Chemistry The Central Science Fourteenth Edition Chapter 16




![I. p. H p. OH = -Log [OH-] = 10 -p. OH p. H I. p. H p. OH = -Log [OH-] = 10 -p. OH p. H](https://slidetodoc.com/presentation_image_h2/951f030b08d69c3dc46c37a508f36790/image-20.jpg)
![p. H & Water Due to auto ionization of water, Kw = [H+] [OH-] p. H & Water Due to auto ionization of water, Kw = [H+] [OH-]](https://slidetodoc.com/presentation_image_h2/951f030b08d69c3dc46c37a508f36790/image-25.jpg)


![WEAK ACIDS/BASES & EQUILIBRIUM HA(aq) H+ (aq) + A- (aq) Ka = [H+] [A-] WEAK ACIDS/BASES & EQUILIBRIUM HA(aq) H+ (aq) + A- (aq) Ka = [H+] [A-]](https://slidetodoc.com/presentation_image_h2/951f030b08d69c3dc46c37a508f36790/image-29.jpg)
![Weak Acids [Table 16. 2 continued] *The proton that ionizes is shown in red. Weak Acids [Table 16. 2 continued] *The proton that ionizes is shown in red.](https://slidetodoc.com/presentation_image_h2/951f030b08d69c3dc46c37a508f36790/image-35.jpg)


![RECALL Percent HA dissociation = [HA]dissociated x 100 [HA]initial Polyprotic acids with more than RECALL Percent HA dissociation = [HA]dissociated x 100 [HA]initial Polyprotic acids with more than](https://slidetodoc.com/presentation_image_h2/951f030b08d69c3dc46c37a508f36790/image-40.jpg)
![Base-Dissociation Constants [Table 16. 4 continued] Conjugate Acid K sub b Base Structural Formula* Base-Dissociation Constants [Table 16. 4 continued] Conjugate Acid K sub b Base Structural Formula*](https://slidetodoc.com/presentation_image_h2/951f030b08d69c3dc46c37a508f36790/image-47.jpg)










- Slides: 68

Chemistry: The Central Science Fourteenth Edition Chapter 16 Acid–Base Equilibria

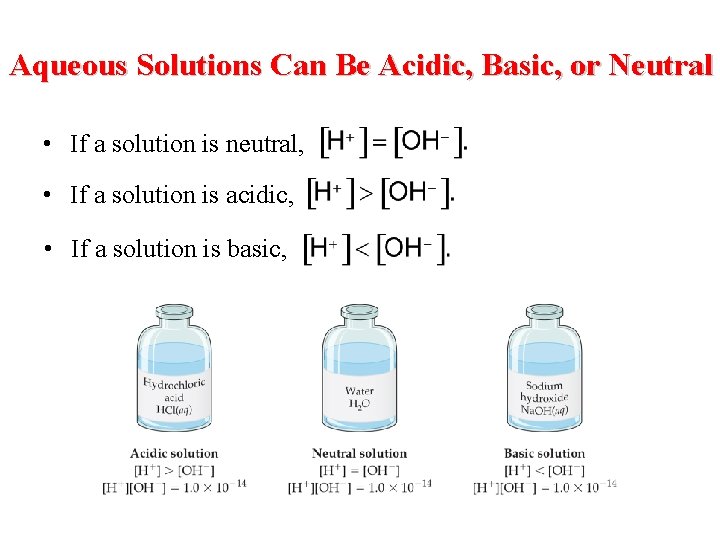
Aqueous Solutions Can Be Acidic, Basic, or Neutral • If a solution is neutral, • If a solution is acidic, • If a solution is basic,

ACIDS & BASES Click on topic for greater detail Acids: - acids are sour tasting - Arrhenius acid: Any substance that when dissolved in water, increases the concentration of hydronium ion (H 3 O+) - Bronsted-Lowry acid: A proton donor - Lewis Acid: An Electron acceptor Bases: - bases are bitter tasting and slippery - Arrhenius base: Any substance that when dissolved in water, increases the concentration of hydroxide ion (OH-) - Bronsted-Lowery base: A proton acceptor - Lewis base: An electron donor

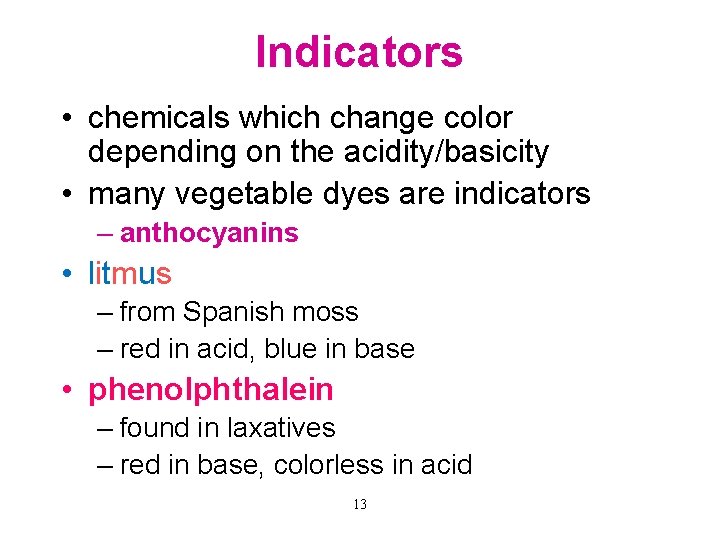
Indicators • chemicals which change color depending on the acidity/basicity • many vegetable dyes are indicators – anthocyanins • litmus – from Spanish moss – red in acid, blue in base • phenolphthalein – found in laxatives – red in base, colorless in acid 13

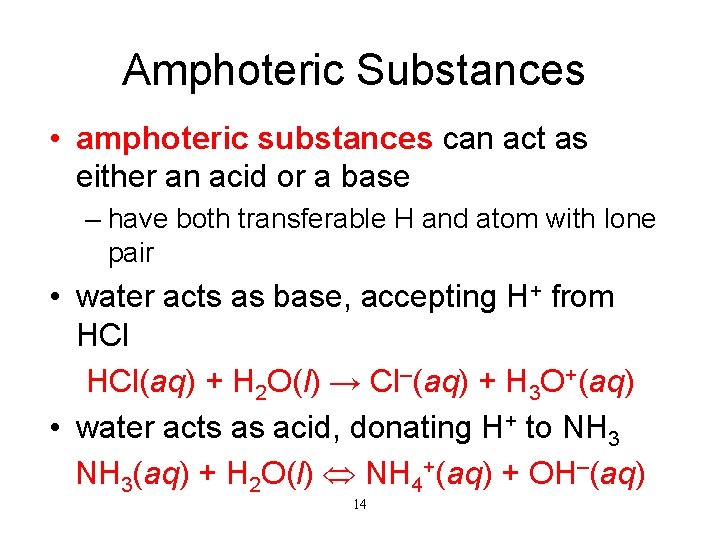
Amphoteric Substances • amphoteric substances can act as either an acid or a base – have both transferable H and atom with lone pair • water acts as base, accepting H+ from HCl(aq) + H 2 O(l) → Cl–(aq) + H 3 O+(aq) • water acts as acid, donating H+ to NH 3(aq) + H 2 O(l) NH 4+(aq) + OH–(aq) 14

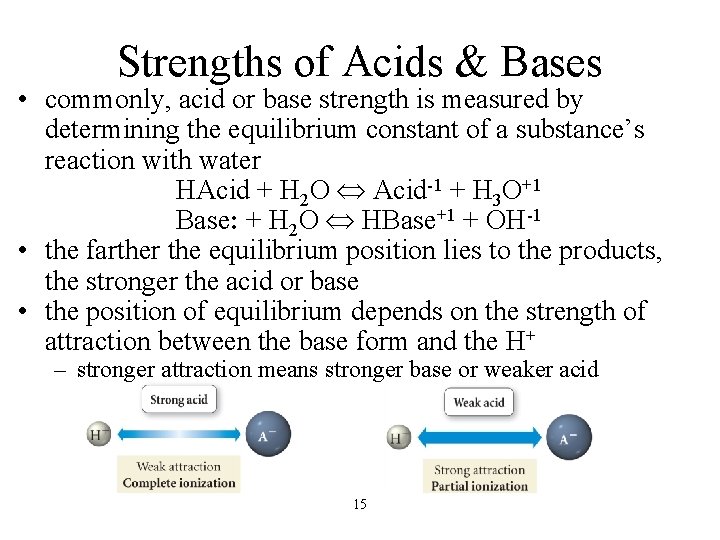
Strengths of Acids & Bases • commonly, acid or base strength is measured by determining the equilibrium constant of a substance’s reaction with water HAcid + H 2 O Acid-1 + H 3 O+1 Base: + H 2 O HBase+1 + OH-1 • the farther the equilibrium position lies to the products, the stronger the acid or base • the position of equilibrium depends on the strength of attraction between the base form and the H+ – stronger attraction means stronger base or weaker acid 15

