MILD COGNITIVE IMPAIRMENT DIFFERENTIAL DIAGNOSIS J Wesson Ashford









































- Slides: 71

MILD COGNITIVE IMPAIRMENT DIFFERENTIAL DIAGNOSIS J. Wesson Ashford, M. D. , Ph. D. Stanford / VA Alzheimer’s Center VAMC, Palo Alto, California May 14, 2004

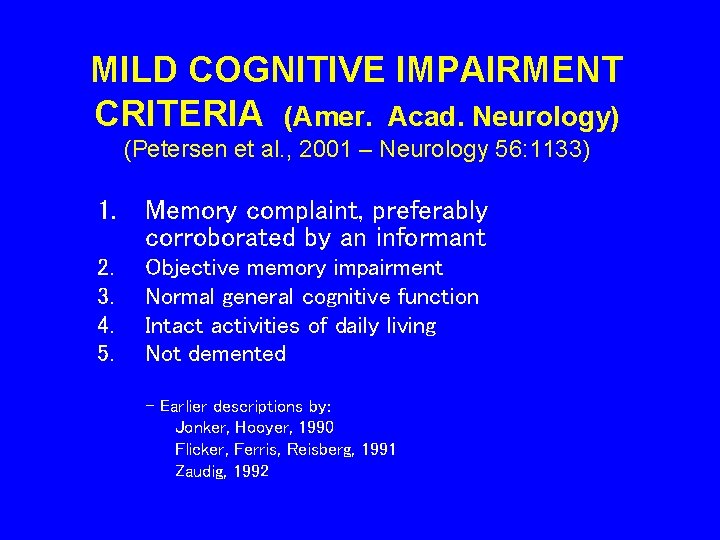
MILD COGNITIVE IMPAIRMENT CRITERIA (Amer. Acad. Neurology) (Petersen et al. , 2001 – Neurology 56: 1133) 1. Memory complaint, preferably corroborated by an informant 2. 3. 4. 5. Objective memory impairment Normal general cognitive function Intact activities of daily living Not demented - Earlier descriptions by: Jonker, Hooyer, 1990 Flicker, Ferris, Reisberg, 1991 Zaudig, 1992

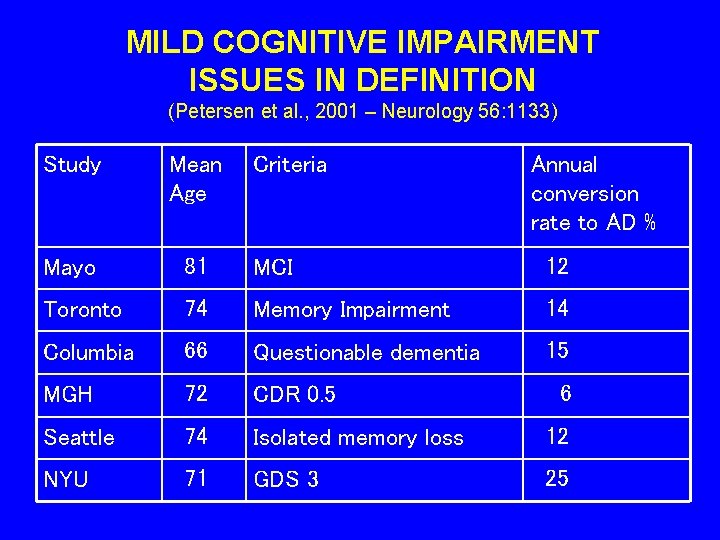
MILD COGNITIVE IMPAIRMENT ISSUES IN DEFINITION (Petersen et al. , 2001 – Neurology 56: 1133) Study Mean Age Criteria Annual conversion rate to AD % Mayo 81 MCI 12 Toronto 74 Memory Impairment 14 Columbia 66 Questionable dementia 15 MGH 72 CDR 0. 5 Seattle 74 Isolated memory loss 12 NYU 71 GDS 3 25 6

Markov Chain model Yesavavage et al. , 2002

ALZHEIMER’S DISEASE AAMI / MCI DEMENTIA Ashford et al. , 1995

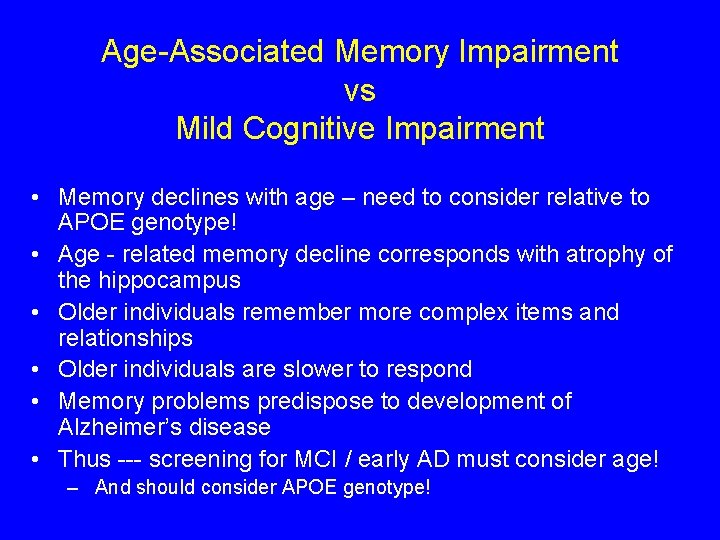
Age-Associated Memory Impairment vs Mild Cognitive Impairment • Memory declines with age – need to consider relative to APOE genotype! • Age - related memory decline corresponds with atrophy of the hippocampus • Older individuals remember more complex items and relationships • Older individuals are slower to respond • Memory problems predispose to development of Alzheimer’s disease • Thus --- screening for MCI / early AD must consider age! – And should consider APOE genotype!

Early Recognition of AD: Consensus Statement (AAGP, AGS, Alzheimer’s Association) • AD continues to be missed as diagnosis • AD is unrecognized and under-reported – patients do not realized – families tend to compensate • Effective treatment and management techniques are available – (ACh. EIs FDA approved) – Several other approaches are beneficial Small et al. , JAMA, 1997

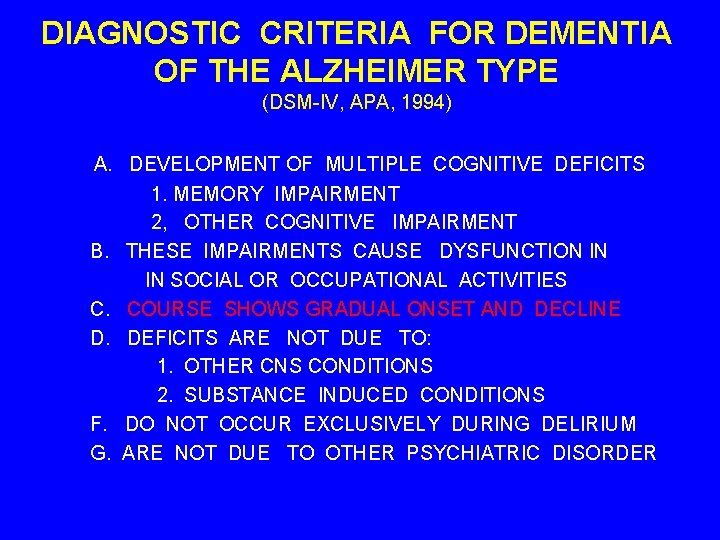
DIAGNOSTIC CRITERIA FOR DEMENTIA OF THE ALZHEIMER TYPE (DSM-IV, APA, 1994) A. DEVELOPMENT OF MULTIPLE COGNITIVE DEFICITS 1. MEMORY IMPAIRMENT 2, OTHER COGNITIVE IMPAIRMENT B. THESE IMPAIRMENTS CAUSE DYSFUNCTION IN IN SOCIAL OR OCCUPATIONAL ACTIVITIES C. COURSE SHOWS GRADUAL ONSET AND DECLINE D. DEFICITS ARE NOT DUE TO: 1. OTHER CNS CONDITIONS 2. SUBSTANCE INDUCED CONDITIONS F. DO NOT OCCUR EXCLUSIVELY DURING DELIRIUM G. ARE NOT DUE TO OTHER PSYCHIATRIC DISORDER

