Differential diagnosis and treatment strategy of neuromuscular disorders


















- Slides: 69

Differential diagnosis and treatment strategy of neuromuscular disorders 2017住院醫師教育課程 中醫大附醫 郭育呈 20170820

Outline • Classification and clinical manifestations of dysimmune neuropathies • Diagnosis of dysimmune neuropathies • Treatment of dysimmune neuropathies • Autoimmune myasthenia gravis • ACh. R Ab, Mu. SK Ab, LRP 4 Ab • Lambert-Eaton myasthenic syndrome (LEMS) 2

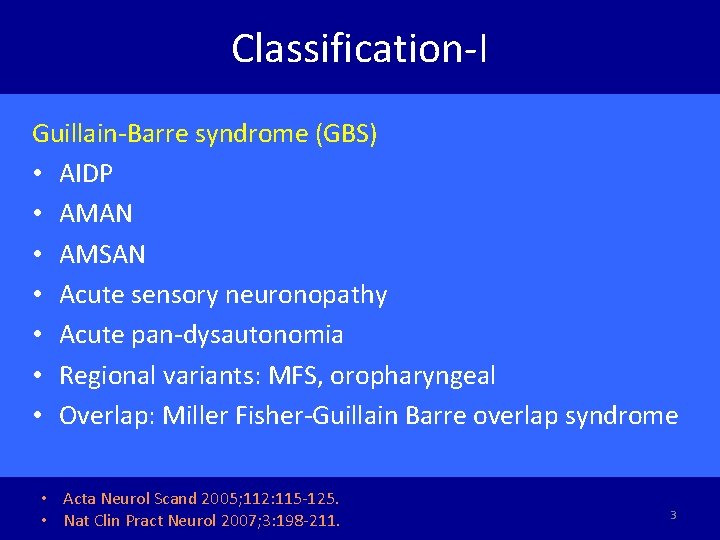
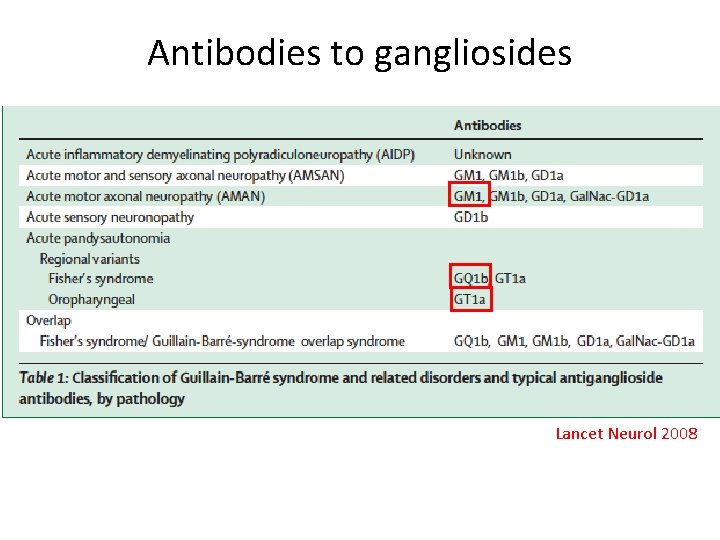
Classification-I Guillain-Barre syndrome (GBS) • AIDP • AMAN • AMSAN • Acute sensory neuronopathy • Acute pan-dysautonomia • Regional variants: MFS, oropharyngeal • Overlap: Miller Fisher-Guillain Barre overlap syndrome • Acta Neurol Scand 2005; 112: 115 -125. • Nat Clin Pract Neurol 2007; 3: 198 -211. 3

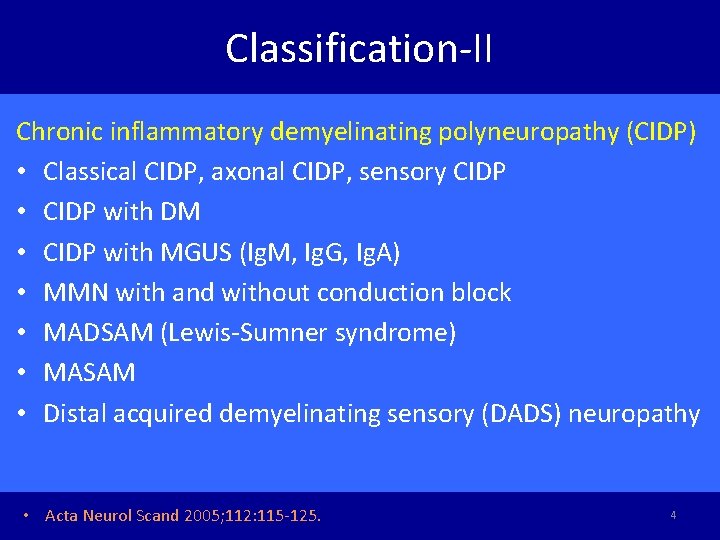
Classification-II Chronic inflammatory demyelinating polyneuropathy (CIDP) • Classical CIDP, axonal CIDP, sensory CIDP • CIDP with DM • CIDP with MGUS (Ig. M, Ig. G, Ig. A) • MMN with and without conduction block • MADSAM (Lewis-Sumner syndrome) • MASAM • Distal acquired demyelinating sensory (DADS) neuropathy • Acta Neurol Scand 2005; 112: 115 -125. 4

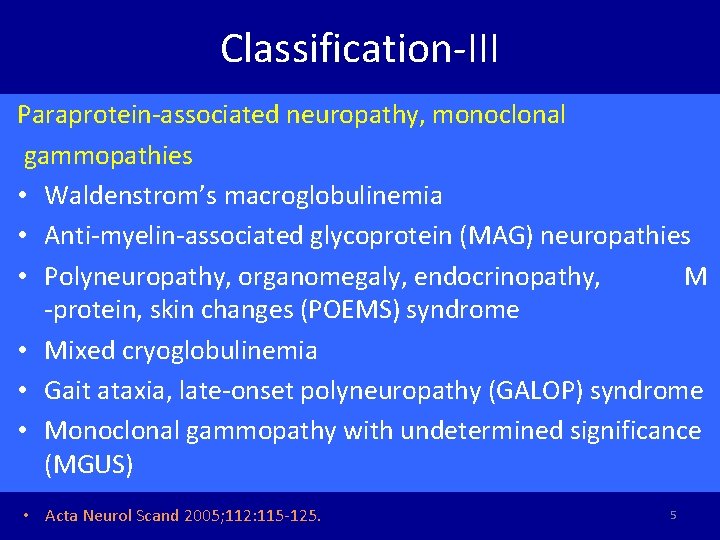
Classification-III Paraprotein-associated neuropathy, monoclonal gammopathies • Waldenstrom’s macroglobulinemia • Anti-myelin-associated glycoprotein (MAG) neuropathies • Polyneuropathy, organomegaly, endocrinopathy, M -protein, skin changes (POEMS) syndrome • Mixed cryoglobulinemia • Gait ataxia, late-onset polyneuropathy (GALOP) syndrome • Monoclonal gammopathy with undetermined significance (MGUS) • Acta Neurol Scand 2005; 112: 115 -125. 5

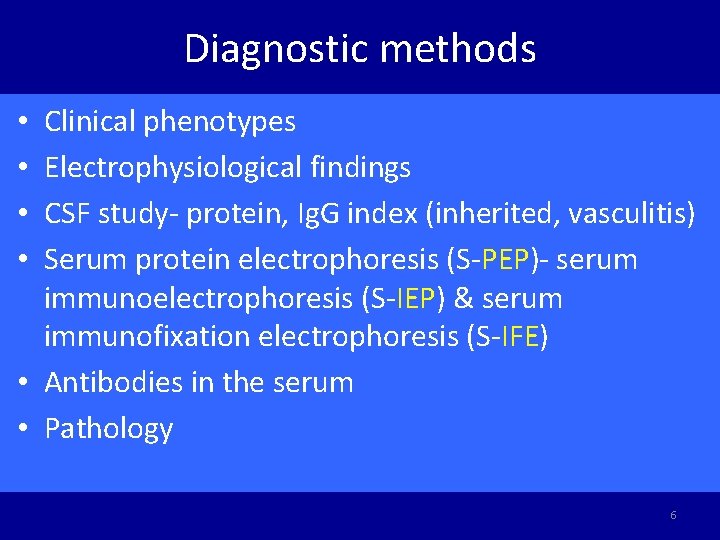
Diagnostic methods Clinical phenotypes Electrophysiological findings CSF study- protein, Ig. G index (inherited, vasculitis) Serum protein electrophoresis (S-PEP)- serum immunoelectrophoresis (S-IEP) & serum immunofixation electrophoresis (S-IFE) • Antibodies in the serum • Pathology • • 6

Autoantibodies in the serum • Monoclonal antibodies without identifiable antigenic targets (M proteins) • Monoclonal and polyclonal autoantibodies that bind to specific neural components • Diagnostic utility • Provide clues to the pathogenesis of neuropathic syndrome • Suggest specific avenues of therapy 7

Guillain Barre syndrome • GBS always follows an infection as URI or GI infection • Campylobacter jejuni, CMV, EBV, mycoplasma pneumonia • Vaccination and stress events debated • 25% artificial ventilation (vital capacity < 15 -20 ml/kg) • 2/3 ANS dysfunction • Urinary retention and constipation are unusual at the onset of the disease but commonly develop at the nadir of the disease. • Autoimmunity Reviews 2014; 13: 525 -530. 8