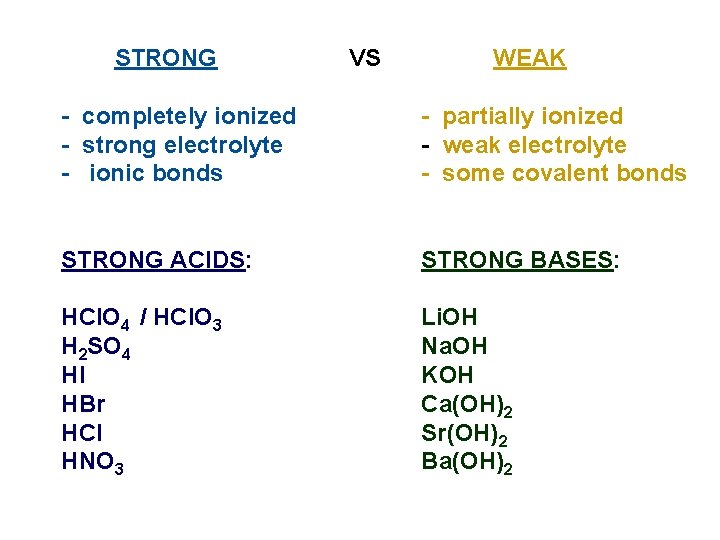
STRONG VS WEAK - completely ionized - strong electrolyte - ionic bonds - partially ionized - weak electrolyte - some covalent bonds STRONG ACIDS: STRONG BASES: HCl. O 4 / HCl. O 3 H 2 SO 4 Hl HBr HCl HNO 3 Li. OH Na. OH KOH Ca(OH)2 Sr(OH)2 Ba(OH)2

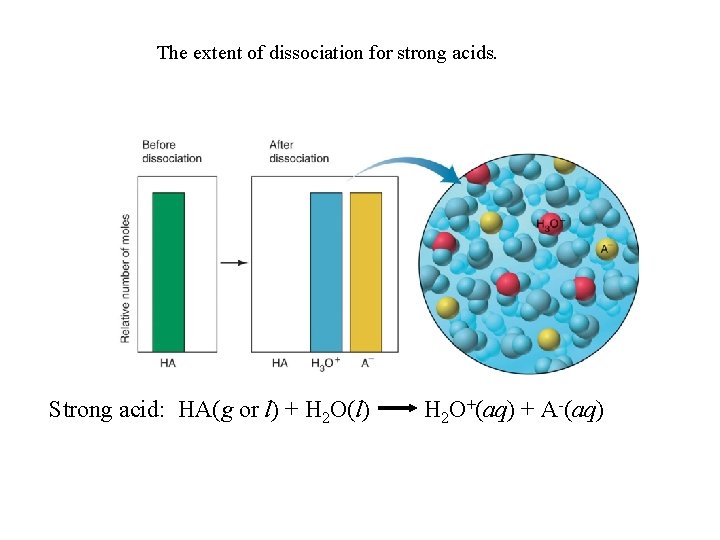
The extent of dissociation for strong acids. Strong acid: HA(g or l) + H 2 O(l) H 2 O+(aq) + A-(aq)

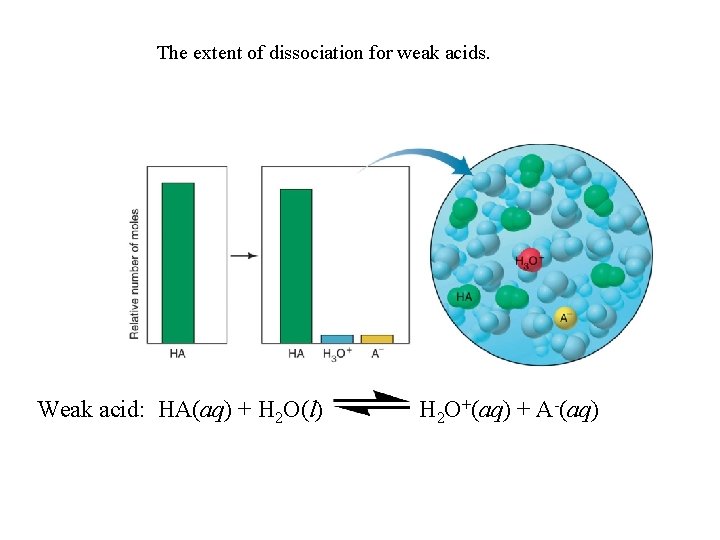
The extent of dissociation for weak acids. Weak acid: HA(aq) + H 2 O(l) H 2 O+(aq) + A-(aq)

General Trends in Acidity • the stronger an acid is at donating H, the weaker the conjugate base is at accepting H • higher oxidation number = stronger oxyacid – H 2 SO 4 > H 2 SO 3; HNO 3 > HNO 2 • cation stronger acid than neutral molecule; neutral stronger acid than anion – H 3 O+1 > H 2 O > OH-1; NH 4+1 > NH 3 > NH 2 -1 – base trend opposite 19

Predict the product and describe each species as strong or weak acids or bases. HI + Sr(OH)2 H 3 O+ + C 5 H 5 N

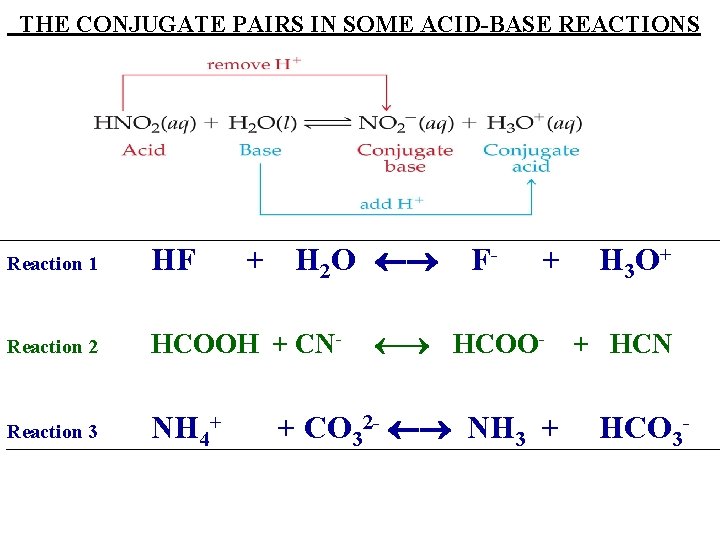
THE CONJUGATE PAIRS IN SOME ACID-BASE REACTIONS + H 2 O Reaction 1 HF Reaction 2 HCOOH + CN- Reaction 3 NH 4+ F- + HCOO- + CO 32 - NH 3 + H 3 O+ + HCN HCO 3 -