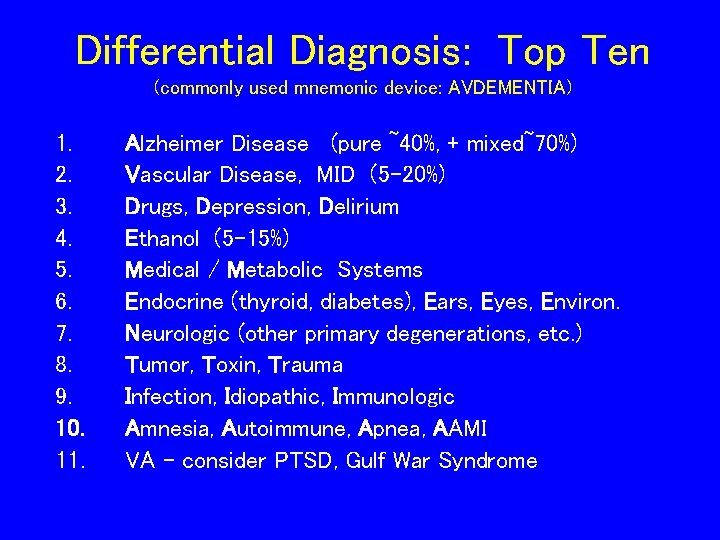
Differential Diagnosis: Top Ten (commonly used mnemonic device: AVDEMENTIA) 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. Alzheimer Disease (pure ~40%, + mixed~70%) Vascular Disease, MID (5 -20%) Drugs, Depression, Delirium Ethanol (5 -15%) Medical / Metabolic Systems Endocrine (thyroid, diabetes), Ears, Eyes, Environ. Neurologic (other primary degenerations, etc. ) Tumor, Toxin, Trauma Infection, Idiopathic, Immunologic Amnesia, Autoimmune, Apnea, AAMI VA – consider PTSD, Gulf War Syndrome

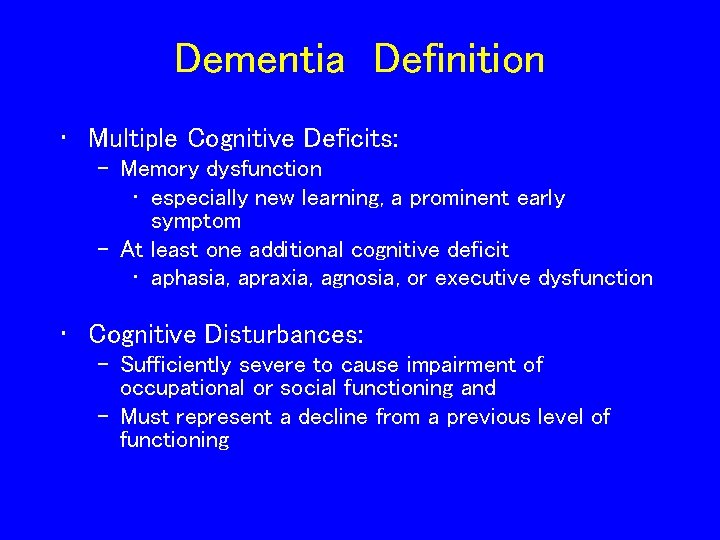
Dementia Definition • Multiple Cognitive Deficits: – Memory dysfunction • especially new learning, a prominent early symptom – At least one additional cognitive deficit • aphasia, apraxia, agnosia, or executive dysfunction • Cognitive Disturbances: – Sufficiently severe to cause impairment of occupational or social functioning and – Must represent a decline from a previous level of functioning

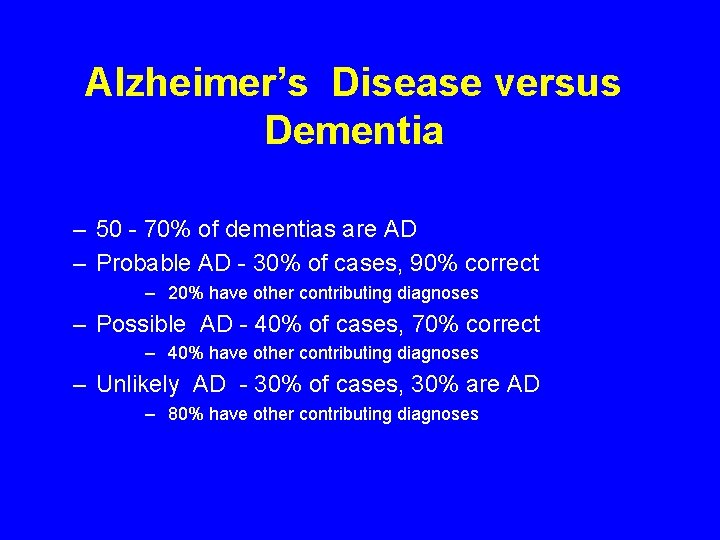
Alzheimer’s Disease versus Dementia – 50 - 70% of dementias are AD – Probable AD - 30% of cases, 90% correct – 20% have other contributing diagnoses – Possible AD - 40% of cases, 70% correct – 40% have other contributing diagnoses – Unlikely AD - 30% of cases, 30% are AD – 80% have other contributing diagnoses

Vascular Dementia (DSM-IV - APA, 1994) A. Multiple Cogntive Impairments 1. 2. Memory Impairment Other Cognitive Disturbances B. Deficits Impair Social/Occupational C. Focal Neurological Signs and Symptoms or Laboratory Evidence Indicating Cerebrovascular Disease Etiologically Related to the Deficits D. Not Due to Delirium

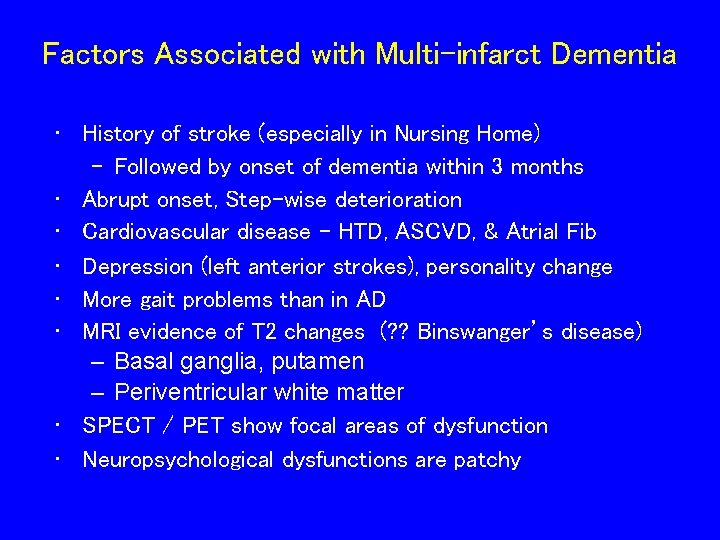
Factors Associated with Multi-infarct Dementia • History of stroke (especially in Nursing Home) – Followed by onset of dementia within 3 months • Abrupt onset, Step-wise deterioration • Cardiovascular disease - HTD, ASCVD, & Atrial Fib • Depression (left anterior strokes), personality change • More gait problems than in AD • MRI evidence of T 2 changes (? ? Binswanger’s disease) – Basal ganglia, putamen – Periventricular white matter • SPECT / PET show focal areas of dysfunction • Neuropsychological dysfunctions are patchy