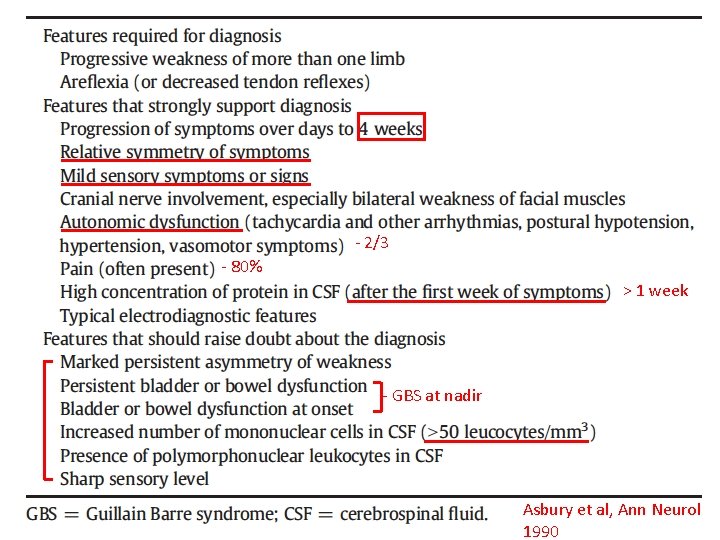
- 2/3 - 80% > 1 week - GBS at nadir Asbury et al, Ann Neurol 1990

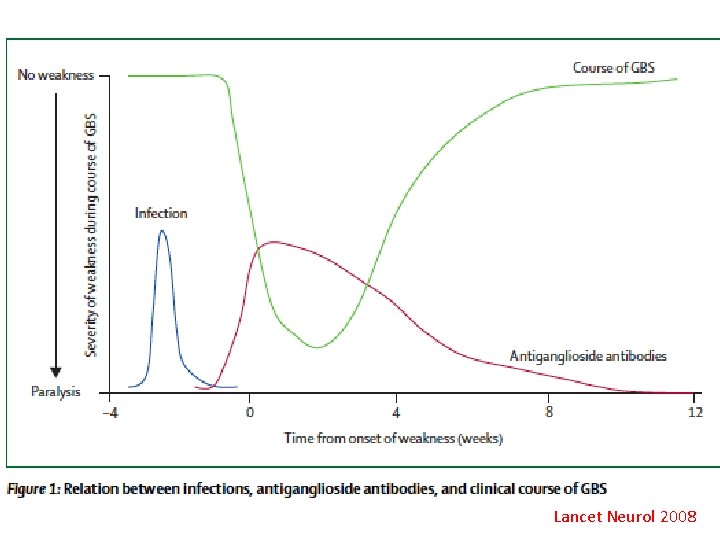
Lancet Neurol 2008

Antibodies to gangliosides Lancet Neurol 2008

Guillain Barre syndrome • 20% disability after 1 year • Adverse prognostic factors: advanced age, the severity of disease at nadir, bed-bound, artificial ventilation. • Plasma exchange or IVIg (0. 4 gm/kg/day in 5 days) • Oral steroid or pulse therapy with methylprednisolone alone not beneficial • Autoimmunity Reviews 2014; 13: 525 -530. 12

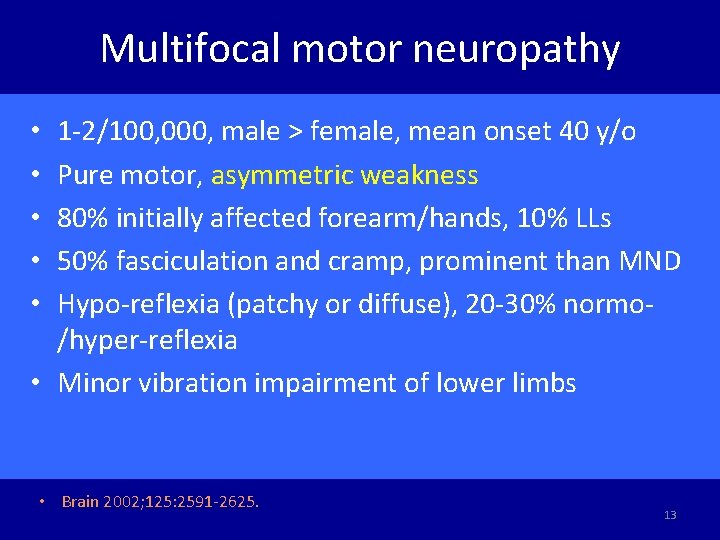
Multifocal motor neuropathy 1 -2/100, 000, male > female, mean onset 40 y/o Pure motor, asymmetric weakness 80% initially affected forearm/hands, 10% LLs 50% fasciculation and cramp, prominent than MND Hypo-reflexia (patchy or diffuse), 20 -30% normo/hyper-reflexia • Minor vibration impairment of lower limbs • • • Brain 2002; 125: 2591 -2625. 13

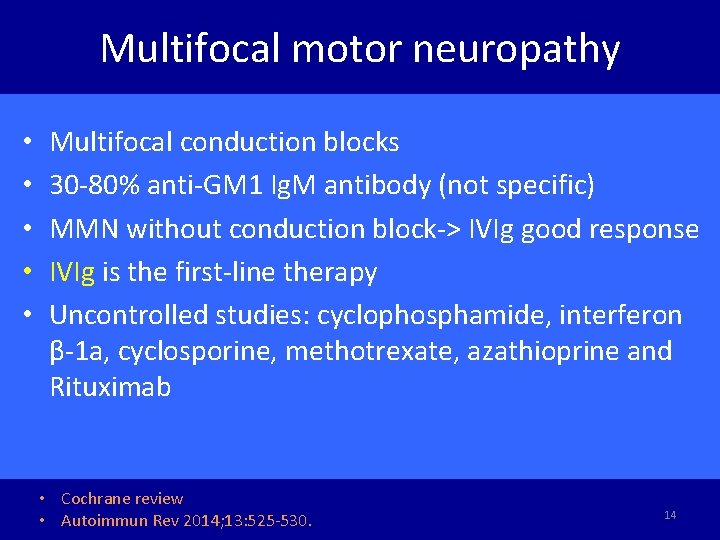
Multifocal motor neuropathy • • • Multifocal conduction blocks 30 -80% anti-GM 1 Ig. M antibody (not specific) MMN without conduction block-> IVIg good response IVIg is the first-line therapy Uncontrolled studies: cyclophosphamide, interferon β-1 a, cyclosporine, methotrexate, azathioprine and Rituximab • Cochrane review • Autoimmun Rev 2014; 13: 525 -530. 14

EFNS criteria -JPNS 2010; 15: 295 -301. Autoimmun Rev 2014; 13: 525 -530 15

16

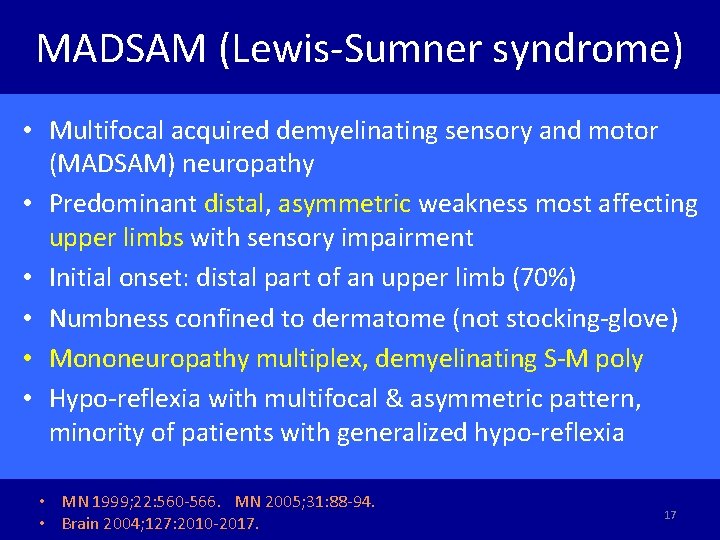
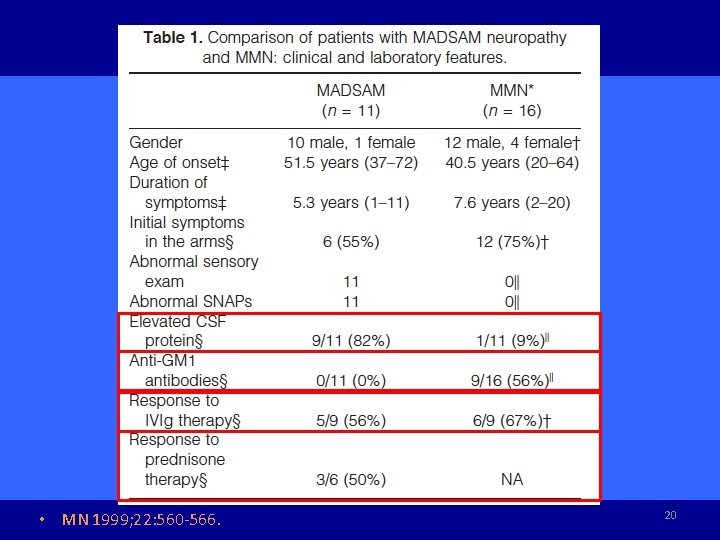
MADSAM (Lewis-Sumner syndrome) • Multifocal acquired demyelinating sensory and motor (MADSAM) neuropathy • Predominant distal, asymmetric weakness most affecting upper limbs with sensory impairment • Initial onset: distal part of an upper limb (70%) • Numbness confined to dermatome (not stocking-glove) • Mononeuropathy multiplex, demyelinating S-M poly • Hypo-reflexia with multifocal & asymmetric pattern, minority of patients with generalized hypo-reflexia • MN 1999; 22: 560 -566. MN 2005; 31: 88 -94. • Brain 2004; 127: 2010 -2017. 17

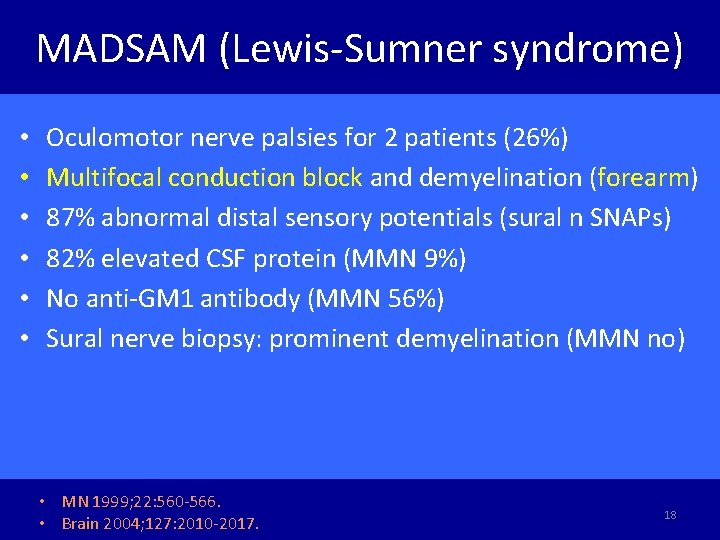
MADSAM (Lewis-Sumner syndrome) • • • Oculomotor nerve palsies for 2 patients (26%) Multifocal conduction block and demyelination (forearm) 87% abnormal distal sensory potentials (sural n SNAPs) 82% elevated CSF protein (MMN 9%) No anti-GM 1 antibody (MMN 56%) Sural nerve biopsy: prominent demyelination (MMN no) • MN 1999; 22: 560 -566. • Brain 2004; 127: 2010 -2017. 18