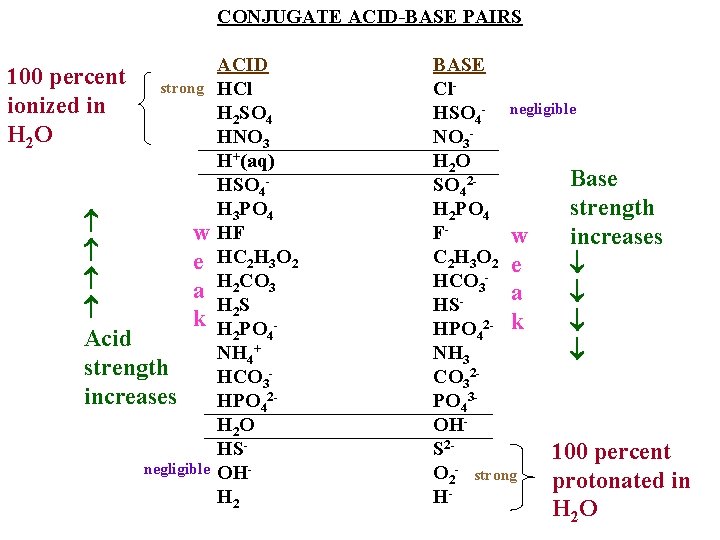
CONJUGATE ACID-BASE PAIRS 100 percent ionized in H 2 O strong Acid strength increases w e a k negligible ACID HCl H 2 SO 4 HNO 3 H+(aq) HSO 4 H 3 PO 4 HF HC 2 H 3 O 2 H 2 CO 3 H 2 S H 2 PO 4 NH 4+ HCO 3 HPO 42 H 2 O HSOHH 2 BASE Cl. HSO 4 - negligible NO 3 H 2 O Base SO 42 strength H 2 PO 4 Fw increases C 2 H 3 O 2 e HCO 3 a HS HPO 42 - k NH 3 CO 32 PO 43 OHS 2100 percent O 2 - strong protonated in H H 2 O

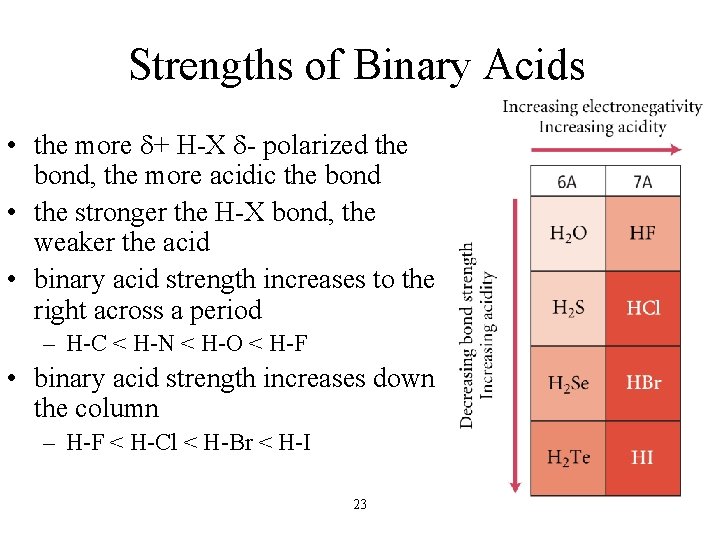
Strengths of Binary Acids • the more d+ H-X d- polarized the bond, the more acidic the bond • the stronger the H-X bond, the weaker the acid • binary acid strength increases to the right across a period – H-C < H-N < H-O < H-F • binary acid strength increases down the column – H-F < H-Cl < H-Br < H-I 23

Strengths of Oxyacids, H-O-Y • the more electronegative the Y atom, the stronger the acid – helps weakens the H-O bond • the more oxygens attached to Y, the stronger the acid – further weakens and polarizes the H-O bond 24

The strength of an acid depends on how easily the proton, H+, is lost or removed from an H - X bond. Greater Acid Strength: - more polar bonds - larger “X” atom - oxo acids: higher electronegativity - oxo acids: more oxygen atoms - oxo acids: more hydrogen atoms List the following in order of increasing strength: l. HI, HF, HCl 2. H 2 O, CH 4, HF 3. HIO 3, HCl. O 3, HBr. O 3 4. HBr. O, HBr. O 3, HBr. O 2 5. HI, H 2 SO 4, HCl. O 4, HNO 3

SAMPLE PROBLEM Classifying Acid and Base Strength from the Chemical Formula Classify each of the following compounds as a strong acid, weak acid, strong base, or weak base. (a) H 2 SO 4 PLAN: (b) (CH 3)2 CHCOOH (c) KOH (d) (CH 3)2 CHNH 2 Pay attention to the text definitions of acids and bases. Look at O for acids as well as the -COOH group; watch for amine groups and cations in bases. SOLUTION:

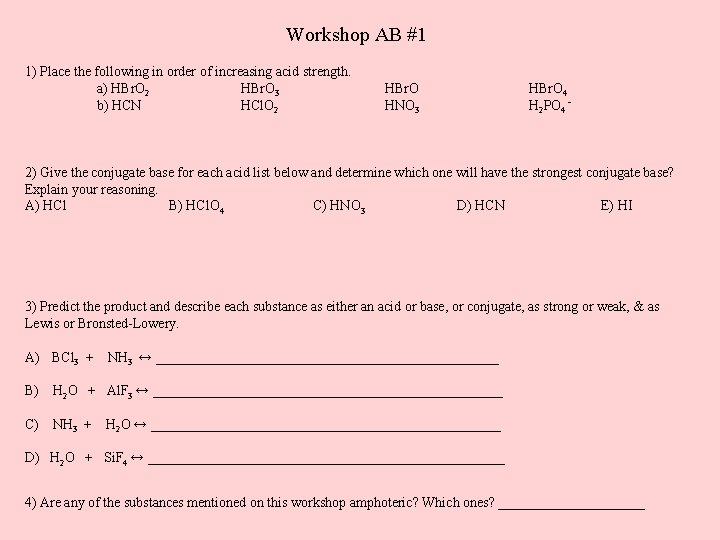
Workshop AB #1 1) Place the following in order of increasing acid strength. a) HBr. O 2 HBr. O 3 b) HCN HCl. O 2 HBr. O HNO 3 HBr. O 4 H 2 PO 4⁻ 2) Give the conjugate base for each acid list below and determine which one will have the strongest conjugate base? Explain your reasoning. A) HCl B) HCl. O 4 C) HNO 3 D) HCN E) HI 3) Predict the product and describe each substance as either an acid or base, or conjugate, as strong or weak, & as Lewis or Bronsted-Lowery. A) BCl 3 + NH 3 ↔ _________________________ B) H 2 O + Al. F 3 ↔ _________________________ C) NH 3 + H 2 O ↔ _________________________ D) H 2 O + Si. F 4 ↔ __________________________ 4) Are any of the substances mentioned on this workshop amphoteric? Which ones? ___________

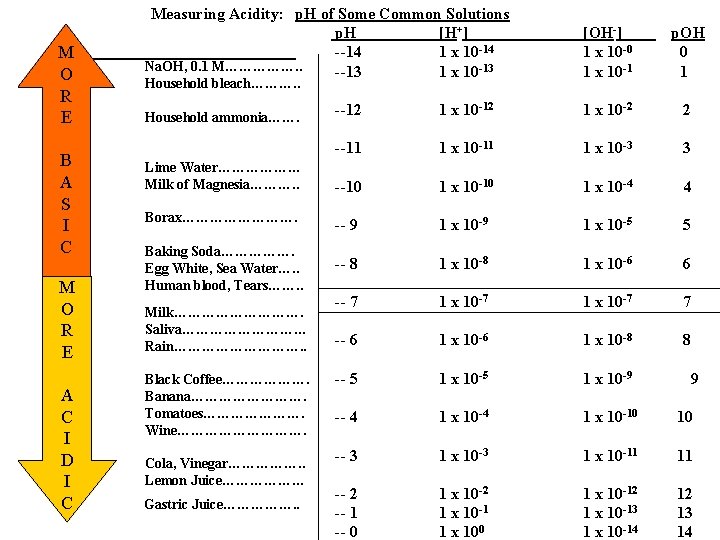
M O R E B A S I C M O R E A C I D I C Measuring Acidity: p. H of Some Common Solutions p. H [H+] --14 1 x 10 -14 Na. OH, 0. 1 M……………. . --13 1 x 10 -13 Household bleach………. . Household ammonia……. Lime Water……………… Milk of Magnesia………. . Borax…………. Baking Soda……………. Egg White, Sea Water…. . Human blood, Tears……. . Milk……………. Saliva…………… Rain……………. . Black Coffee………………. Banana…………. Tomatoes…………………. Wine……………. Cola, Vinegar……………. . Lemon Juice……………… Gastric Juice……………. . [OH-] 1 x 10 -0 1 x 10 -1 p. OH 0 1 --12 1 x 10 -2 2 --11 1 x 10 -3 3 --10 1 x 10 -4 4 -- 9 1 x 10 -5 5 -- 8 1 x 10 -6 6 -- 7 1 x 10 -7 7 -- 6 1 x 10 -8 8 -- 5 1 x 10 -9 -- 4 1 x 10 -10 10 -- 3 1 x 10 -11 11 -- 2 -- 1 -- 0 1 x 10 -2 1 x 10 -1 1 x 100 1 x 10 -12 1 x 10 -13 1 x 10 -14 12 13 14 9
![I p H p OH Log OH 10 p OH p H I. p. H p. OH = -Log [OH-] = 10 -p. OH p. H](https://slidetodoc.com/presentation_image_h2/951f030b08d69c3dc46c37a508f36790/image-20.jpg)
I. p. H p. OH = -Log [OH-] = 10 -p. OH p. H = -Log [H+] = 10 -p. H Practice Problems on p. H: 1. A 0. 0015 M Na. OH solution has what p. OH? [OH-]? 2. A 0. 00015 M nitric acid solution has what p. H? [H+]?