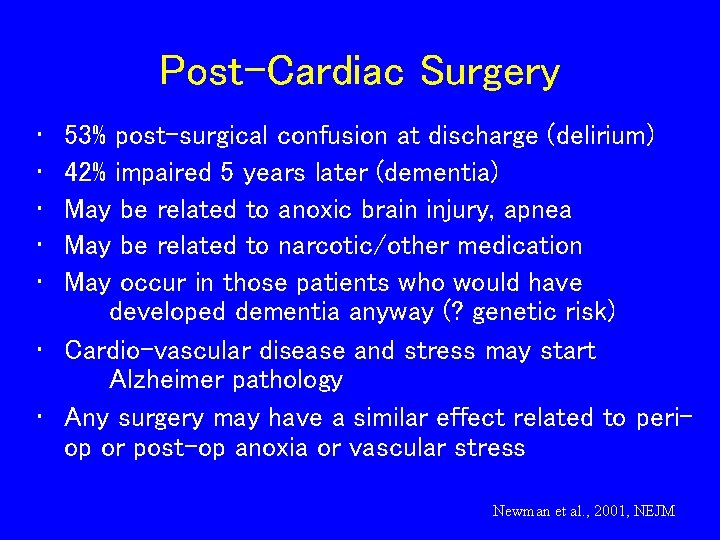
Post-Cardiac Surgery • • • 53% post-surgical confusion at discharge (delirium) 42% impaired 5 years later (dementia) May be related to anoxic brain injury, apnea May be related to narcotic/other medication May occur in those patients who would have developed dementia anyway (? genetic risk) • Cardio-vascular disease and stress may start Alzheimer pathology • Any surgery may have a similar effect related to periop or post-op anoxia or vascular stress Newman et al. , 2001, NEJM

Drug Interactions • Anticholinergics: amitriptyline, atropine, benztropine, scopolamine, hyoscyamine, oxybutynin, diphenhydramine, chlorpheniramine, many anti-histaminics – May aggravate Alzheimer pathology • GABA agonists: benzodiazepines, barbiturates, ethanol, anti-convulsants • Beta-blockers: propranolol • Dopaminergics: l-dopa, alpha-methyl-dopa • Narcotics: may contribute to dementia

Drug Toxicity • Anti-cholinergic – Peripheral: blurred vision, dry mouth, constipation, urinary obstruction – Central: confusion, memory encoding block • Gaba-agonist: – Muscle relaxant, anti-convulsant, sedative, anti-anxiety, amnesic, confusion • Medication induced electrolyte imbalance – Confusion (watch for in nursing home)

Depression • • Onset: rapid Precipitants: psycho-social (not organic) Duration: less than 3 months to presentation Mood: depressed, anxious Behavior: decreased activity or agitation Cognition: unimpaired or poor responses Somatic symptoms: fatigue, lethargy, sleep, appetite disruption • Course: rapid resolution with treatment, but may precede Alzheimer’s disease

Delirium Definition (more often a problem in medical in-patients) § Disturbance of consciousness § § i. e. , reduced clarity of awareness of the environment with reduced ability to focus, sustain, or shift attention Change in cognition (memory, orientation, language, perception) Development over a short period (hours to days), tends to fluctuate Evidence of medical etiology

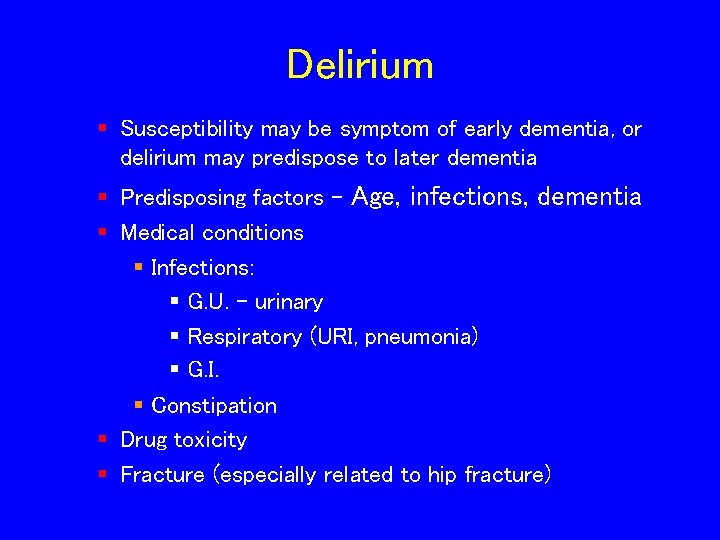
Delirium § Susceptibility may be symptom of early dementia, or delirium may predispose to later dementia § Predisposing factors - Age, infections, dementia § Medical conditions § Infections: § G. U. - urinary § Respiratory (URI, pneumonia) § G. I. § Constipation § Drug toxicity § Fracture (especially related to hip fracture)

Ethanol • Possibly Neuroprotective – May not kill neurons directly (? Dietary recommendation? ) • Accidents, Head Injury • Dietary Deficiency – Thiamine – Wernicke-Korsakoff syndrome • Hepatic Encephalopathy • Withdrawal Damage (seizures) Delayed Alcohol Withdrawal – Watch for in hospitalized patients • Chronic Neurodegeneration – Cerebellum, gray matter nuclei

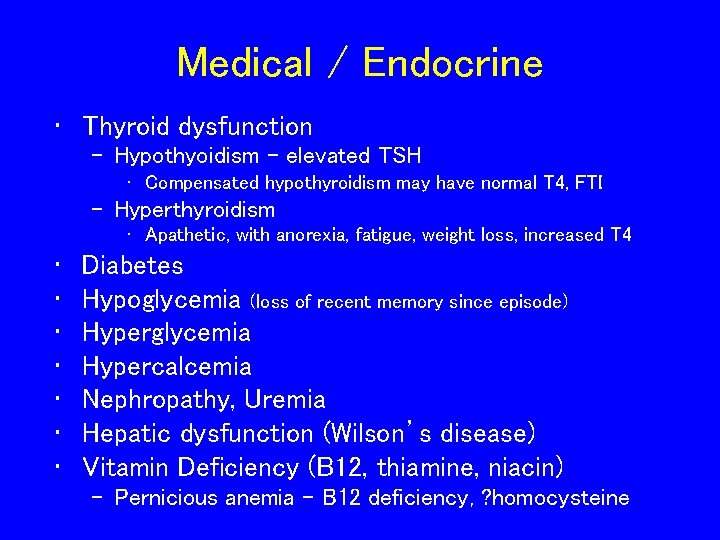
Medical / Endocrine • Thyroid dysfunction – Hypothyoidism – elevated TSH • Compensated hypothyroidism may have normal T 4, FTI – Hyperthyroidism • Apathetic, with anorexia, fatigue, weight loss, increased T 4 • • Diabetes Hypoglycemia (loss of recent memory since episode) Hyperglycemia Hypercalcemia Nephropathy, Uremia Hepatic dysfunction (Wilson’s disease) Vitamin Deficiency (B 12, thiamine, niacin) – Pernicious anemia – B 12 deficiency, ? homocysteine

Eyes, Ears, Environment • Must consider sensory deficits might contribute to the appearance of the patient being demented • Central Auditory Processing Deficits (CAPD) • Hearing problems are socially isolating • Visual problems are difficult to accommodate by a demented patient, ? To do cataract op? • Environmental stress factors can predispose to a variety of conditions • Nutritional deficiencies (tea & toast syndrome)

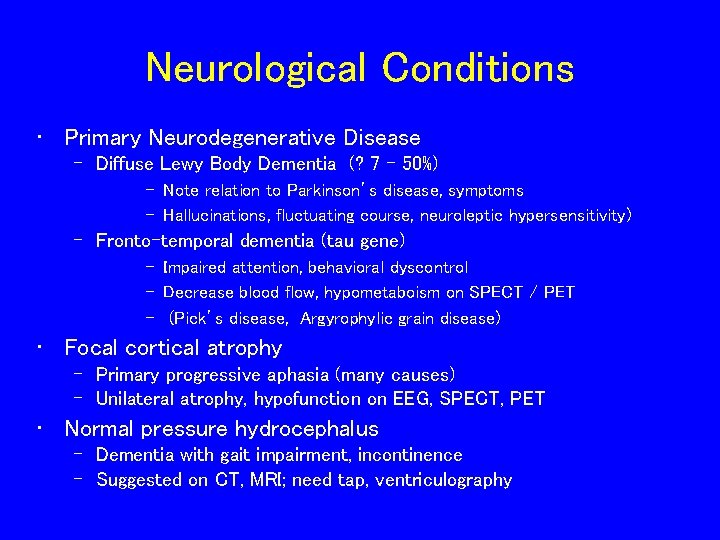
Neurological Conditions • Primary Neurodegenerative Disease – Diffuse Lewy Body Dementia (? 7 - 50%) – Note relation to Parkinson’s disease, symptoms – Hallucinations, fluctuating course, neuroleptic hypersensitivity) – Fronto-temporal dementia (tau gene) – Impaired attention, behavioral dyscontrol – Decrease blood flow, hypometaboism on SPECT / PET – (Pick’s disease, Argyrophylic grain disease) • Focal cortical atrophy – Primary progressive aphasia (many causes) – Unilateral atrophy, hypofunction on EEG, SPECT, PET • Normal pressure hydrocephalus – Dementia with gait impairment, incontinence – Suggested on CT, MRI; need tap, ventriculography