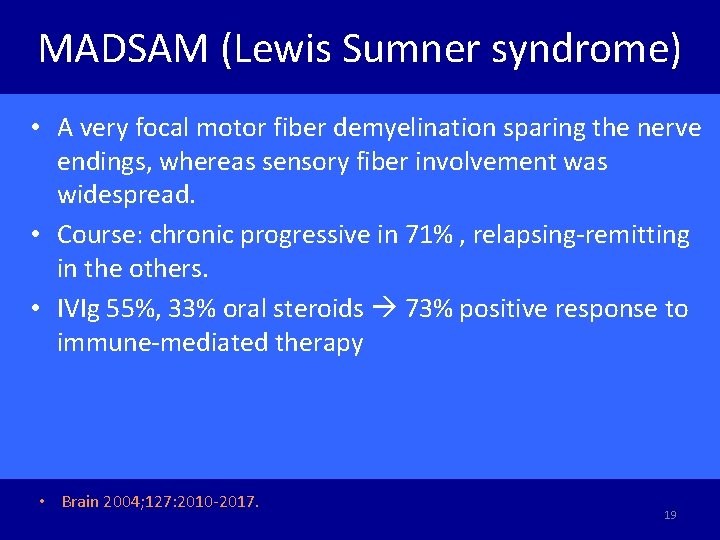
MADSAM (Lewis Sumner syndrome) • A very focal motor fiber demyelination sparing the nerve endings, whereas sensory fiber involvement was widespread. • Course: chronic progressive in 71% , relapsing-remitting in the others. • IVIg 55%, 33% oral steroids 73% positive response to immune-mediated therapy • Brain 2004; 127: 2010 -2017. 19

• MN 1999; 22: 560 -566. 20

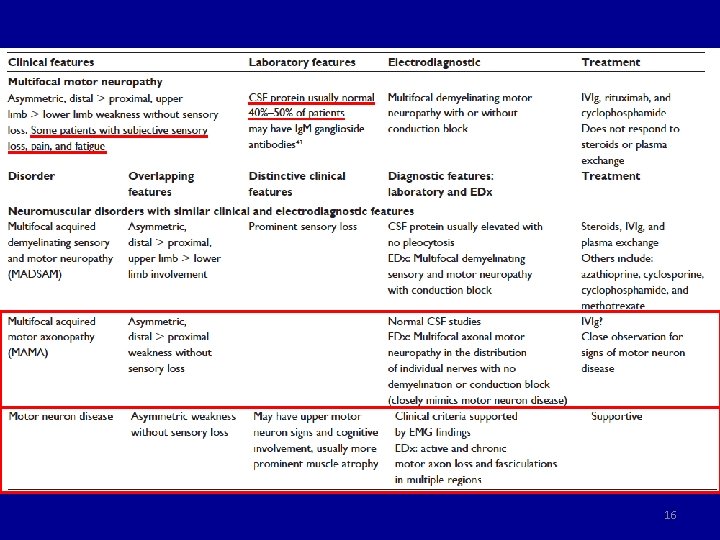
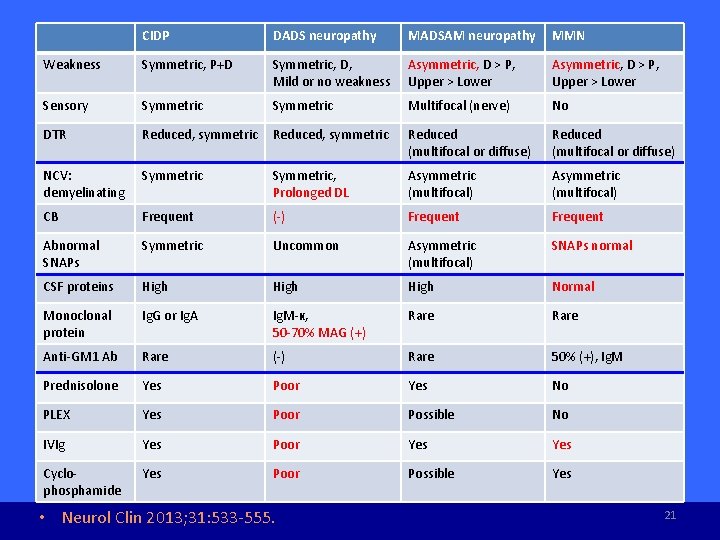
CIDP DADS neuropathy MADSAM neuropathy MMN Weakness Symmetric, P+D Symmetric, D, Mild or no weakness Asymmetric, D > P, Upper > Lower Sensory Symmetric Multifocal (nerve) No DTR Reduced, symmetric Reduced (multifocal or diffuse) NCV: demyelinating Symmetric, Prolonged DL Asymmetric (multifocal) CB Frequent (-) Frequent Abnormal SNAPs Symmetric Uncommon Asymmetric (multifocal) SNAPs normal CSF proteins High Normal Monoclonal protein Ig. G or Ig. A Ig. M-κ, 50 -70% MAG (+) Rare Anti-GM 1 Ab Rare (-) Rare 50% (+), Ig. M Prednisolone Yes Poor Yes No PLEX Yes Poor Possible No IVIg Yes Poor Yes Cyclophosphamide Yes Poor Possible Yes • Neurol Clin 2013; 31: 533 -555. 21

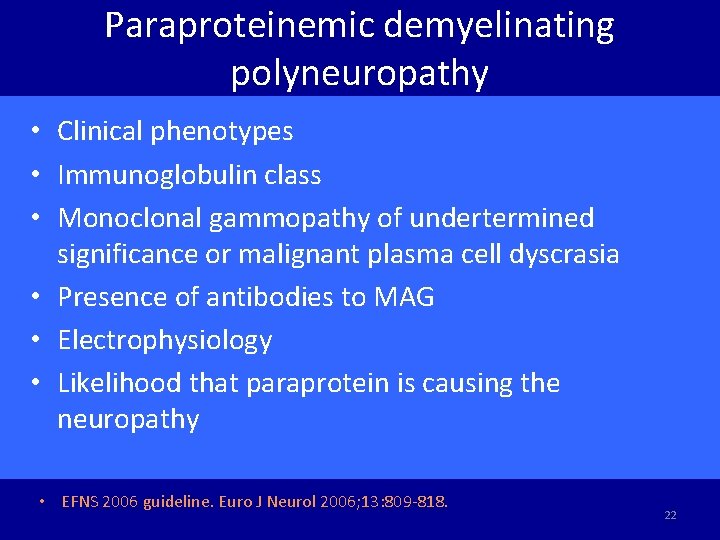
Paraproteinemic demyelinating polyneuropathy • Clinical phenotypes • Immunoglobulin class • Monoclonal gammopathy of undertermined significance or malignant plasma cell dyscrasia • Presence of antibodies to MAG • Electrophysiology • Likelihood that paraprotein is causing the neuropathy • EFNS 2006 guideline. Euro J Neurol 2006; 13: 809 -818. 22

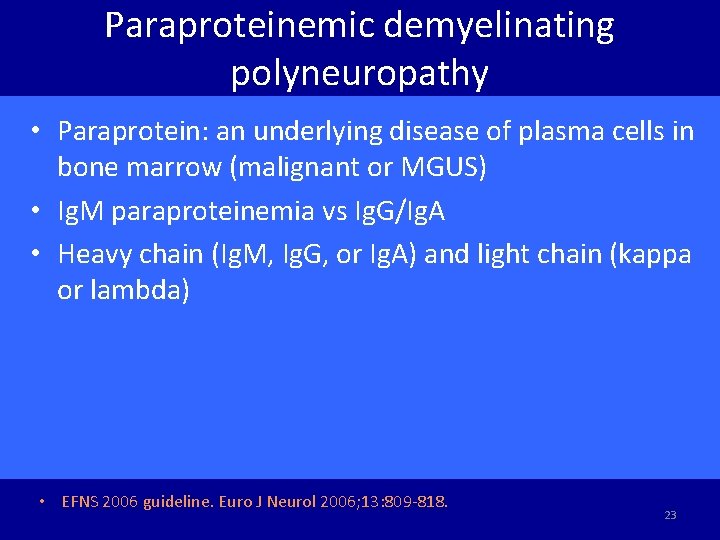
Paraproteinemic demyelinating polyneuropathy • Paraprotein: an underlying disease of plasma cells in bone marrow (malignant or MGUS) • Ig. M paraproteinemia vs Ig. G/Ig. A • Heavy chain (Ig. M, Ig. G, or Ig. A) and light chain (kappa or lambda) • EFNS 2006 guideline. Euro J Neurol 2006; 13: 809 -818. 23