How Do We Measure p. H? • One method is with indicators. • They give less accurate, but quick measurements. • An indicator is a compound that has one color in its acid form and another color in its basic form.

Methods for measuring the p. H of an aqueous solution Another way to measure p. H is with a p. H meters are used for accurate measurement of p. H; electrodes indicate small changes in voltage to detect p. H meter

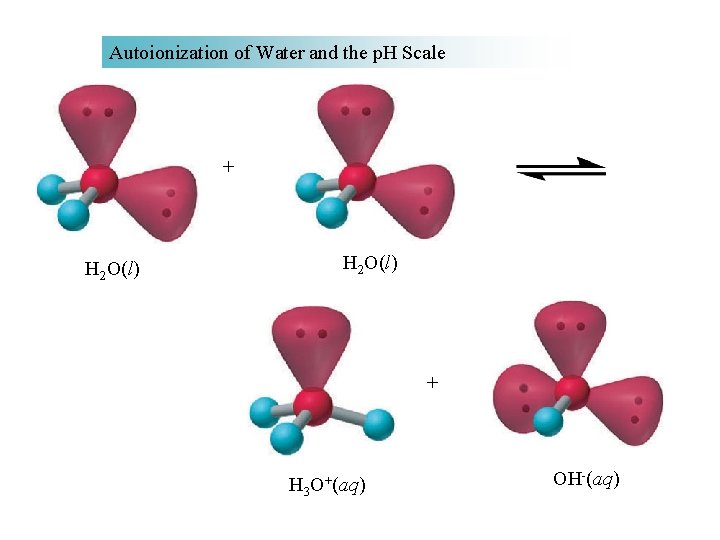
Autoionization of Water and the p. H Scale + H 2 O(l) + H 3 O+(aq) OH-(aq)

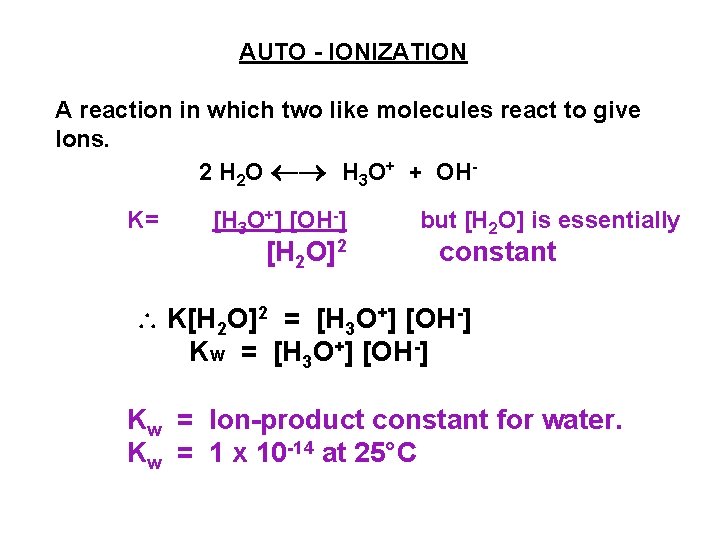
AUTO - IONIZATION A reaction in which two like molecules react to give Ions. 2 H 2 O H 3 O+ + OHK= [H 3 O+] [OH-] [H 2 O]2 but [H 2 O] is essentially constant K[H 2 O]2 = [H 3 O+] [OH-] Kw = Ion-product constant for water. Kw = 1 x 10 -14 at 25°C
![p H Water Due to auto ionization of water Kw H OH p. H & Water Due to auto ionization of water, Kw = [H+] [OH-]](https://slidetodoc.com/presentation_image_h2/951f030b08d69c3dc46c37a508f36790/image-25.jpg)
p. H & Water Due to auto ionization of water, Kw = [H+] [OH-] so take the log Log Kw = Log [H+] [OH-] = Log [H+] + log [OH-] therefore: p Kw = p. H + p. OH or 14 = p. H + p. OH

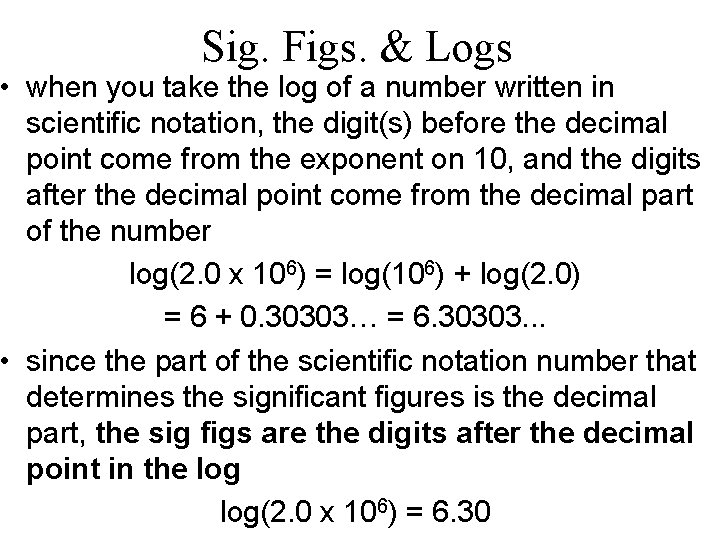
Sig. Figs. & Logs • when you take the log of a number written in scientific notation, the digit(s) before the decimal point come from the exponent on 10, and the digits after the decimal point come from the decimal part of the number log(2. 0 x 106) = log(106) + log(2. 0) = 6 + 0. 30303… = 6. 30303. . . • since the part of the scientific notation number that determines the significant figures is the decimal part, the sig figs are the digits after the decimal point in the log(2. 0 x 106) = 6. 30

p. H Lecture Problems on p. H: 1. A 0. 0015 M Na. OH solution has what p. H? [H+]? 2. A 0. 00015 M nitric acid solution has what p. OH? [OH-]? 3. A 6. 44 x 10 -5 M Ca(OH)2 solution has what p. H? p. OH? 4. A solution has p. OH of 12. 7, what is the [H+]?

