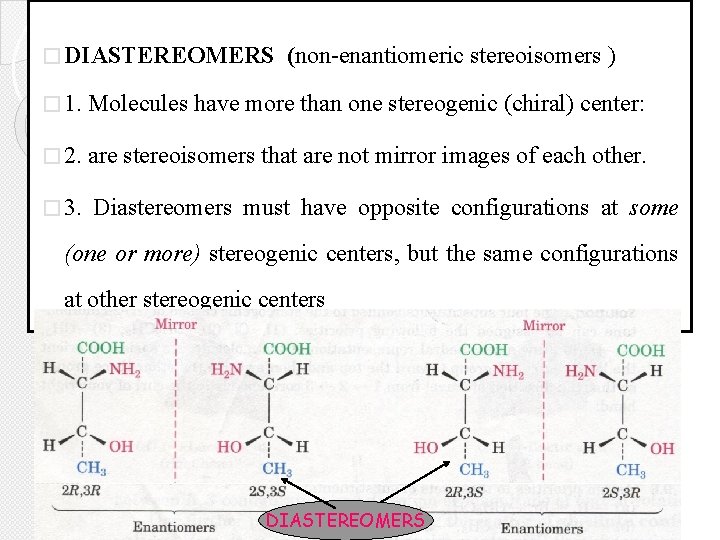
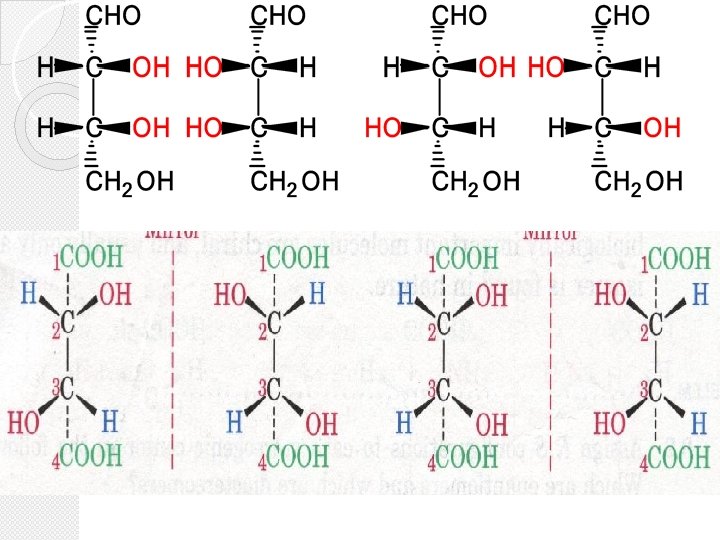
� DIASTEREOMERS (non-enantiomeric stereoisomers ) � 1. Molecules have more than one stereogenic (chiral) center: � 2. are stereoisomers that are not mirror images of each other. � 3. Diastereomers must have opposite configurations at some (one or more) stereogenic centers, but the same configurations at other stereogenic centers DIASTEREOMERS

Enantiomers & Diastereomers �For a molecule with 1 chiral center, 21 = 2 stereoisomers are possible �For a molecule with 2 chiral centers, a maximum of 22 = 4 stereoisomers are possible �For a molecule with n chiral centers, a maximum of 2 n stereoisomers are possible

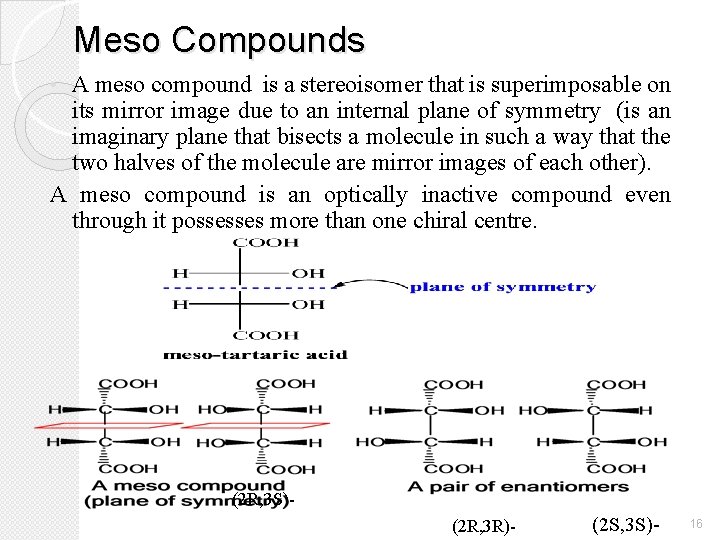
Meso Compounds A meso compound is a stereoisomer that is superimposable on its mirror image due to an internal plane of symmetry (is an imaginary plane that bisects a molecule in such a way that the two halves of the molecule are mirror images of each other). A meso compound is an optically inactive compound even through it possesses more than one chiral centre. • (2 R, 3 S)(2 R, 3 R)- (2 S, 3 S)- 16




� 1) All but one of the 20 amino acids that make up proteins are chiral and left-handed. � 2) The molecules of natural sugars are almost all classified as right-handed (R configuration). � 3) the active site of enzyme is chiral, and only one enantiomer of a chiral reactant fits it �Thus Enzymes are capable of distinguishing between stereoisomers The biological importance of chirality