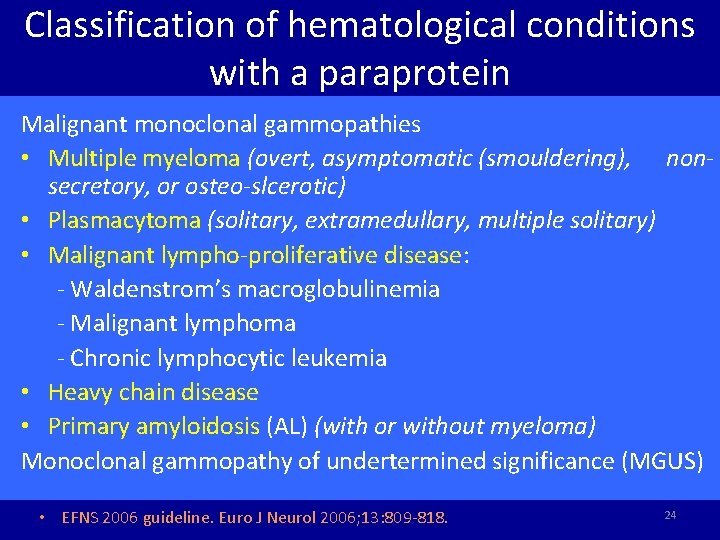
Classification of hematological conditions with a paraprotein Malignant monoclonal gammopathies • Multiple myeloma (overt, asymptomatic (smouldering), nonsecretory, or osteo-slcerotic) • Plasmacytoma (solitary, extramedullary, multiple solitary) • Malignant lympho-proliferative disease: - Waldenstrom’s macroglobulinemia - Malignant lymphoma - Chronic lymphocytic leukemia • Heavy chain disease • Primary amyloidosis (AL) (with or without myeloma) Monoclonal gammopathy of undertermined significance (MGUS) • EFNS 2006 guideline. Euro J Neurol 2006; 13: 809 -818. 24

Investigation of paraprotein • Serum IFE • CBC-DC, BUN/Cr, AST/ALT, Ca, P, UA, ESR, CRP, LDH, RF, β 2 -microglobulin, RF and cryoglobulins • Immunoglobulin Ig. G, Ig. A and Ig. M • Urine for Bence-Jones protein (free light chains), and, if positive 24 hour urine for protein quantification • Skeletal X ray: skull, pelvis, spine, ribs & long bones (shoulder to wrist & hip to ankle), 99 m. Tc MIBI scan. • Sonography or CT scan of abdomen & chest • Bone marrow aspiration • EFNS 2006 guideline. Euro J Neurol 2006; 13: 809 -818. 25

Investigation of paraprotein- PE • • Lymphadenopathy Hepato-splenomegaly Macroglossia Signs of POEMS syndrome • EFNS 2006 guideline. Eur J Neurol 2006; 13: 809 -818. 26

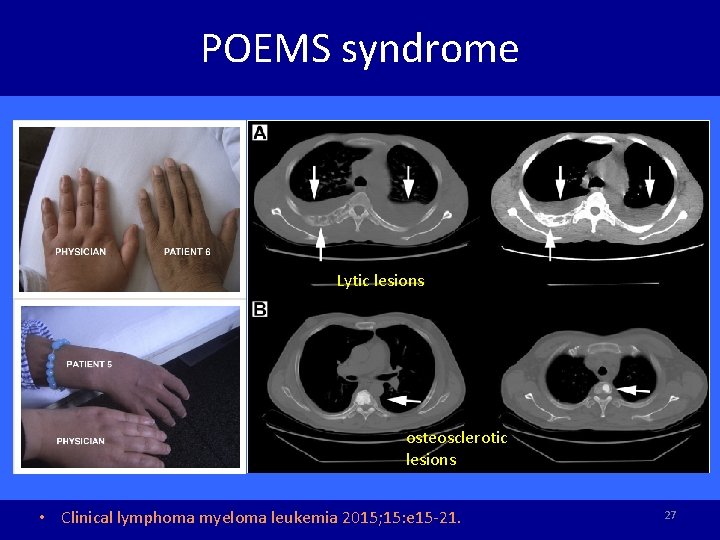
POEMS syndrome Lytic lesions osteosclerotic lesions • Clinical lymphoma myeloma leukemia 2015; 15: e 15 -21. 27

POEMS syndrome • Demyelinating polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy and skin changes • Plasma cell dyscrasia and multiple organ disorder • Overproduction of VEGF by plasmacytomas • 50% initial presentation with polyneuropathy, many are misdiagnosed as CIDP. • CSF: albuminocytological dissociation • JNNP 2012; 83: 476 -479. 28

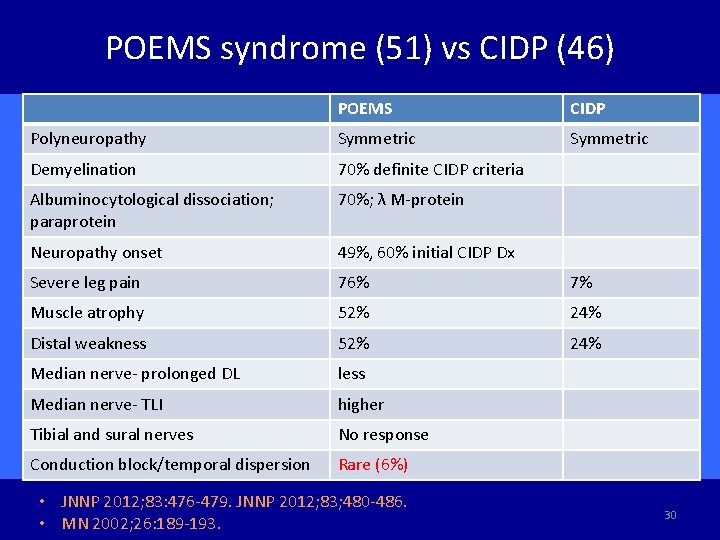
POEMS syndrome (51) vs CIDP (46) POEMS CIDP Polyneuropathy Symmetric Demyelination 70% definite CIDP criteria Albuminocytological dissociation; paraprotein 70%; λ M-protein Neuropathy onset 49%, 60% initial CIDP Dx Severe leg pain 76% 7% Muscle atrophy 52% 24% Distal weakness 52% 24% Median nerve- prolonged DL less Median nerve- TLI higher Tibial and sural nerves No response Conduction block/temporal dispersion Rare (6%) • JNNP 2012; 83: 476 -479. JNNP 2012; 83; 480 -486. • MN 2002; 26: 189 -193. 30

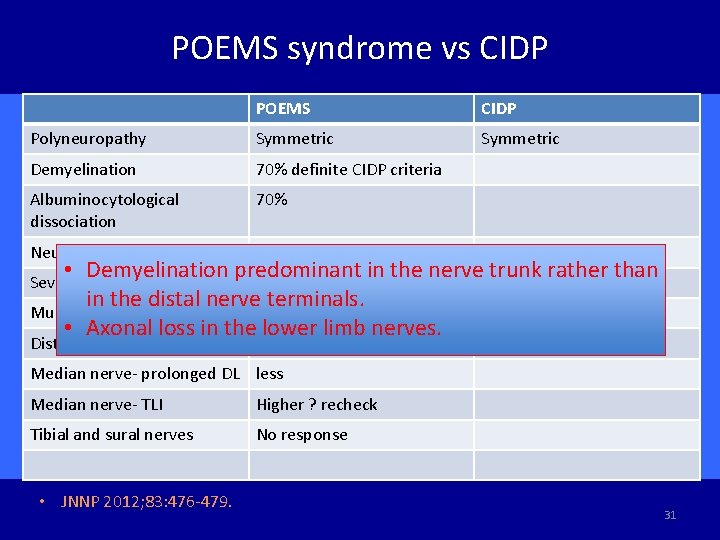
POEMS syndrome vs CIDP POEMS CIDP Polyneuropathy Symmetric Demyelination 70% definite CIDP criteria Albuminocytological dissociation 70% Neuropathy onset 49%, 60% initial CIDP Dx • Demyelination predominant in the nerve 7% trunk rather than 76% in the distal nerve terminals. Muscle atrophy 52% 24% • Axonal loss in the lower limb nerves. Severe leg pain Distal weakness 52% 24% Median nerve- prolonged DL less Median nerve- TLI Higher ? recheck Tibial and sural nerves No response • JNNP 2012; 83: 476 -479. 31

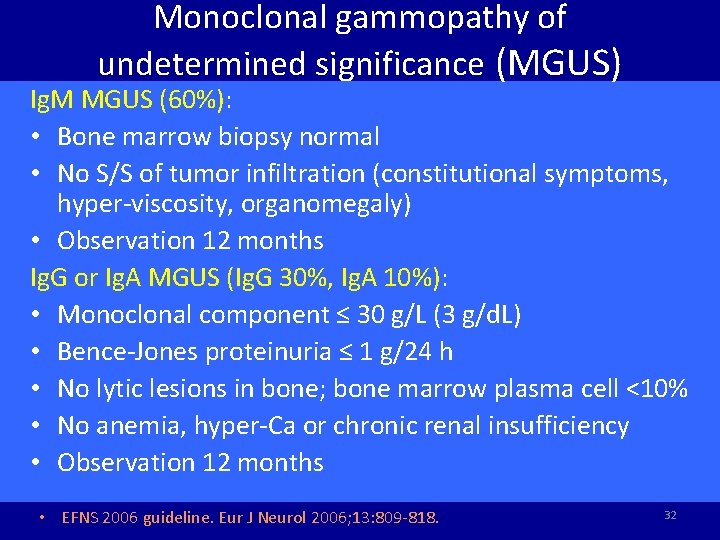
Monoclonal gammopathy of undetermined significance (MGUS) Ig. M MGUS (60%): • Bone marrow biopsy normal • No S/S of tumor infiltration (constitutional symptoms, hyper-viscosity, organomegaly) • Observation 12 months Ig. G or Ig. A MGUS (Ig. G 30%, Ig. A 10%): • Monoclonal component ≤ 30 g/L (3 g/d. L) • Bence-Jones proteinuria ≤ 1 g/24 h • No lytic lesions in bone; bone marrow plasma cell <10% • No anemia, hyper-Ca or chronic renal insufficiency • Observation 12 months • EFNS 2006 guideline. Eur J Neurol 2006; 13: 809 -818. 32