Workshop AB#2 on p. H 1. A 3. 998 x 10 -9 M Ba(OH)2 solution has what p. H? p. OH? 2. An unknown solution has p. H of 2. 7, what is the [H+] & [OH-]? 3. In an art restoration project, a conservator prepares copper-plate etching solutions by diluting concentrated HNO 3 to 2. 0 M, 0. 30 M, and 0. 0063 M HNO 3. Calculate [H 3 O+], p. H, [OH-], and p. OH of the three solutions at 25 o. C.
![WEAK ACIDSBASES EQUILIBRIUM HAaq H aq A aq Ka H A WEAK ACIDS/BASES & EQUILIBRIUM HA(aq) H+ (aq) + A- (aq) Ka = [H+] [A-]](https://slidetodoc.com/presentation_image_h2/951f030b08d69c3dc46c37a508f36790/image-29.jpg)
WEAK ACIDS/BASES & EQUILIBRIUM HA(aq) H+ (aq) + A- (aq) Ka = [H+] [A-] Ka = acid dissociation constant [HA] B- + H 2 O HB+ + OH- Kb = K[H 2 O] = [HB+] [OH-] [B-] K = [HB+] [OH-] [B-] [H 2 O] Kb = base dissociation constant The magnitude of Ka or Kb refers to the strength of the acid. Small Ka value = weak acid Small Kb value = weak base

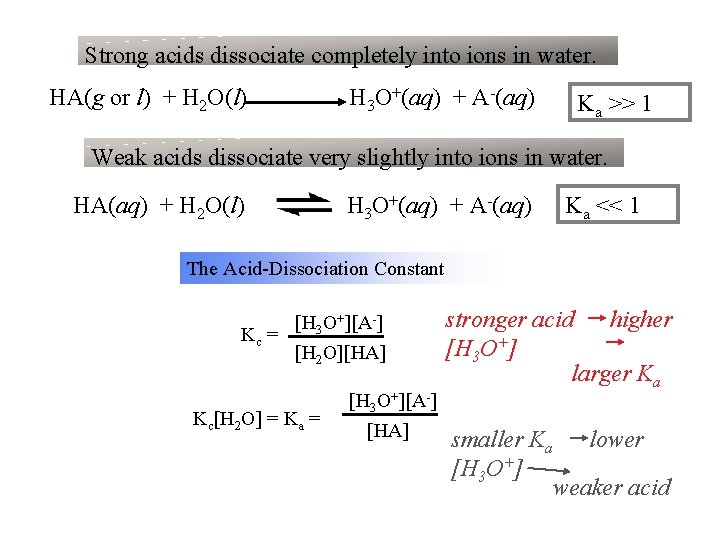
Strong acids dissociate completely into ions in water. HA(g or l) + H 2 O(l) H 3 O+(aq) + A-(aq) Ka >> 1 Weak acids dissociate very slightly into ions in water. HA(aq) + H 2 O(l) H 3 O+(aq) + A-(aq) Ka << 1 The Acid-Dissociation Constant [H 3 O+][A-] Kc = [H 2 O][HA] Kc[H 2 O] = Ka = [H 3 O+][A-] [HA] stronger acid higher [H 3 O+] larger Ka smaller Ka [H 3 O+] lower weaker acid

SAMPLE PROBLEM: Predicting the Net Direction of an Acid-Base Reaction Predict the net direction and whether Ka is greater or less than 1 for each of the following reactions (assume equal initial concentrations of all species): (a) H 2 PO 4 -(aq) + NH 3(aq) (b) H 2 O(l) + HS-(aq) PLAN: HPO 42 -(aq) + NH 4+(aq) OH-(aq) + H 2 S(aq) Identify the conjugate acid-base pairs and then consult SLIDE 24 to determine the relative strength of each. The stronger the species, the more preponderant its conjugate. SOLUTION:

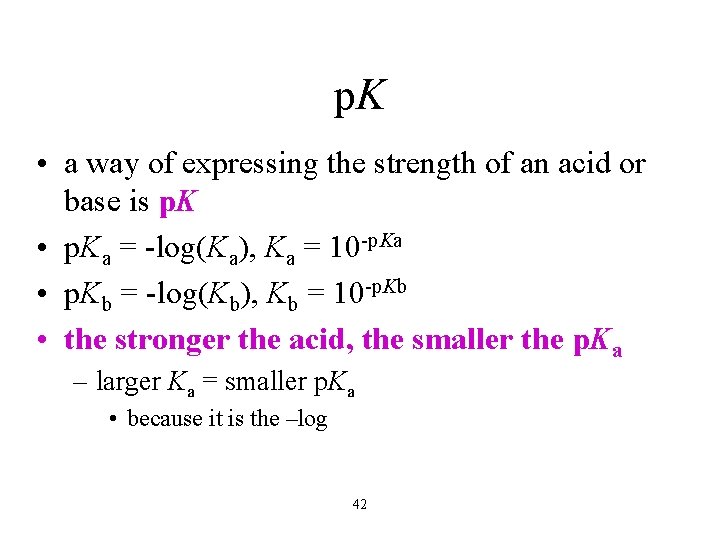
p. K • a way of expressing the strength of an acid or base is p. K • p. Ka = -log(Ka), Ka = 10 -p. Ka • p. Kb = -log(Kb), Kb = 10 -p. Kb • the stronger the acid, the smaller the p. Ka – larger Ka = smaller p. Ka • because it is the –log 42

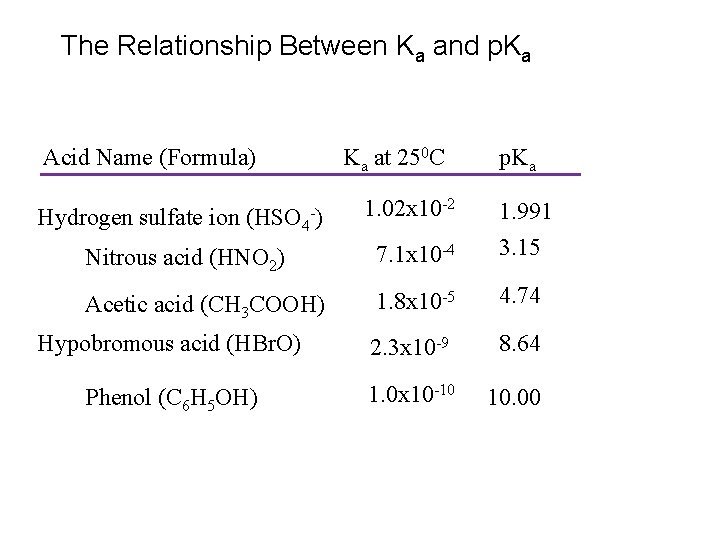
The Relationship Between Ka and p. Ka Acid Name (Formula) Ka at 250 C 1. 02 x 10 -2 p. Ka Nitrous acid (HNO 2) 7. 1 x 10 -4 1. 991 3. 15 Acetic acid (CH 3 COOH) 1. 8 x 10 -5 4. 74 Hypobromous acid (HBr. O) 2. 3 x 10 -9 8. 64 Phenol (C 6 H 5 OH) 1. 0 x 10 -10 10. 00 Hydrogen sulfate ion (HSO 4 -)

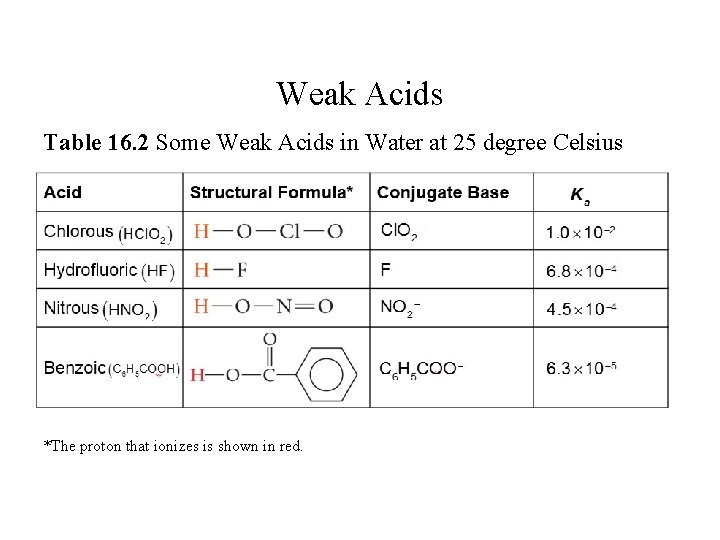
Weak Acids Table 16. 2 Some Weak Acids in Water at 25 degree Celsius *The proton that ionizes is shown in red.
![Weak Acids Table 16 2 continued The proton that ionizes is shown in red Weak Acids [Table 16. 2 continued] *The proton that ionizes is shown in red.](https://slidetodoc.com/presentation_image_h2/951f030b08d69c3dc46c37a508f36790/image-35.jpg)
Weak Acids [Table 16. 2 continued] *The proton that ionizes is shown in red.