Other Neurologic Conditions – Subdural hematoma – Huntington’s disease – Creutzfeldt-Jakob disease • Rapid progression • Characteristic EEG changes – Multiple sclerosis – Corticobasal degeneraton – Cerebellar degeneration – Progressive supranuclear palsey

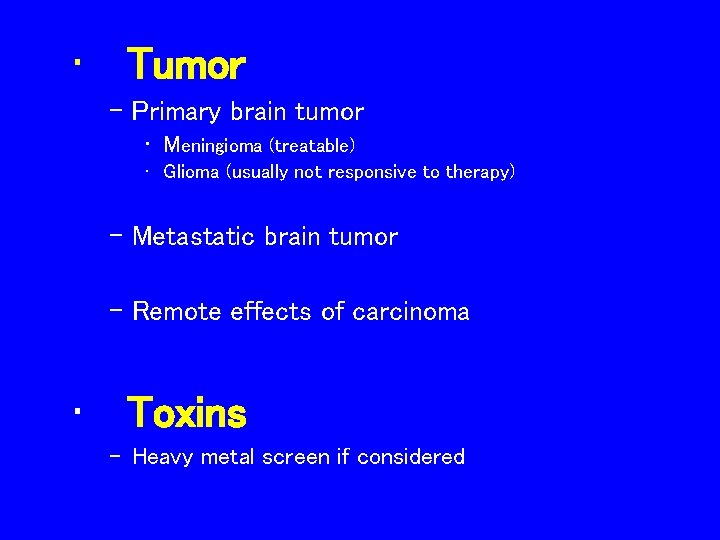
• Tumor – Primary brain tumor • Meningioma (treatable) • Glioma (usually not responsive to therapy) – Metastatic brain tumor – Remote effects of carcinoma • Toxins – Heavy metal screen if considered

Trauma – Concussion, Contusion • Occult head trauma if recent fall – Subdural hematoma – Hydrocephalus: • Normal pressure (late effect of bleed) – Dementia pugilistica – Possible contributor to Alzheimer’s disease initiation and progression (? 4% of cases) – Concern re: physical abuse by caretakers

Infectious Conditions Affecting the Brain – HIV – Neurosyphilis – Viral encephalitis (herpes) – Bacterial meningitis – Fungal (cryptococcus) – Prion (Creutzfeldt-Jakob disease); (mad cow disease)

AMNESIC DISORDER DSM-IV A. Memory impairment - inability to learn new information, or - Inability to recall previously learned information • • • Memory disturbance significantly impairs social, occupational function, deterioration from past Memory not due to delirium, dementia Physiological basis or substance induced - Distinguish from dissociative disorders, dissociative amnesia, dissociative identity disorders • Specify - Transient – less than 1 month - Chronic - more than 1 month

Causes of Amnesic Disorders • Amnesia – Dissociative: localized, selective, generalized – Organic - damage to CA 1 of hippocampus • thiamine deficiency (WKE), hypoglycemia, hypoxia • Epileptic events – Partial complex seizures • Specific brain diseases – Transient global amnesia – Multiple sclerosis

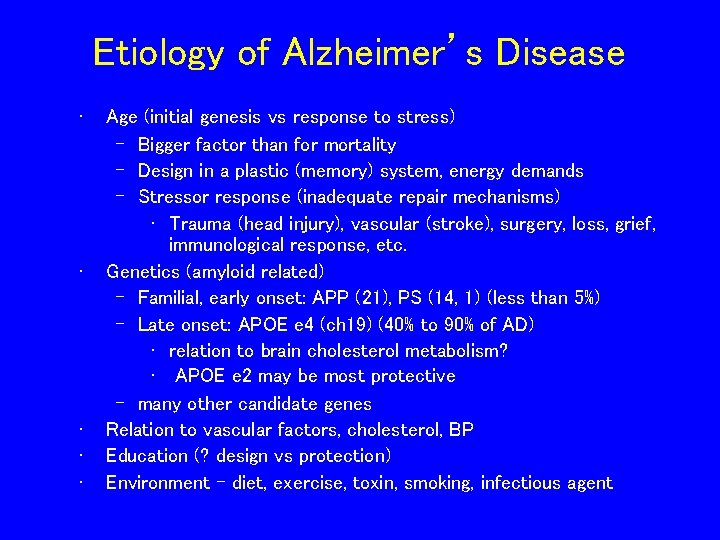
Etiology of Alzheimer’s Disease • • • Age (initial genesis vs response to stress) – Bigger factor than for mortality – Design in a plastic (memory) system, energy demands – Stressor response (inadequate repair mechanisms) • Trauma (head injury), vascular (stroke), surgery, loss, grief, immunological response, etc. Genetics (amyloid related) – Familial, early onset: APP (21), PS (14, 1) (less than 5%) – Late onset: APOE e 4 (ch 19) (40% to 90% of AD) • relation to brain cholesterol metabolism? • APOE e 2 may be most protective – many other candidate genes Relation to vascular factors, cholesterol, BP Education (? design vs protection) Environment - diet, exercise, toxin, smoking, infectious agent

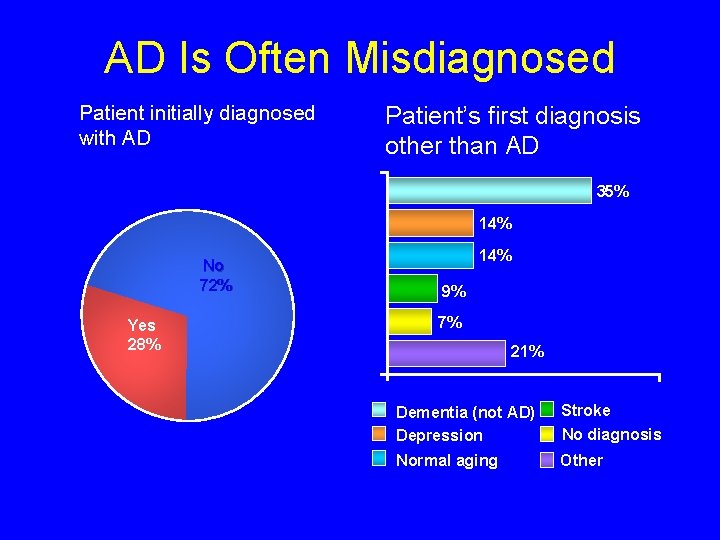
AD Is Often Misdiagnosed Patient initially diagnosed with AD Patient’s first diagnosis other than AD 35% 14% No 72% Yes 28% 14% 9% 7% 21% Dementia (not AD) Depression Stroke No diagnosis Normal aging Other Source: Consumer Health Sciences, LLC. Alzheimer’s Caregiver Project. 1999.