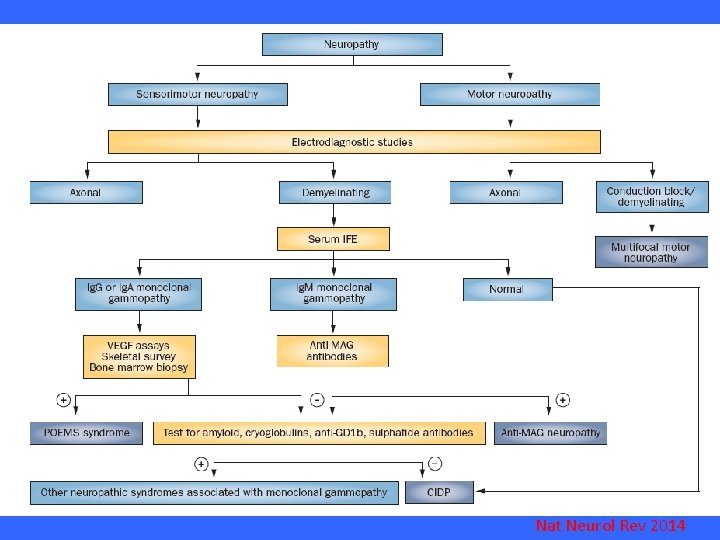
Nat Neurol Rev 2014

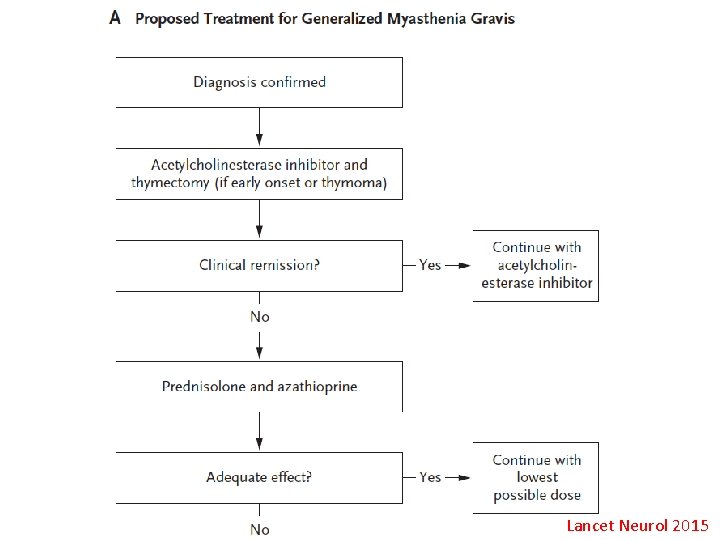
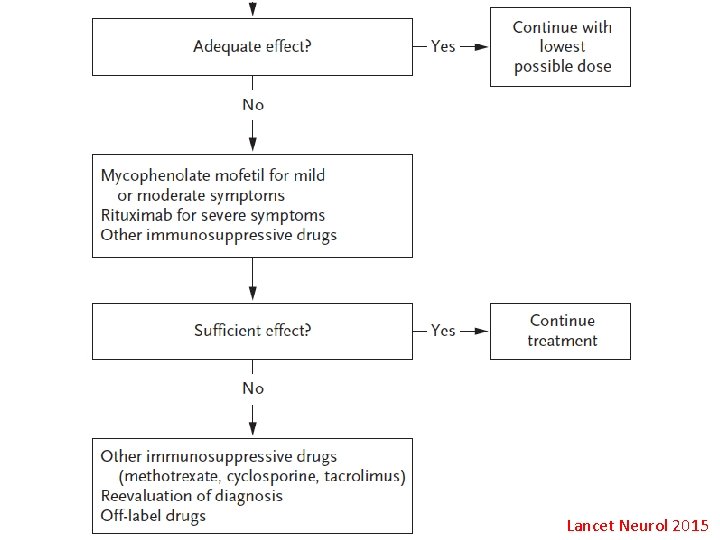
Treatment • • • GBS: IVIg, plasmapheresis CIDP: IVIg, steroid, plasmapheresis MMN: IVIg MADSAM: IVIg, steroid POEMS: steroid, add-on IS, plasmapheresis MGUS: steroid, plasmapheresis, 2 nd imuran 34

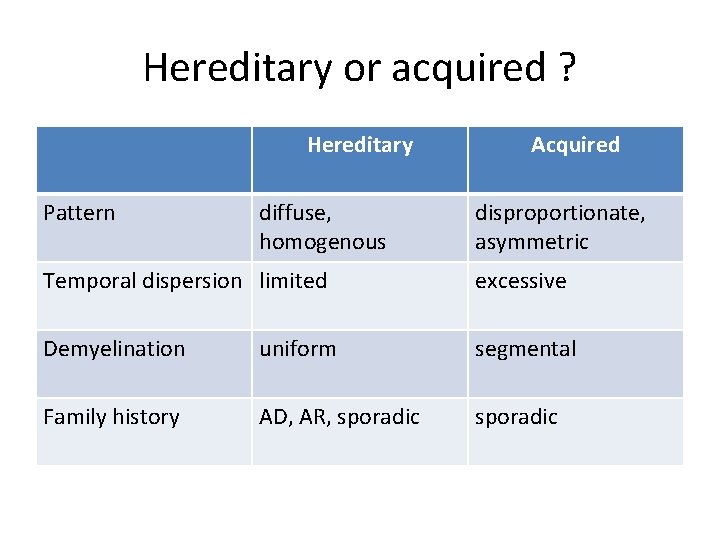
Hereditary or acquired ? Hereditary Pattern diffuse, homogenous Acquired disproportionate, asymmetric Temporal dispersion limited excessive Demyelination uniform segmental Family history AD, AR, sporadic


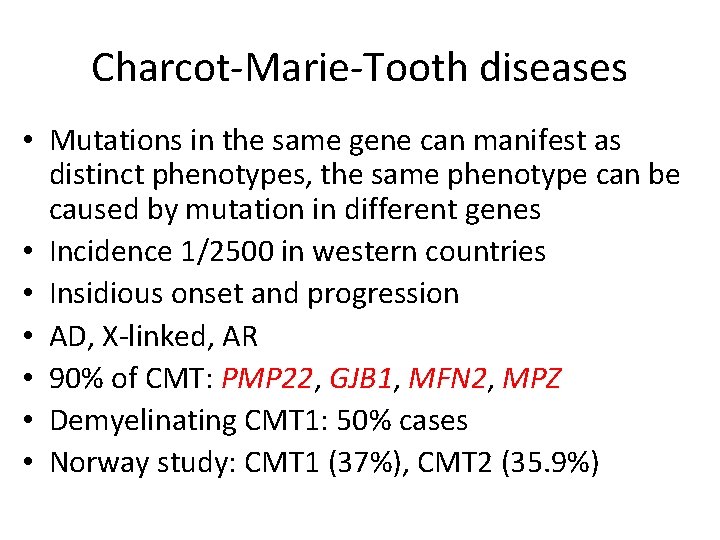
Charcot-Marie-Tooth diseases • Mutations in the same gene can manifest as distinct phenotypes, the same phenotype can be caused by mutation in different genes • Incidence 1/2500 in western countries • Insidious onset and progression • AD, X-linked, AR • 90% of CMT: PMP 22, GJB 1, MFN 2, MPZ • Demyelinating CMT 1: 50% cases • Norway study: CMT 1 (37%), CMT 2 (35. 9%)

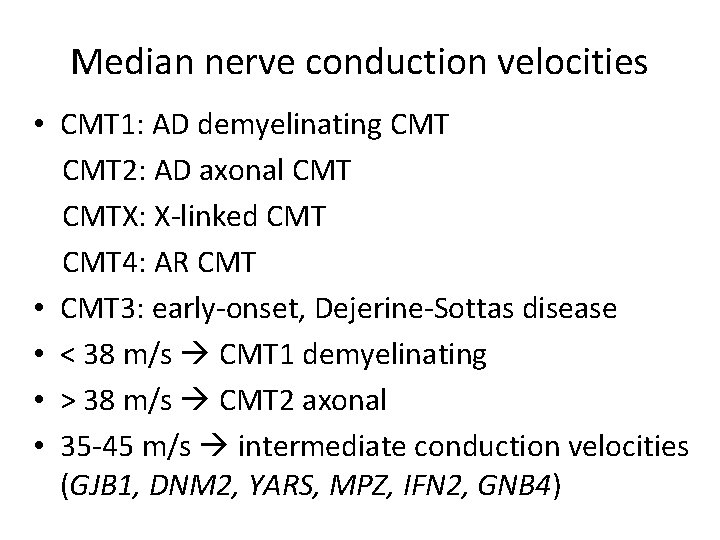
Median nerve conduction velocities • CMT 1: AD demyelinating CMT 2: AD axonal CMTX: X-linked CMT 4: AR CMT • CMT 3: early-onset, Dejerine-Sottas disease • < 38 m/s CMT 1 demyelinating • > 38 m/s CMT 2 axonal • 35 -45 m/s intermediate conduction velocities (GJB 1, DNM 2, YARS, MPZ, IFN 2, GNB 4)

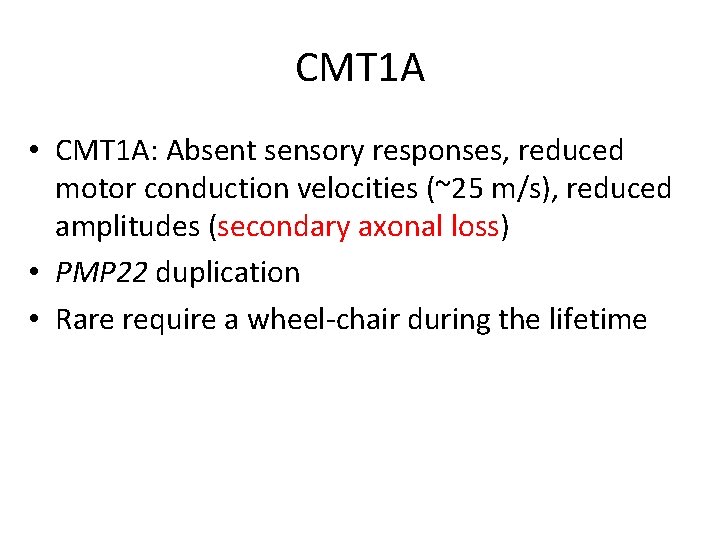
CMT 1 A • CMT 1 A: Absent sensory responses, reduced motor conduction velocities (~25 m/s), reduced amplitudes (secondary axonal loss) • PMP 22 duplication • Rare require a wheel-chair during the lifetime