GENERAL STEPS FOR CALCULATING THE p. H (p. OH) OF A WEAK ACID (BASE) Step 1: Write a balanced chemical equation describing the “action”. Step 2: Make a list of given and implied information. Step 3: Write the equilibrium constant equation associated with the balanced chemical equation in Step 1. Step 4: An equilibrium table should be set up since we are dealing with a weak acid (partially dissociated species). The table should describe the changes which occurred in order to establish equilibrium. Step 5: Substitute the equilibrium values from Step 4 into the equilibrium constant equation in Step 3. Solve for x. If the expression can not be solved with basic algebra, try either the quadratic equation or the successive-approximation method. Step 6: Calculate the p. H (p. OH) using the expression: p. H = -Log [H+] or p. OH = -Log [OH-]

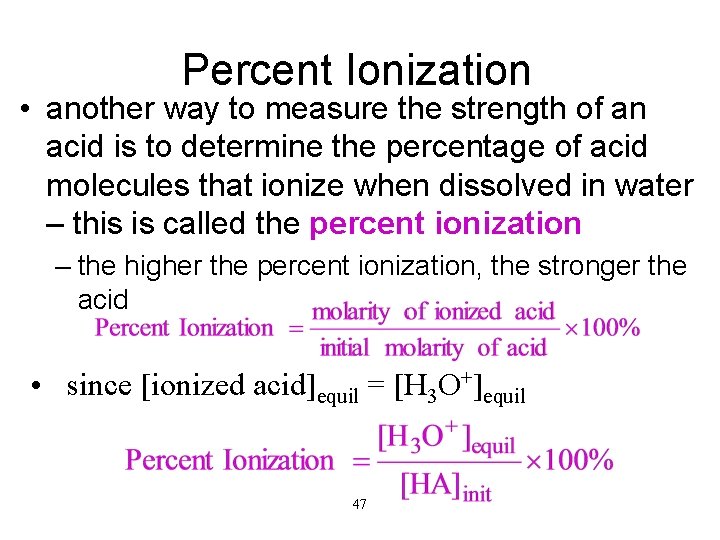
Percent Ionization • another way to measure the strength of an acid is to determine the percentage of acid molecules that ionize when dissolved in water – this is called the percent ionization – the higher the percent ionization, the stronger the acid • since [ionized acid]equil = [H 3 O+]equil 47

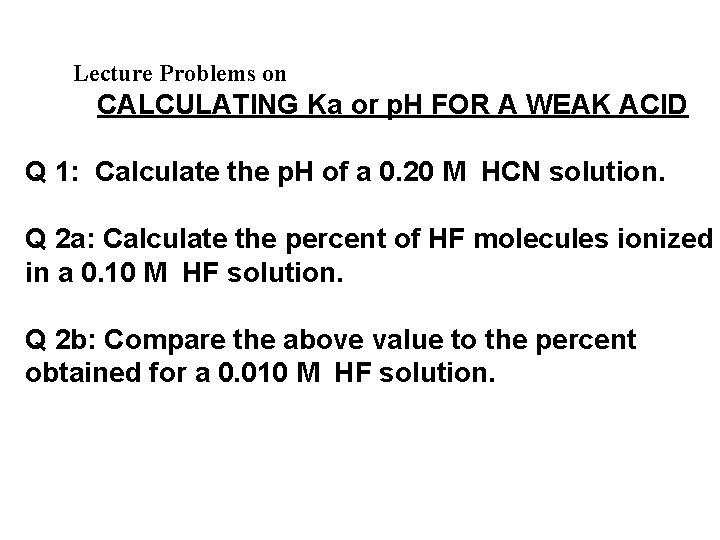
Lecture Problems on CALCULATING Ka or p. H FOR A WEAK ACID Q 1: Calculate the p. H of a 0. 20 M HCN solution. Q 2 a: Calculate the percent of HF molecules ionized in a 0. 10 M HF solution. Q 2 b: Compare the above value to the percent obtained for a 0. 010 M HF solution.

Workshop AB #3 on CALCULATING Ka or p. H FOR A WEAK ACID Q 1: Calculate the p. H of a 0. 035 M H 2 S solution. Q 2. A student prepared a 0. 10 M solution of formic acid HCHO 2 and measured it’s p. H, at 25°C, p. H = 2. 38 a) calculate Ka b) what percent of acid Ionizes?
![RECALL Percent HA dissociation HAdissociated x 100 HAinitial Polyprotic acids with more than RECALL Percent HA dissociation = [HA]dissociated x 100 [HA]initial Polyprotic acids with more than](https://slidetodoc.com/presentation_image_h2/951f030b08d69c3dc46c37a508f36790/image-40.jpg)
RECALL Percent HA dissociation = [HA]dissociated x 100 [HA]initial Polyprotic acids with more than more ionizable proton H 3 PO 4(aq) + H 2 O(l) H 2 PO 4 -(aq) + H 2 O(l) HPO 42 -(aq) + H 2 O(l) +][H PO -] [H O 3 2 4 H 2 PO 4 + H 3 Ka 1 = [H 3 PO 4] = 7. 2 x 10 -3 HPO 42 -(aq) + H 3 O+(aq) [H 3 O+][HPO 42 -] Ka 2 = [H 2 PO 4 -] = 6. 3 x 10 -8 +][PO 3 -] [H O 3+ 3 4 PO 4 (aq) + H 3 O (aq) Ka 3 = [HPO 42 -] Ka 1 > Ka 2 > Ka 3 = 4. 2 x 10 -13 -(aq) O+(aq)

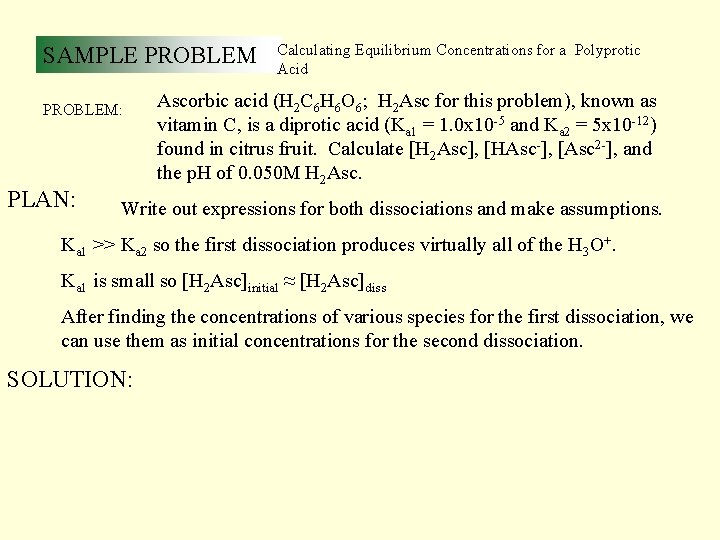
SAMPLE PROBLEM: PLAN: Calculating Equilibrium Concentrations for a Polyprotic Acid Ascorbic acid (H 2 C 6 H 6 O 6; H 2 Asc for this problem), known as vitamin C, is a diprotic acid (Ka 1 = 1. 0 x 10 -5 and Ka 2 = 5 x 10 -12) found in citrus fruit. Calculate [H 2 Asc], [HAsc-], [Asc 2 -], and the p. H of 0. 050 M H 2 Asc. Write out expressions for both dissociations and make assumptions. Ka 1 >> Ka 2 so the first dissociation produces virtually all of the H 3 O+. Ka 1 is small so [H 2 Asc]initial ≈ [H 2 Asc]diss After finding the concentrations of various species for the first dissociation, we can use them as initial concentrations for the second dissociation. SOLUTION:

Lecture Problems on POLYPROTIC ACIDS The solubility of CO 2 in pure H 2 O at 25ºC and 0. 1 atm is 0. 0037 M. a) What is the p. H of a 0. 0037 M solution of H 2 CO 3? b) What is the [CO 32 -] produced?