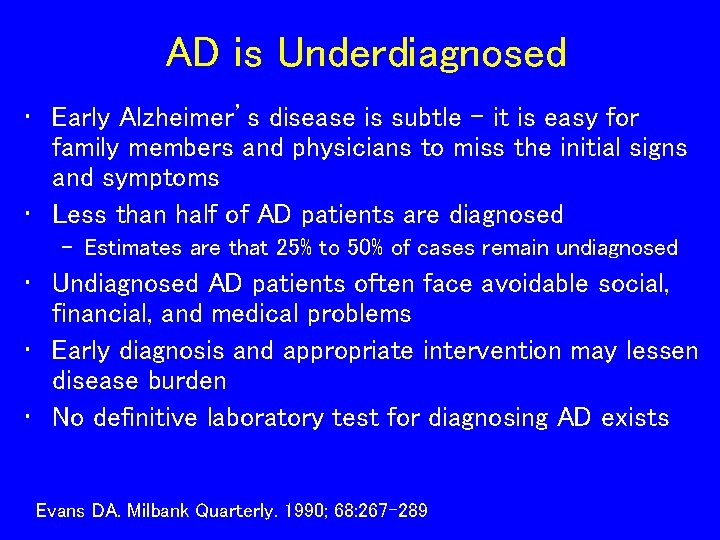
AD is Underdiagnosed • Early Alzheimer’s disease is subtle – it is easy for family members and physicians to miss the initial signs and symptoms • Less than half of AD patients are diagnosed – Estimates are that 25% to 50% of cases remain undiagnosed • Undiagnosed AD patients often face avoidable social, financial, and medical problems • Early diagnosis and appropriate intervention may lessen disease burden • No definitive laboratory test for diagnosing AD exists Evans DA. Milbank Quarterly. 1990; 68: 267 -289

AD Can Be Readily Diagnosed Mc. Khann G et al. Neurology. 1984; 34: 939 -944. Kazee AM et al. Alzheimer Dis Assoc Disord. 1993; 7: 152 -164. • A diagnosis of Alzheimer’s disease can be made with a high degree of certainty • Using NINCDS-ADRDA criteria, accuracy in autopsy-verified cases is approximately 90% • Diagnosis is a 2 -step process: – Detection through screening – Confirmation through patient history and physical, caregiver interview, brain imaging, and appropriate laboratory studies

Assessment History Of The Development Of The Dementia – – – Ask the Patient What Problem Has Brought Him to See You Ask the Family, Companion about the Problem Specifically Ask about Memory Problems Ask about the First Symptoms Enquire about Time of Onset Ask about Any Unusual Events Around the Time of Onset, e. g. , stress, trauma, surgery – Ask about Nature and Rate of Progression • Physical Examination • Neurological Examination

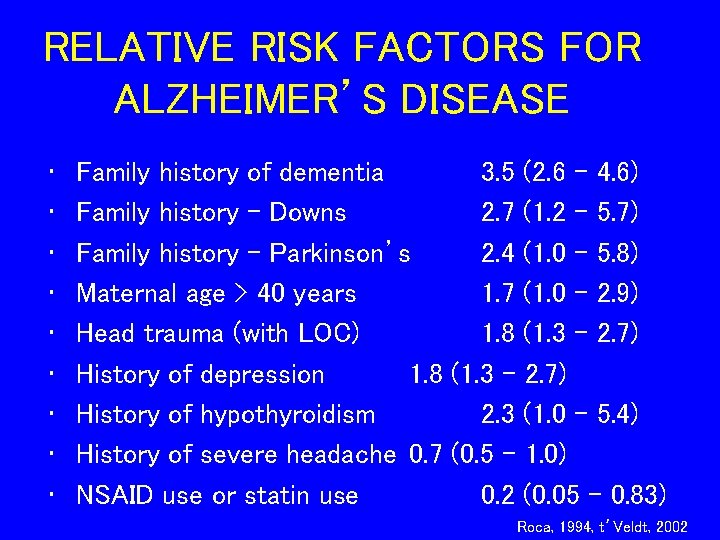
RELATIVE RISK FACTORS FOR ALZHEIMER’S DISEASE • • • Family history of dementia 3. 5 (2. 6 - 4. 6) Family history – Downs 2. 7 (1. 2 - 5. 7) Family history - Parkinson’s 2. 4 (1. 0 - 5. 8) Maternal age > 40 years 1. 7 (1. 0 - 2. 9) Head trauma (with LOC) 1. 8 (1. 3 - 2. 7) History of depression 1. 8 (1. 3 - 2. 7) History of hypothyroidism 2. 3 (1. 0 - 5. 4) History of severe headache 0. 7 (0. 5 - 1. 0) NSAID use or statin use 0. 2 (0. 05 – 0. 83) Roca, 1994, t’Veldt, 2002

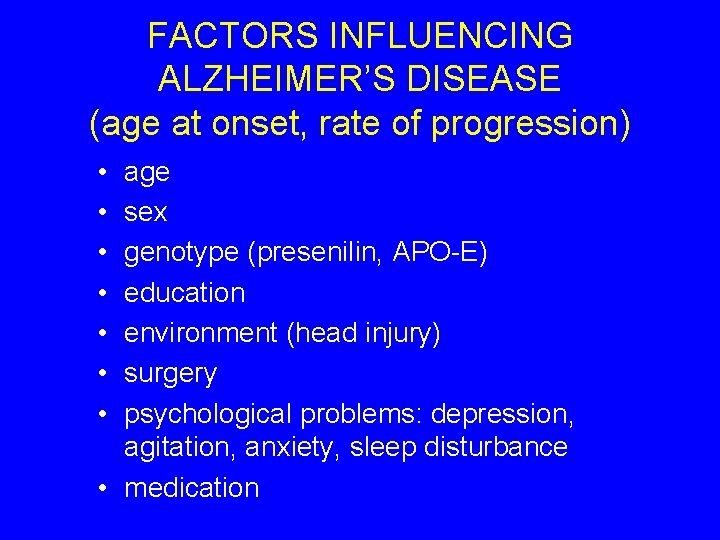
FACTORS INFLUENCING ALZHEIMER’S DISEASE (age at onset, rate of progression) • • age sex genotype (presenilin, APO-E) education environment (head injury) surgery psychological problems: depression, agitation, anxiety, sleep disturbance • medication

PHYSICAL/NEUROLOGICAL EXAMINATION • CHECK BLOOD PRESSURE • IDENTIFY SYSTEMIC DISORDERS • CRANIAL NERVES – Olfactory dysfunction, poor eye tracking – Check for hearing, vision deficits • SENSORY DEFICITS – Proprioception, vibration • DEEP TENDON REFLEXES – Brisk, check for focal reflexes • PATHOLOGIC REFLEXES – Hyperactive snout reflex, Gegenhalten

NEUROPSYCHOLOGICAL TESTING (WAIS, WECHSLER) • • MEMORY: SHORT-TERM, REMOTE VERBAL FUNCTION, FLUENCY VISUO-SPATIAL FUNCTION ATTENTION EXECUTIVE FUNCTION ABSTRACT THINKING ACCOUNT FOR EDUCATION ACCOUNT FOR PRIOR DISFUNCTIONS

CURRENT APPROACHES TO SEVERITY ASSESSMENT • • • MINI-MENTAL STATE EXAM CLOCK DRAWING ANIMAL NAMING (1 minute) MATTIS DEMENTIA RATING SCALE ALZHEIMER’S DISEASE ASSESSEMENT SCALE (ADAS) ACTIVITIES OF DAILY LIVING GLOBAL CLINICAL SCALE CLINICAL DEMENTIA RATING SCALE GLOBAL DETERIORATION SCALE / FAST

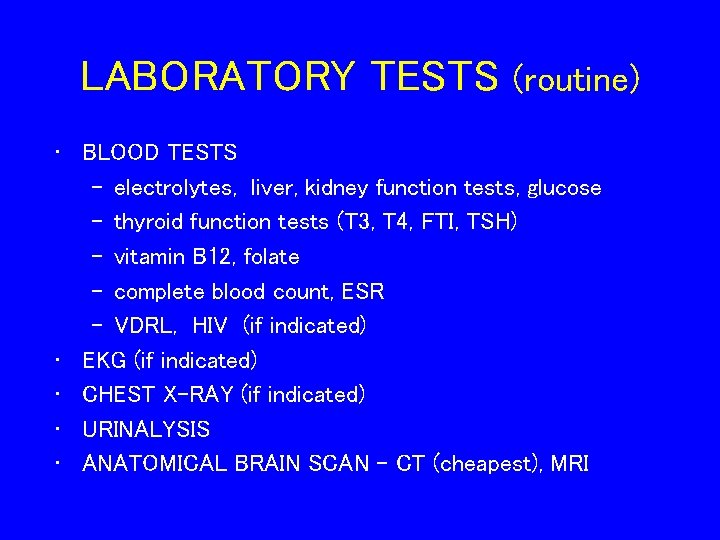
LABORATORY TESTS (routine) • BLOOD TESTS – electrolytes, liver, kidney function tests, glucose – thyroid function tests (T 3, T 4, FTI, TSH) – vitamin B 12, folate – complete blood count, ESR – VDRL, HIV (if indicated) • EKG (if indicated) • CHEST X-RAY (if indicated) • URINALYSIS • ANATOMICAL BRAIN SCAN – CT (cheapest), MRI