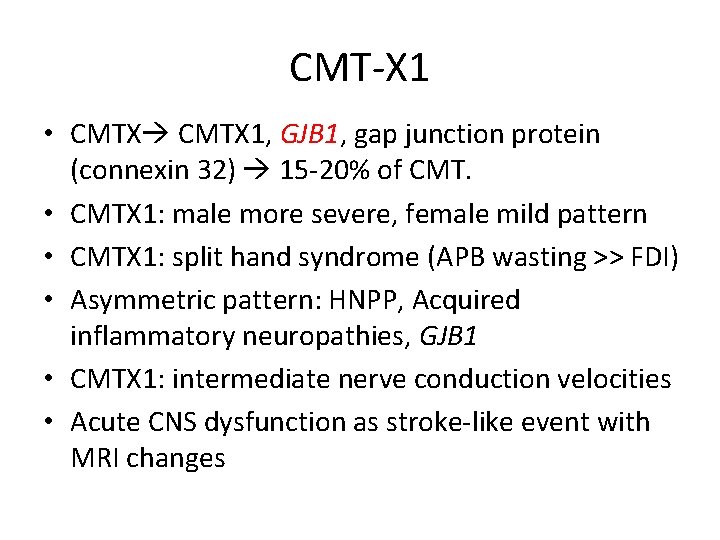
CMT-X 1 • CMTX 1, GJB 1, gap junction protein (connexin 32) 15 -20% of CMT. • CMTX 1: male more severe, female mild pattern • CMTX 1: split hand syndrome (APB wasting >> FDI) • Asymmetric pattern: HNPP, Acquired inflammatory neuropathies, GJB 1 • CMTX 1: intermediate nerve conduction velocities • Acute CNS dysfunction as stroke-like event with MRI changes


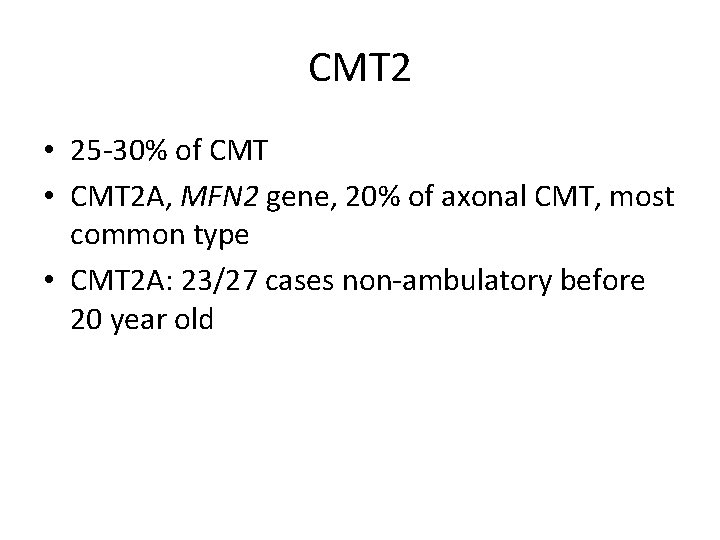
CMT 2 • 25 -30% of CMT • CMT 2 A, MFN 2 gene, 20% of axonal CMT, most common type • CMT 2 A: 23/27 cases non-ambulatory before 20 year old


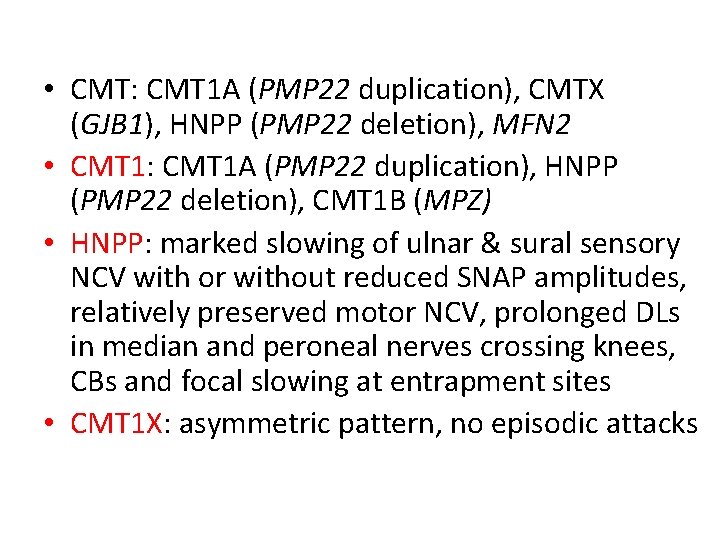
• CMT: CMT 1 A (PMP 22 duplication), CMTX (GJB 1), HNPP (PMP 22 deletion), MFN 2 • CMT 1: CMT 1 A (PMP 22 duplication), HNPP (PMP 22 deletion), CMT 1 B (MPZ) • HNPP: marked slowing of ulnar & sural sensory NCV with or without reduced SNAP amplitudes, relatively preserved motor NCV, prolonged DLs in median and peroneal nerves crossing knees, CBs and focal slowing at entrapment sites • CMT 1 X: asymmetric pattern, no episodic attacks





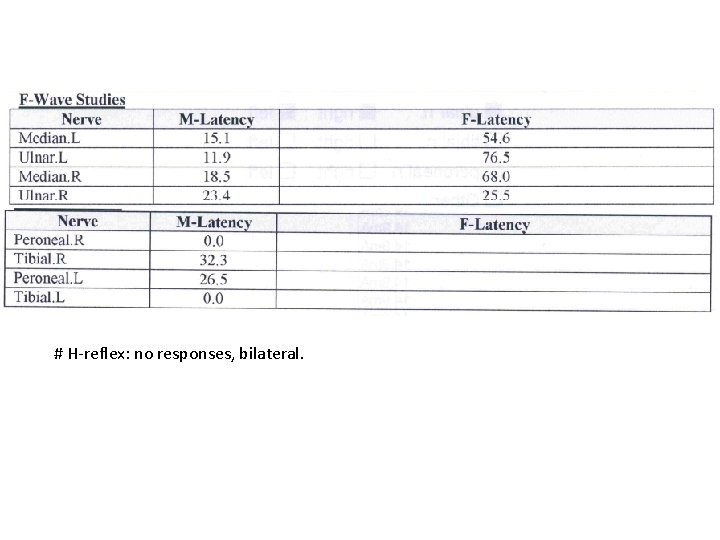
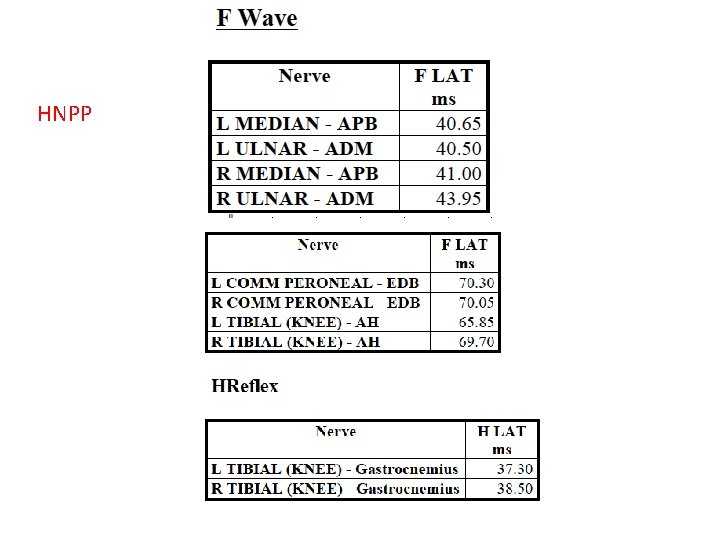
# H-reflex: no responses, bilateral.

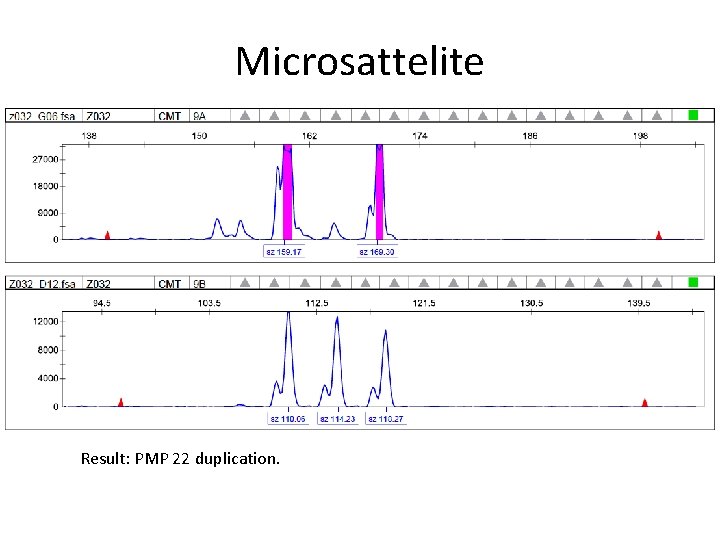
Microsattelite Result: PMP 22 duplication.

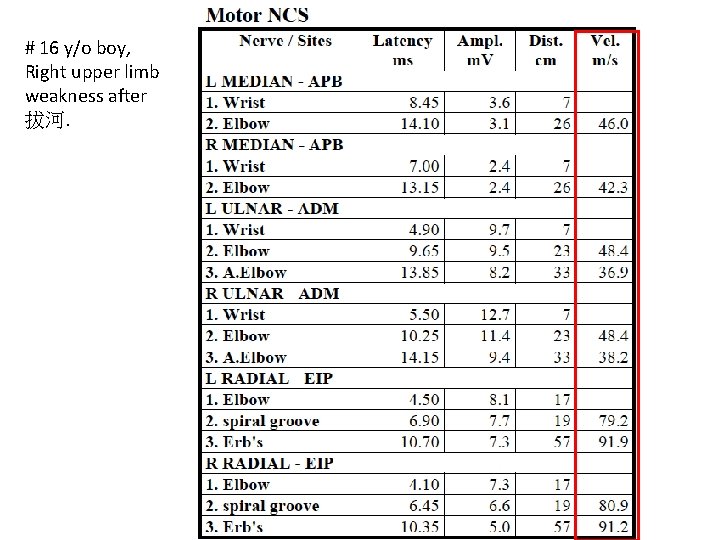
# 16 y/o boy, Right upper limb weakness after 拔河.