Workshop AB #4 on POLYPROTIC ACIDS Q 1. Calculate the p. H of a 0. 045 M sulfurous acid solution. H 2 SO 3 H+ + HSO 3 Ka 1 = 1. 7 x 10 -2 HSO 3 - H+ + SO 32 Ka 2 = 6. 4 x 10 -8 Ka 1 > Ka 2

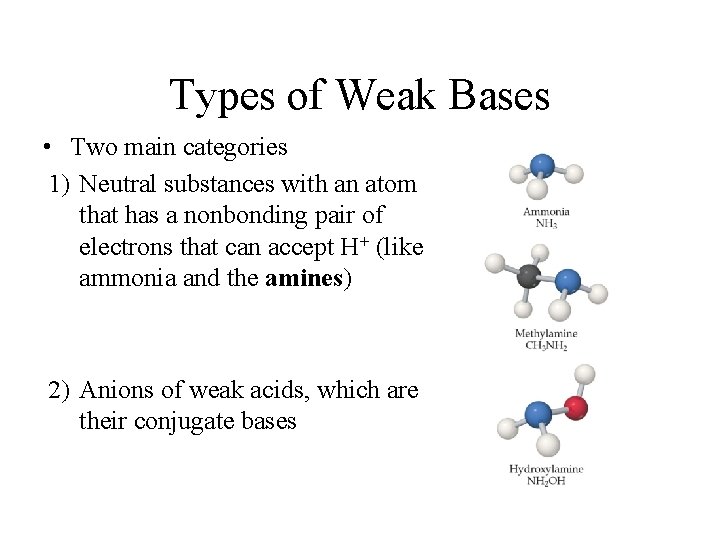
Types of Weak Bases • Two main categories 1) Neutral substances with an atom that has a nonbonding pair of electrons that can accept H+ (like ammonia and the amines) 2) Anions of weak acids, which are their conjugate bases

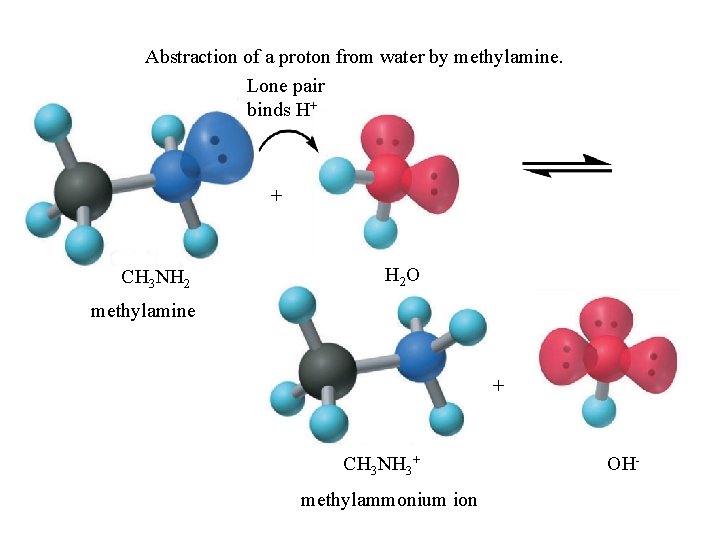
Abstraction of a proton from water by methylamine. Lone pair binds H+ + CH 3 NH 2 O methylamine + CH 3 NH 3+ methylammonium ion OH-

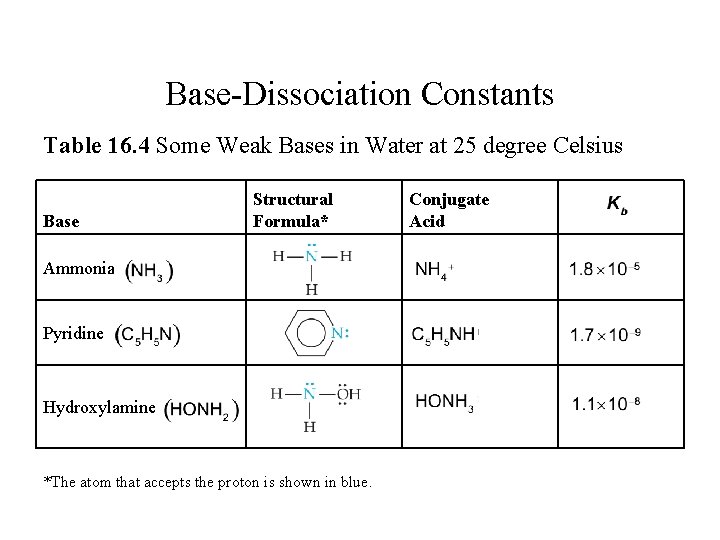
Base-Dissociation Constants Table 16. 4 Some Weak Bases in Water at 25 degree Celsius Base Ammonia N H 3 Pyridine C 5 H 5 N Hydroxylamine H O N H 2 Structural Formula* Conjugate Acid Structural formula, N with a lone pair single bonded leftward, rightward, and downward to H atoms. N is the atom that accepts the proton, shown in blue. N H 4, plus 1. 8 times 10 to the negative fifth Structural formula, N with a lone pair is shown on the right side of a hexagonal ring. N is the atom that accepts the proton, shown in blue. C 5 H 5 N H, plus 1. 7 times 10 to the negative ninth Structural formula, N with a lone pair single bonded leftward and downward to H atoms, and rightward to O H. The O has 2 lone pairs. N is the atom that accepts the proton, shown in blue. H O N H 3, plus 1. 1 times 10 to the negative eighth *The atom that accepts the proton is shown in blue. K sub b
![BaseDissociation Constants Table 16 4 continued Conjugate Acid K sub b Base Structural Formula Base-Dissociation Constants [Table 16. 4 continued] Conjugate Acid K sub b Base Structural Formula*](https://slidetodoc.com/presentation_image_h2/951f030b08d69c3dc46c37a508f36790/image-47.jpg)
Base-Dissociation Constants [Table 16. 4 continued] Conjugate Acid K sub b Base Structural Formula* C H 3, N H 3, plus Methylamine C H 3, N H 2 Structural formula, N with one lone pair single bonded leftward and downward to H atoms, and rightward to C H 3. N is the atom that accepts the proton, shown in blue. 4. 4 times 10 to the negative fourth H 2 S Hydrosulfide ion H S, minus Structural formula, H single bonded to S, with 3 lone pairs. The structure has a negative charge. S is the atom that accepts the proton, shown in blue. 1. 8 times 10 to the negative seventh Structural formula, a central C single bonded upward to O with 3 lone pairs, downward and leftward to O with 3 lone pairs, and double bonded downward and rightward to O with 2 lone pairs. The structure has a charge of 2 minus. The O with 3 lone pairs on the lower left is the atom that accepts the proton, shown in blue. H C O 3, minus 1. 8 times 10 to the negative fourth Structural formula, C l with 3 lone pairs single bonded to O with 3 lone pairs. The structure has a negative charge. O is the atom that accepts the proton, shown in blue. HCl. O 3. 3 times 10 to the negative seventh Carbonate ion C O 3, 2 minus Hypochlorite ion C l O, minus

WEAK BASES & EQUILIBRIUM B - + H 2 O HB+ + OH- K = [HB+] [OH-] [B-] [H 2 O] Kb = K[H 2 O] = [HB+] [OH-] [B-] Kb = base dissociation constant Q. Calculate [OH-] and p. H of a 0. 15 M NH 3 solution.