SPECIAL LABORATORY TESTS • FUNCTIONAL BRAIN IMAGING (SPECT, PET) • EEG, Evoked Potentials (P 300) • REACTION TIMES (slowed in the elderly, especially when complex response is required • CSF ANALYSIS - ROUTINE STUDIES – ELEVATED TAU (future possible) – DECREASED AMYLOID (future possible) • HEAVY METAL SCREEN (24 hr urine) • GENOTYPING – APO-LIPOPROTEIN-E (for supporting diagnosis) – AUTOSOMAL DOMINANT (young onset)

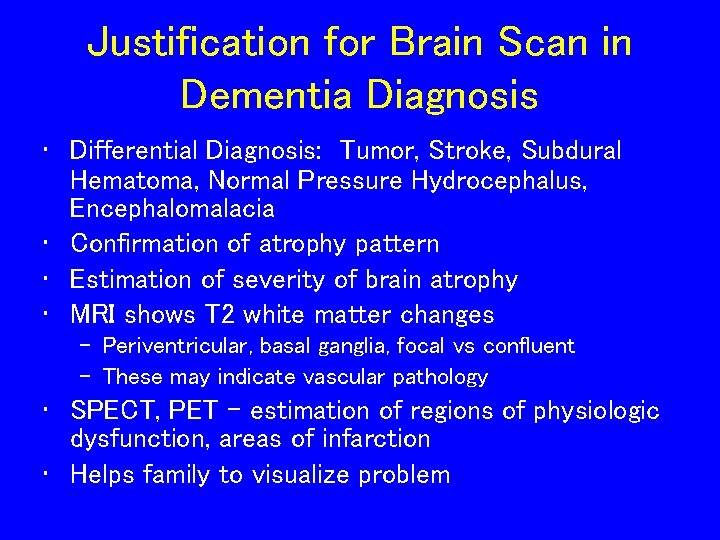
Justification for Brain Scan in Dementia Diagnosis • Differential Diagnosis: Tumor, Stroke, Subdural Hematoma, Normal Pressure Hydrocephalus, Encephalomalacia • Confirmation of atrophy pattern • Estimation of severity of brain atrophy • MRI shows T 2 white matter changes – Periventricular, basal ganglia, focal vs confluent – These may indicate vascular pathology • SPECT, PET - estimation of regions of physiologic dysfunction, areas of infarction • Helps family to visualize problem

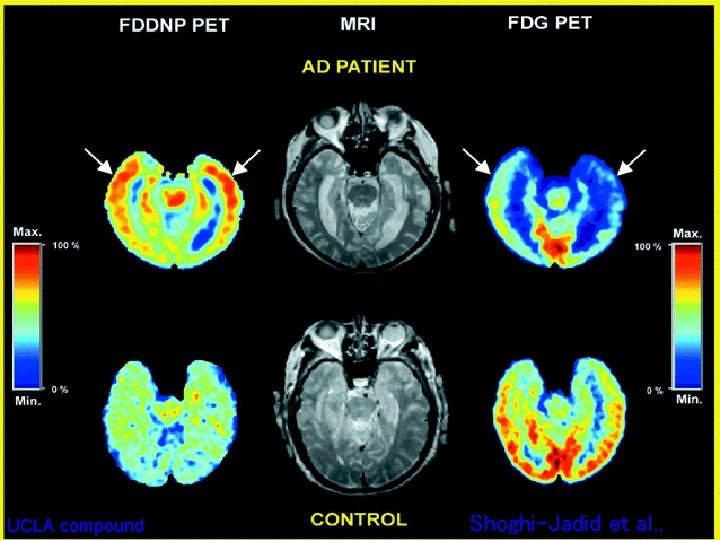
UCLA compound Shoghi-Jadid et al. ,

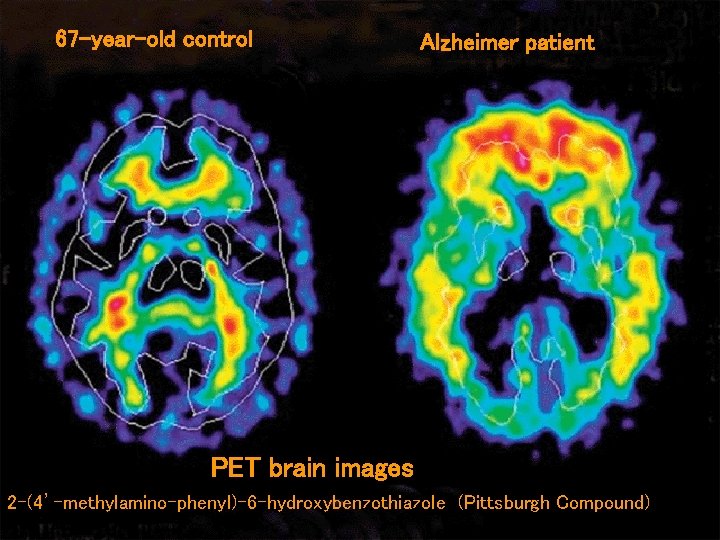
67 -year-old control Alzheimer patient PET brain images 2 -(4’-methylamino-phenyl)-6 -hydroxybenzothiazole (Pittsburgh Compound)

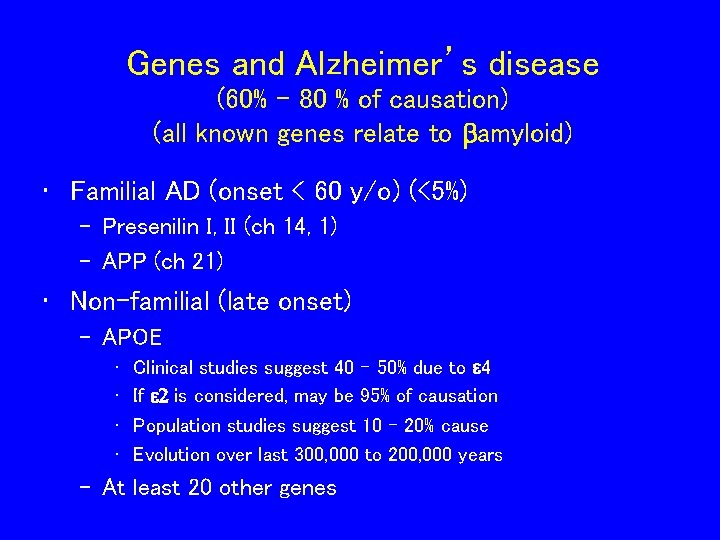
Genes and Alzheimer’s disease (60% - 80 % of causation) (all known genes relate to bamyloid) • Familial AD (onset < 60 y/o) (<5%) – Presenilin I, II (ch 14, 1) – APP (ch 21) • Non-familial (late onset) – APOE • • Clinical studies suggest 40 – 50% due to e 4 If e 2 is considered, may be 95% of causation Population studies suggest 10 – 20% cause Evolution over last 300, 000 to 200, 000 years – At least 20 other genes

APO-E genotype and AD risk 46 Million in US > 60 y/o //// 4 Million have AD (data from Saunders et al. , 1993; Farrer et al. , 1997) JW Ashford, MD Ph. D, 2003

Are we ready to do genetic testing to predict AD? • The family members want it – They consider recommendations against genetic testing to be “paternalistic” • Family members can make more powerful financial decisions based on this knowledge than the relevance of insurance companies implementing changes in actuarial calculations • Those at risk can seek more frequent testing – This is the best opportunity for early recognition • Those at risk will be better advocates for research • Specific preventive treatments can be developed for each genetic factor