HNPP

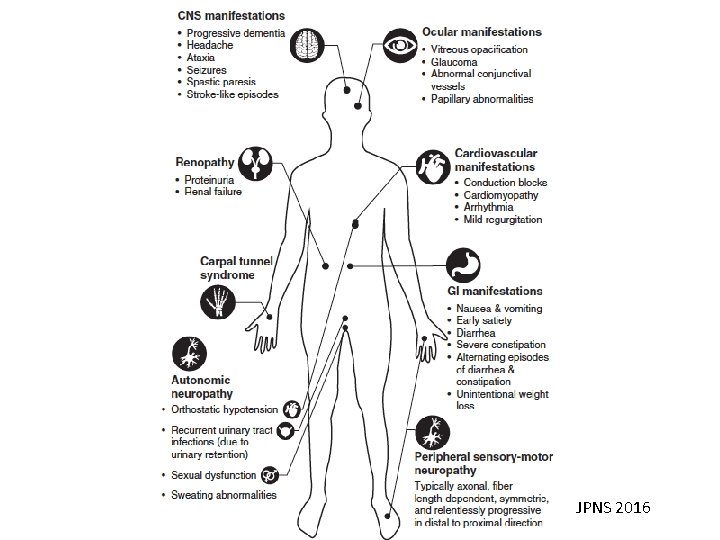
Familial amyloid neuropathy

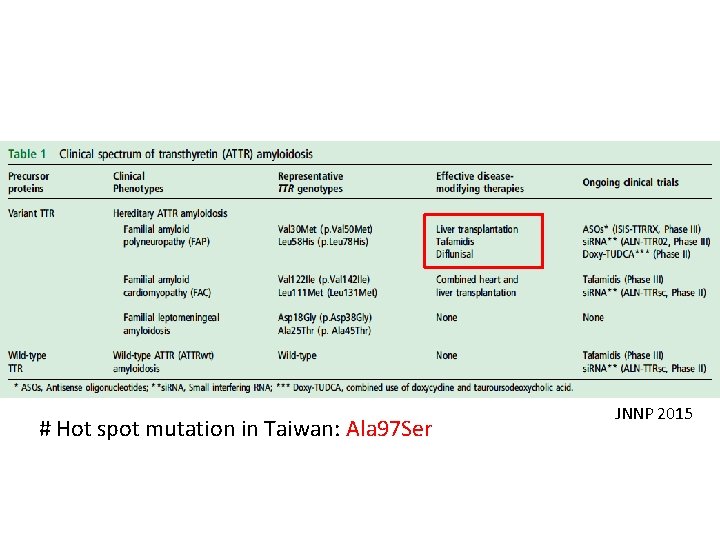
# Hot spot mutation in Taiwan: Ala 97 Ser JNNP 2015

JPNS 2016

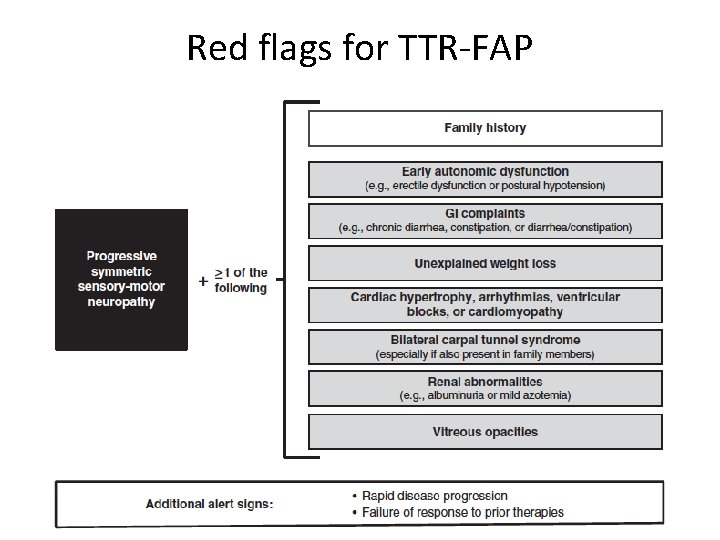
Misdiagnosis of FAP for CIDP • Treatment-unresponsiveness • Severe autonomic dysfunction • Weight loss • Hx of carpal tunnel syndrome Red flags

Red flags for TTR-FAP

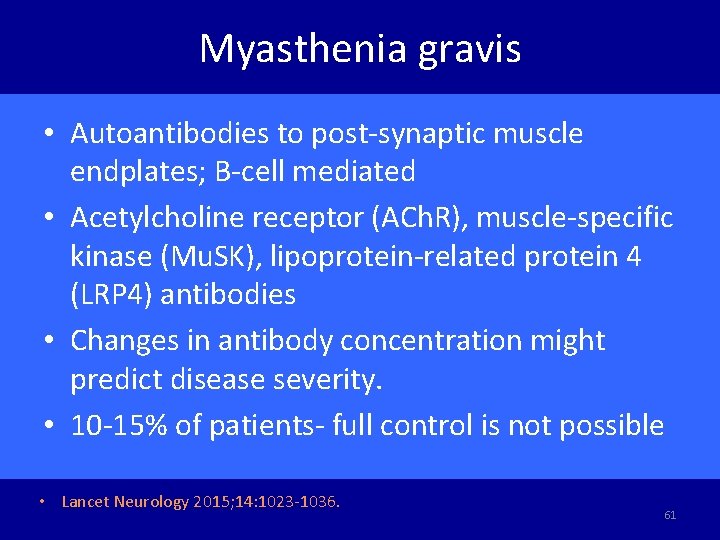
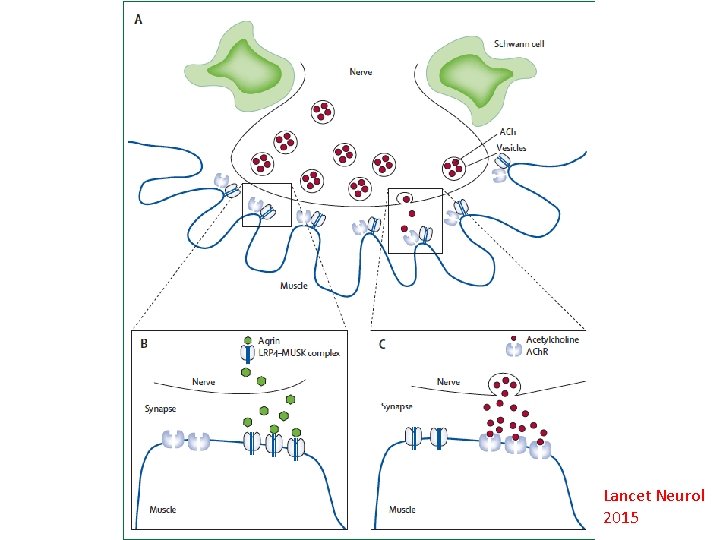
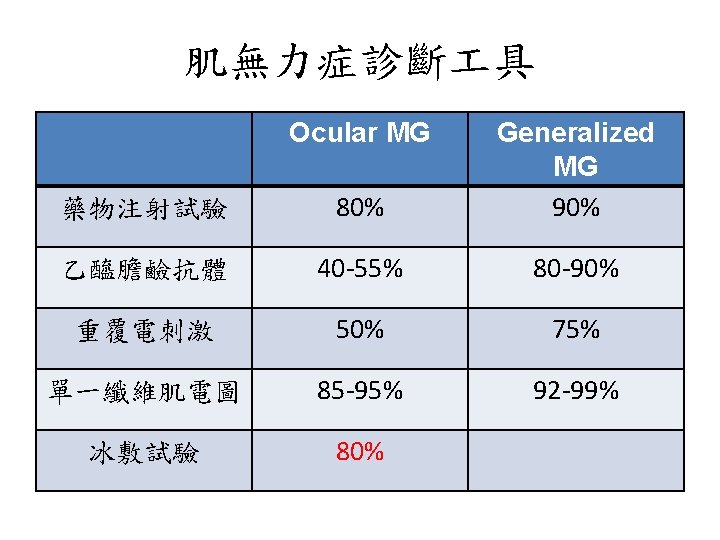
Myasthenia gravis • Autoantibodies to post-synaptic muscle endplates; B-cell mediated • Acetylcholine receptor (ACh. R), muscle-specific kinase (Mu. SK), lipoprotein-related protein 4 (LRP 4) antibodies • Changes in antibody concentration might predict disease severity. • 10 -15% of patients- full control is not possible • Lancet Neurology 2015; 14: 1023 -1036. 61

Lancet Neurol 2015

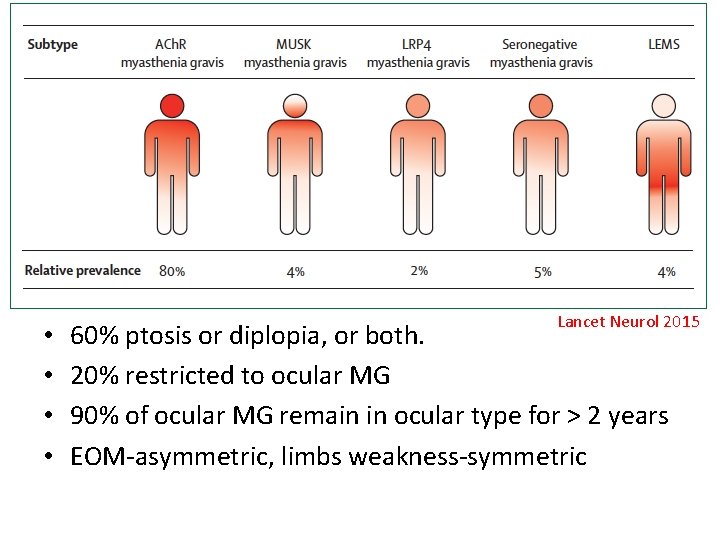
• • Lancet Neurol 2015 60% ptosis or diplopia, or both. 20% restricted to ocular MG 90% of ocular MG remain in ocular type for > 2 years EOM-asymmetric, limbs weakness-symmetric


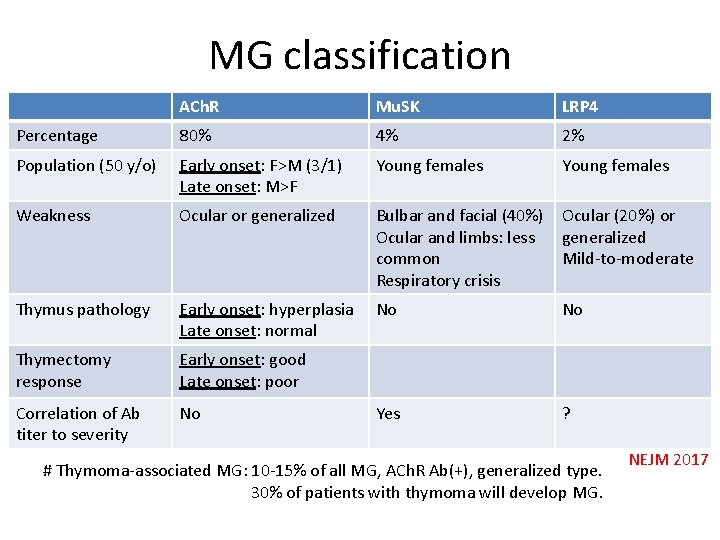
MG classification ACh. R Mu. SK LRP 4 Percentage 80% 4% 2% Population (50 y/o) Early onset: F>M (3/1) Late onset: M>F Young females Weakness Ocular or generalized Bulbar and facial (40%) Ocular and limbs: less common Respiratory crisis Ocular (20%) or generalized Mild-to-moderate Thymus pathology Early onset: hyperplasia Late onset: normal No No Thymectomy response Early onset: good Late onset: poor Correlation of Ab titer to severity No Yes ? # Thymoma-associated MG: 10 -15% of all MG, ACh. R Ab(+), generalized type. 30% of patients with thymoma will develop MG. NEJM 2017

Lancet Neurol 2015

Lancet Neurol 2015

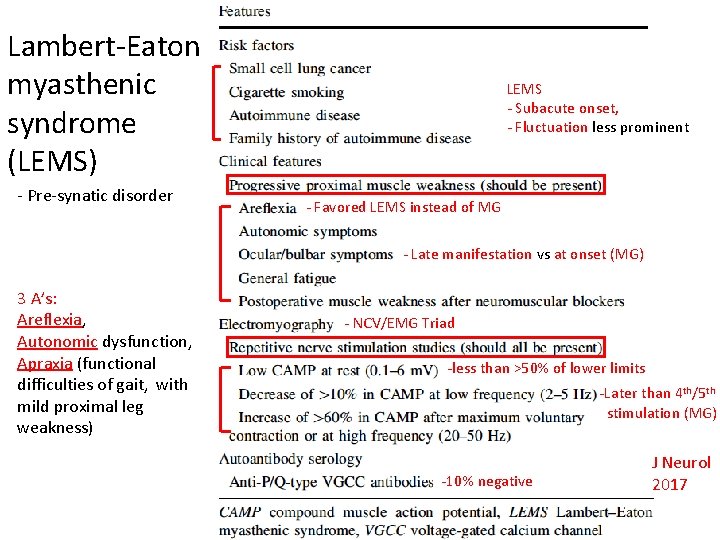
Lambert-Eaton myasthenic syndrome (LEMS) - Pre-synatic disorder LEMS - Subacute onset, - Fluctuation less prominent - Favored LEMS instead of MG - Late manifestation vs at onset (MG) 3 A’s: Areflexia, Autonomic dysfunction, Apraxia (functional difficulties of gait, with mild proximal leg weakness) - NCV/EMG Triad -less than >50% of lower limits -Later than 4 th/5 th stimulation (MG) -10% negative J Neurol 2017

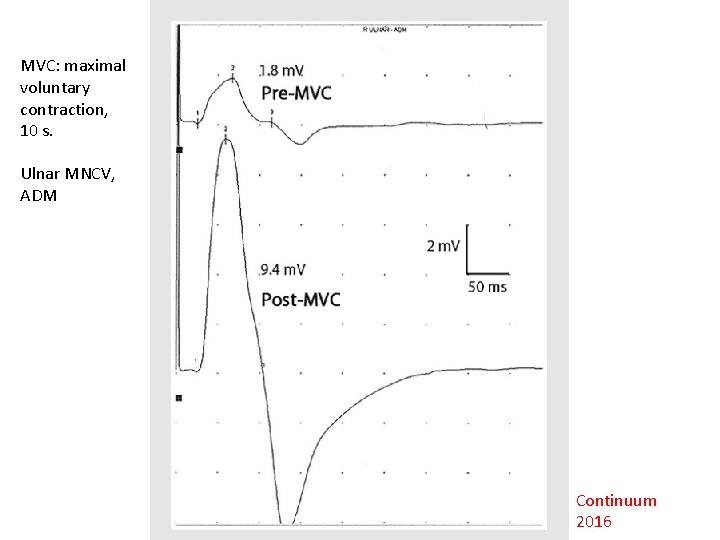
MVC: maximal voluntary contraction, 10 s. Ulnar MNCV, ADM Continuum 2016

Thank you for your attention ! 70
 Myringotomy incision
Myringotomy incision What is the nursing process
What is the nursing process Medical diagnosis and nursing diagnosis difference
Medical diagnosis and nursing diagnosis difference Types of nursing diagnosis
Types of nursing diagnosis Medical diagnosis and nursing diagnosis difference
Medical diagnosis and nursing diagnosis difference Charting exercise chapter 28
Charting exercise chapter 28 Neil classification lateral throat form
Neil classification lateral throat form Endodontic diagnosis and treatment planning
Endodontic diagnosis and treatment planning Chapter 28 oral diagnosis and treatment planning
Chapter 28 oral diagnosis and treatment planning Perbedaan diagnosis gizi dan diagnosis medis
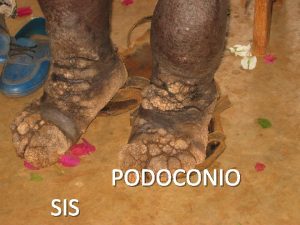
Perbedaan diagnosis gizi dan diagnosis medis What is podoconiosis
What is podoconiosis Dr lapsia
Dr lapsia Dyslexia differential diagnosis
Dyslexia differential diagnosis Intrahepatic jaundice
Intrahepatic jaundice Differential diagnosis of stroke
Differential diagnosis of stroke Describe
Describe Acute productive cough differential diagnosis
Acute productive cough differential diagnosis Differential diagnosis red eye
Differential diagnosis red eye Gastric outlet obstruction differential diagnosis
Gastric outlet obstruction differential diagnosis Gastric ulcer barium swallow
Gastric ulcer barium swallow Haemtemesis
Haemtemesis Anatomy and physiology of peptic ulcer
Anatomy and physiology of peptic ulcer Jaundice physical exam
Jaundice physical exam Vitamin c differential diagnosis mnemonic
Vitamin c differential diagnosis mnemonic Mieloproliferative
Mieloproliferative Parkinson differential diagnosis
Parkinson differential diagnosis Differential diagnosis of stroke
Differential diagnosis of stroke Staging of breast cancer
Staging of breast cancer Supraclavicular lymph nodes swollen
Supraclavicular lymph nodes swollen Odd diagnosis
Odd diagnosis Broadbent sign in constrictive pericarditis
Broadbent sign in constrictive pericarditis Hospital university of pennsylvania
Hospital university of pennsylvania Dd jaundice
Dd jaundice Chicken pix los pinos menu
Chicken pix los pinos menu Nephrotic syndrome differential diagnosis
Nephrotic syndrome differential diagnosis Ohio state lymphedema doctor
Ohio state lymphedema doctor Corte axial
Corte axial Brainstem stroke syndromes
Brainstem stroke syndromes Vitamin d differential diagnosis mnemonic
Vitamin d differential diagnosis mnemonic Down beat nystagmus
Down beat nystagmus Cervical myelopathy wayne
Cervical myelopathy wayne Normocytic hypochromic anemia differential diagnosis
Normocytic hypochromic anemia differential diagnosis Pulmonary hypertension differential diagnosis
Pulmonary hypertension differential diagnosis Bakers cyst medscape
Bakers cyst medscape Differential diagnosis of articular syndrome
Differential diagnosis of articular syndrome Megaloblastic anemia causes
Megaloblastic anemia causes Differential diagnosis for dysphagia
Differential diagnosis for dysphagia 1152020
1152020 Tocolytic drugs
Tocolytic drugs Differential diagnosis for premature rupture of membranes
Differential diagnosis for premature rupture of membranes Epithelial dysplasia
Epithelial dysplasia Epigastric pain differential diagnosis
Epigastric pain differential diagnosis Spotting differential diagnosis
Spotting differential diagnosis Implantation spotting
Implantation spotting Leukocoria differential diagnosis
Leukocoria differential diagnosis Allogeneic stem cell transplant
Allogeneic stem cell transplant Polycythemia differential diagnosis
Polycythemia differential diagnosis Differentials for hydrocephalus
Differentials for hydrocephalus Dka in pregnancy
Dka in pregnancy Spotting differential diagnosis
Spotting differential diagnosis Gurney's tubercle
Gurney's tubercle Conjunctivitis differential diagnosis
Conjunctivitis differential diagnosis Differential diagnosis of vitiligo
Differential diagnosis of vitiligo Molar pregnancy differential diagnosis
Molar pregnancy differential diagnosis Differential diagnosis for leukoplakia
Differential diagnosis for leukoplakia Differential diagnosis of dysphagia
Differential diagnosis of dysphagia Profalam
Profalam Kavram öğretimi örnekleri
Kavram öğretimi örnekleri Differential diagnosis of otitis externa
Differential diagnosis of otitis externa Abdomen quadrants organs
Abdomen quadrants organs