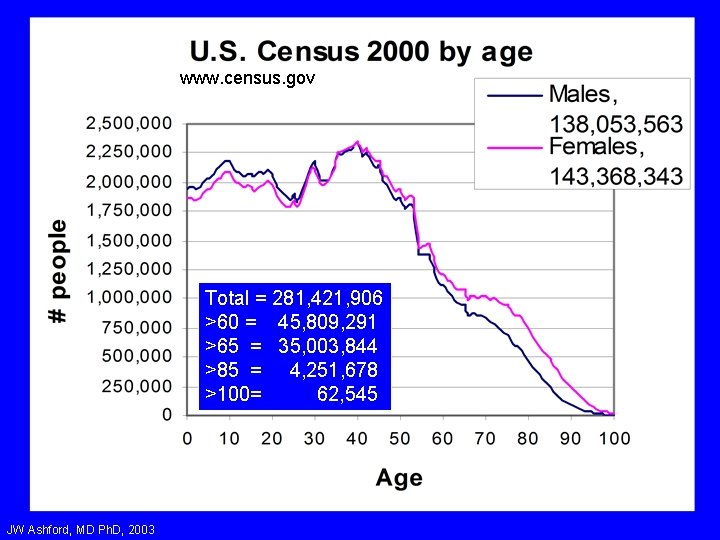
www. census. gov Total = 281, 421, 906 >60 = 45, 809, 291 >65 = 35, 003, 844 >85 = 4, 251, 678 >100= 62, 545 JW Ashford, MD Ph. D, 2003

www. cdc. gov JW Ashford, MD Ph. D, 2003

JW Ashford, MD Ph. D, 2003

JW Ashford, MD Ph. D, 2003

JW Ashford, MD Ph. D, 2003

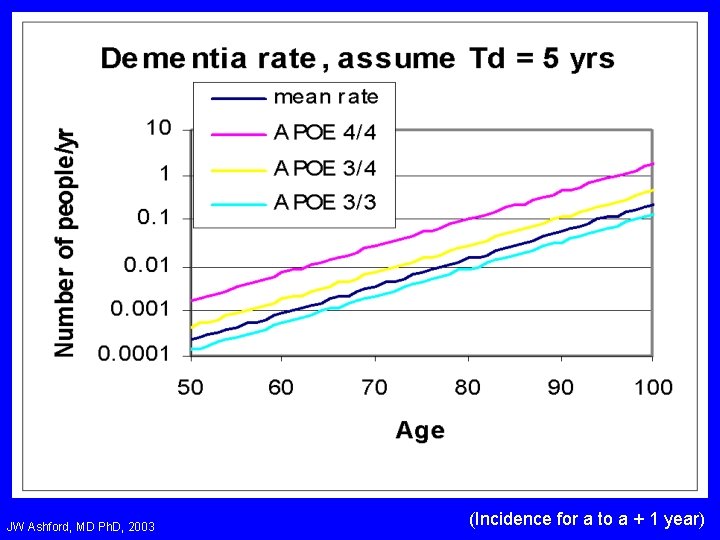
JW Ashford, MD Ph. D, 2003 (Incidence for a to a + 1 year)

JW Ashford, MD Ph. D, 2003

JW Ashford, MD Ph. D, 2000

JW Ashford, MD Ph. D, 2003

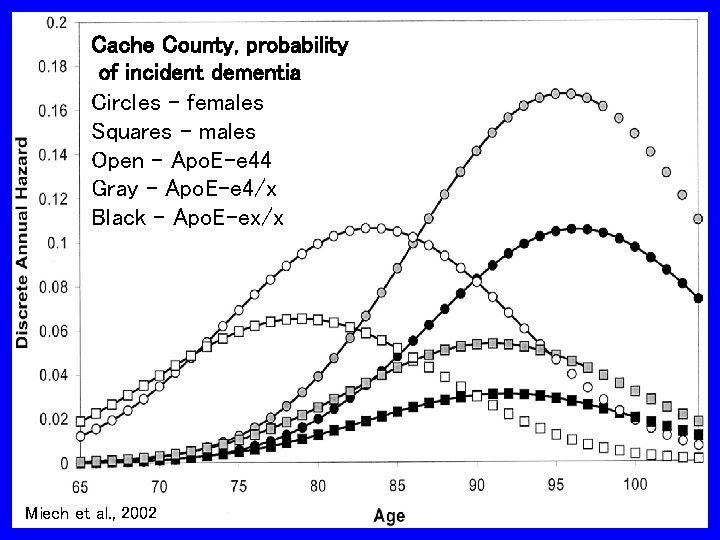
Cache County, probability of incident dementia Circles – females Squares - males Open – Apo. E-e 44 Gray – Apo. E-e 4/x Black – Apo. E-ex/x Miech et al. , 2002

Why Diagnose AD Early? • Safety (driving, compliance, cooking, etc. ) • Family stress and misunderstanding (blame, denial) • Early education of caregivers of how to handle patient (choices, getting started) • Advance planning while patient is competent (will, proxy, power of attorney, advance directives) • Patient’s and Family’s right to know • Specific treatments now available – May slow underlying disease process – May delay nursing home placement longer if started earlier

Need for Better Screening and Early Assessment Tools • Genetic vulnerability testing (trait risk) • Vulnerability factors (education, occupation, head injury) • Early recognition (10 warning signs) • Screening tools (6 th vital sign in elderly) • Positive diagnostic tests – CSF – tau levels elevated, amyloid levels low – Brain scan – PET – DDNP, Congo-red derivatives • Mild Dementia severity assessments • Detecting early change over time – predicting progression, measuring rate

Need for a Brief Screening Test for Alzheimer’s Disease • Recent evidence of benefits of anticholinesterase agents in the treatment of mild Alzheimer’s disease – Improvement of cognition – Slowing of progression

Alzheimer Warning Signs Top Ten Alzheimer Association 1. Recent memory loss affecting job 2. Difficulty performing familiar tasks 3. Problems with language 4. Disorientation to time or place 5. Poor or decreased judgment 6. Problems with abstract thinking 7. Misplacing things 8. Changes in mood or behavior 9. Changes in personality 10. Loss of initiative

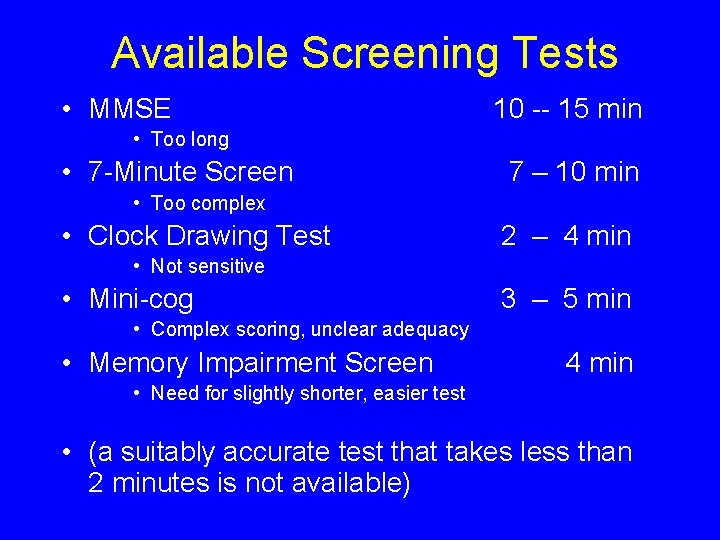
Available Screening Tests • MMSE 10 -- 15 min • Too long • 7 -Minute Screen 7 – 10 min • Too complex • Clock Drawing Test 2 – 4 min • Not sensitive • Mini-cog 3 – 5 min • Complex scoring, unclear adequacy • Memory Impairment Screen 4 min • Need for slightly shorter, easier test • (a suitably accurate test that takes less than 2 minutes is not available)


JW Ashford, MD Ph. D, 2001


Brief Alzheimer Screen (BAS) • Repeat these three words: “apple, table, penny”. • So you will remember these words, repeat them again. • What is today’s date? • D = 1 if within 2 days. • Spell the word “WORLD” backwards • S = 1 point for each word in correct order • “Name as many animals as you can in 30 seconds, GO!” • A = number of animals • “What were the 3 words I asked you to repeat? ” (no prompts) • R = 1 point for each word recalled BAS = 3 x R + 2/3 x A + 5 x D + 2 x S

JW Ashford, MD Ph. D, 2001 Mendiondo et al. , 2004

JW Ashford, MD Ph. D, 2003

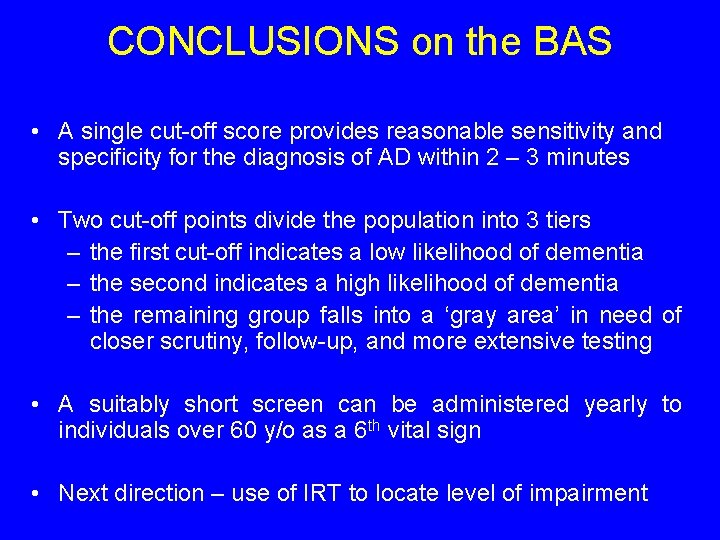
CONCLUSIONS on the BAS • A single cut-off score provides reasonable sensitivity and specificity for the diagnosis of AD within 2 – 3 minutes • Two cut-off points divide the population into 3 tiers – the first cut-off indicates a low likelihood of dementia – the second indicates a high likelihood of dementia – the remaining group falls into a ‘gray area’ in need of closer scrutiny, follow-up, and more extensive testing • A suitably short screen can be administered yearly to individuals over 60 y/o as a 6 th vital sign • Next direction – use of IRT to locate level of impairment

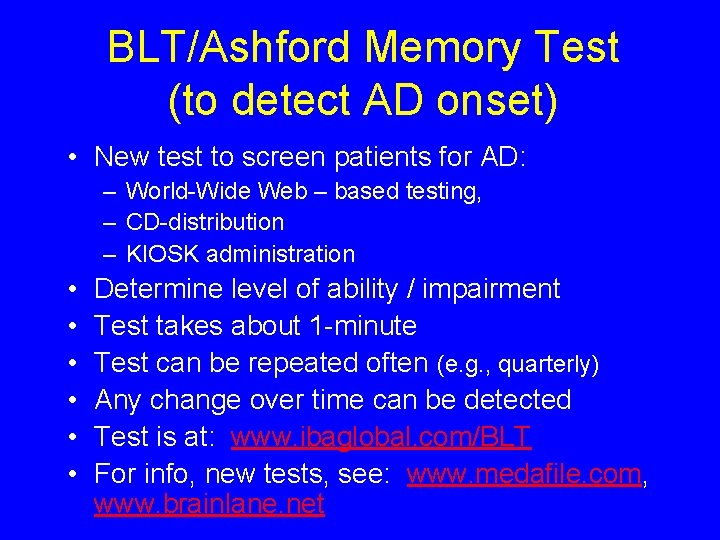
BLT/Ashford Memory Test (to detect AD onset) • New test to screen patients for AD: – World-Wide Web – based testing, – CD-distribution – KIOSK administration • • • Determine level of ability / impairment Test takes about 1 -minute Test can be repeated often (e. g. , quarterly) Any change over time can be detected Test is at: www. ibaglobal. com/BLT For info, new tests, see: www. medafile. com, www. brainlane. net
 Quick mild cognitive impairment screen
Quick mild cognitive impairment screen Gaduh gelisah icd 10
Gaduh gelisah icd 10 Kode icd 10 mata kedutan
Kode icd 10 mata kedutan Nanda nursing diagnosis for eye disorders
Nanda nursing diagnosis for eye disorders Care plan of cataract
Care plan of cataract Cerebrovascular accident
Cerebrovascular accident Nursing diagnosis for vision impairment
Nursing diagnosis for vision impairment Nursing diagnosis for meniere's disease
Nursing diagnosis for meniere's disease Threat modelling consultant
Threat modelling consultant Nina ashford
Nina ashford Mr and mrs ashford
Mr and mrs ashford Ashford school code
Ashford school code Ken wesson
Ken wesson Kyle wesson
Kyle wesson Lauren wesson
Lauren wesson Cognitive and non cognitive religious language
Cognitive and non cognitive religious language Nursing process
Nursing process Perbedaan diagnosis gizi dan diagnosis medis
Perbedaan diagnosis gizi dan diagnosis medis Medical diagnosis and nursing diagnosis difference
Medical diagnosis and nursing diagnosis difference Second phase of nursing process
Second phase of nursing process Types of nursing diagnoses
Types of nursing diagnoses Molar pregnancy symptoms
Molar pregnancy symptoms Differential diagnosis of learning disabilities
Differential diagnosis of learning disabilities Bakers cyst medscape
Bakers cyst medscape Corte axial
Corte axial Papulovesicular rash differential diagnosis
Papulovesicular rash differential diagnosis Kavram öğretiminde dikkat edilmesi gereken noktalar
Kavram öğretiminde dikkat edilmesi gereken noktalar Differential diagnosis of stroke
Differential diagnosis of stroke Hydrocephalus ddx
Hydrocephalus ddx Acute productive cough differential diagnosis
Acute productive cough differential diagnosis Ulcer niche and ulcer notch
Ulcer niche and ulcer notch Differential diagnosis of osmf
Differential diagnosis of osmf Carpal tunnel syndrome differential diagnosis
Carpal tunnel syndrome differential diagnosis Cervical myelopathy wayne
Cervical myelopathy wayne Broadbent sign in constrictive pericarditis
Broadbent sign in constrictive pericarditis Fairy skin
Fairy skin Vitamin c differential diagnosis mnemonic
Vitamin c differential diagnosis mnemonic Polymorphs pour out to sink to bottom of anterior chamber
Polymorphs pour out to sink to bottom of anterior chamber Leukocoria differential diagnosis
Leukocoria differential diagnosis Intrahepatic jaundice
Intrahepatic jaundice Differential diagnosis of articular syndrome
Differential diagnosis of articular syndrome Differential diagnosis of stroke
Differential diagnosis of stroke Differential diagnosis of otitis externa
Differential diagnosis of otitis externa Tnm staging of breast cancer
Tnm staging of breast cancer Dka in pregnancy
Dka in pregnancy Differential diagnosis red eye
Differential diagnosis red eye Hpilora
Hpilora Epigastric pain differential diagnosis
Epigastric pain differential diagnosis What is podoconiosis
What is podoconiosis Differential diagnosis for premature rupture of membranes
Differential diagnosis for premature rupture of membranes Mentzer index
Mentzer index Differential diagnosis for atopic dermatitis
Differential diagnosis for atopic dermatitis Nephrotic syndrome
Nephrotic syndrome Differential diagnosis of dysphagia
Differential diagnosis of dysphagia Differential diagnosis of thrombocytosis
Differential diagnosis of thrombocytosis Differential diagnosis of vitiligo
Differential diagnosis of vitiligo Hormetonia
Hormetonia Leukemia survival rate
Leukemia survival rate Megaloblastic anemia laboratory findings
Megaloblastic anemia laboratory findings Define demetia
Define demetia Non pitting edema differential diagnosis
Non pitting edema differential diagnosis Abdominal quadrants and organs
Abdominal quadrants and organs Ulcer anatomy
Ulcer anatomy Abnormal uterine bleeding in postmenopausal
Abnormal uterine bleeding in postmenopausal Vomiting case presentation
Vomiting case presentation Spotting differential diagnosis
Spotting differential diagnosis Black tarry stool
Black tarry stool Pulmonary hypertension differential diagnosis
Pulmonary hypertension differential diagnosis Phlebolymphedema
Phlebolymphedema Pre-hepatic jaundice
Pre-hepatic jaundice Branchial cyst aspiration
Branchial cyst aspiration Parkinson differential diagnosis
Parkinson differential diagnosis