Workshop AB #5 on Base Equilibria Q 1. What is the p. H of a 0. 012 M solution of nicotinic acid, HC 6 H 4 NO 2? (Ka = 1. 4 x 10 -5 @ 25°C) Q 2. Find the p. H of a 0. 0015 M morphine solution, Kb=1. 6 x 10 -6 Q 3. Calculate the p. H of a 0. 0010 M Ba(OH)2 solution and determine if it is acidic, basic, or neutral 59

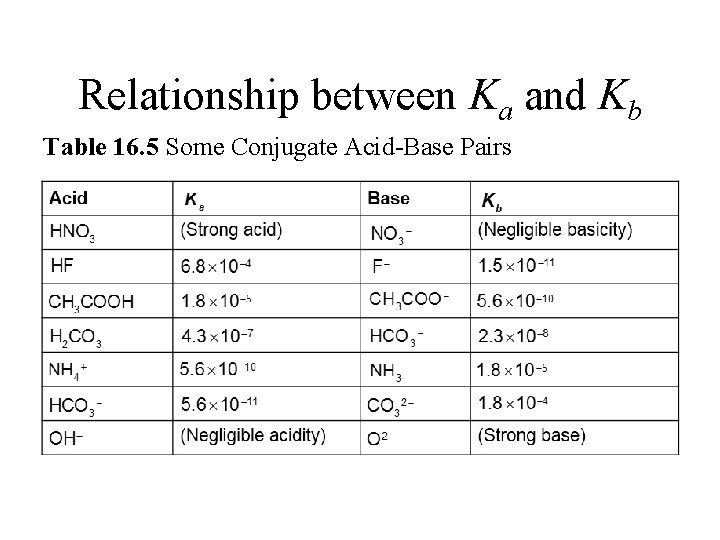
Relationship between Ka and Kb Table 16. 5 Some Conjugate Acid-Base Pairs

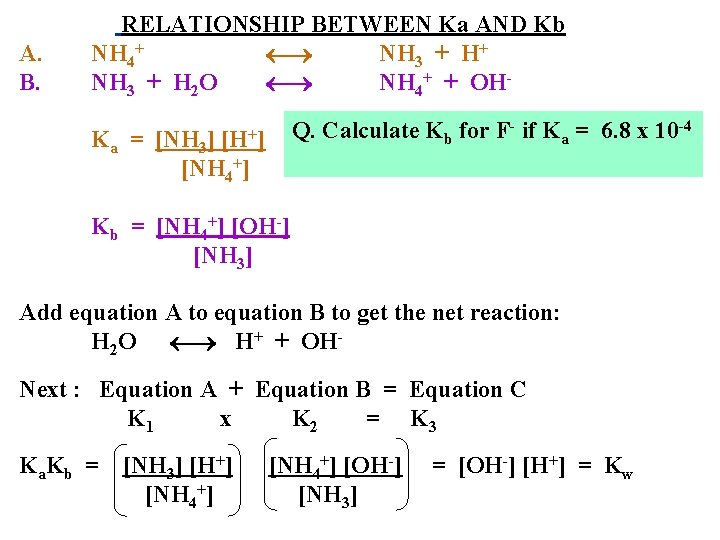
A. B. RELATIONSHIP BETWEEN Ka AND Kb NH 4+ NH 3 + H+ NH 3 + H 2 O NH 4+ + OHQ. Calculate Kb for F- if Ka = 6. 8 x 10 -4 [H+] Ka = [NH 3] [NH 4+] Kb = [NH 4+] [OH-] [NH 3] Add equation A to equation B to get the net reaction: H 2 O H+ + OHNext : Equation A + Equation B = Equation C K 1 x K 2 = K 3 K a. K b = [NH 3] [H+] [NH 4+] [OH-] [NH 3] = [OH-] [H+] = Kw

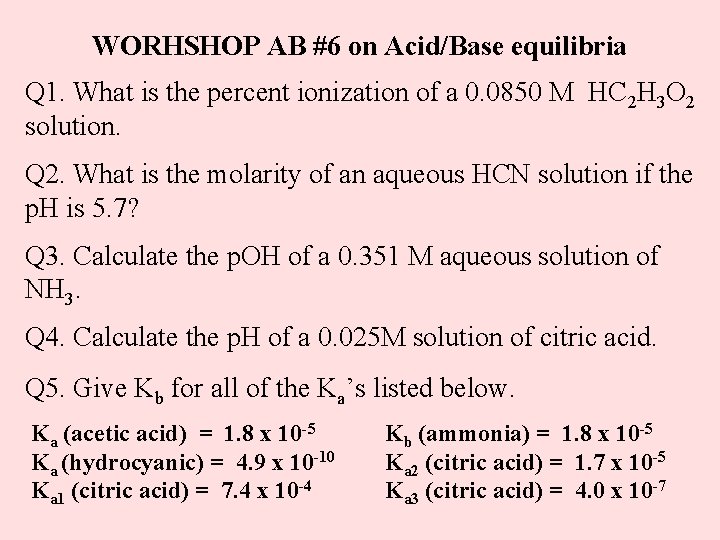
WORHSHOP AB #6 on Acid/Base equilibria Q 1. What is the percent ionization of a 0. 0850 M HC 2 H 3 O 2 solution. Q 2. What is the molarity of an aqueous HCN solution if the p. H is 5. 7? Q 3. Calculate the p. OH of a 0. 351 M aqueous solution of NH 3. Q 4. Calculate the p. H of a 0. 025 M solution of citric acid. Q 5. Give Kb for all of the Ka’s listed below. Ka (acetic acid) = 1. 8 x 10 -5 Ka (hydrocyanic) = 4. 9 x 10 -10 Ka 1 (citric acid) = 7. 4 x 10 -4 Kb (ammonia) = 1. 8 x 10 -5 Ka 2 (citric acid) = 1. 7 x 10 -5 Ka 3 (citric acid) = 4. 0 x 10 -7

Acid–Base Properties of Salts • Many ions react with water to create The reaction with water is often called hydrolysis. • To determine whether a salt is an acid or a base, you need to look at the cation and anion separately. • The cation can be acidic or neutral. • The anion can be acidic, basic, or neutral.

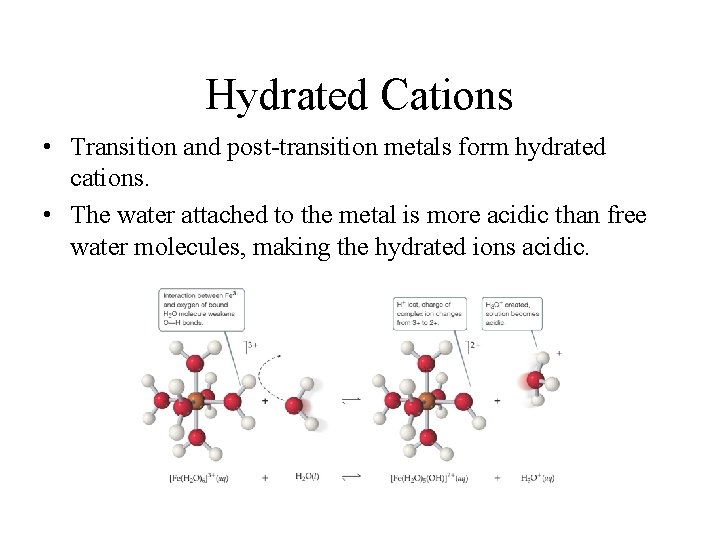
Cations • Group I or group II metal cations are neutral. • Polyatomic cations are typically the conjugate acids of a weak base; for example, • Transition and post-transition metal cations are acidic. Why? (There are no H atoms in these cations!)

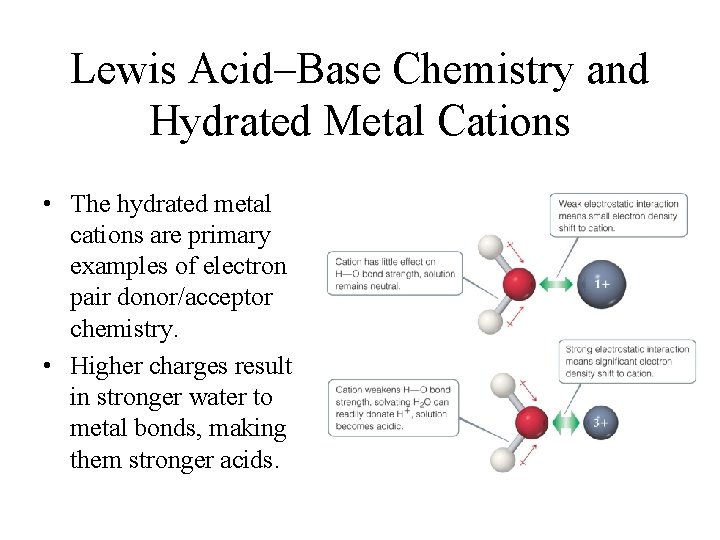
Hydrated Cations • Transition and post-transition metals form hydrated cations. • The water attached to the metal is more acidic than free water molecules, making the hydrated ions acidic.

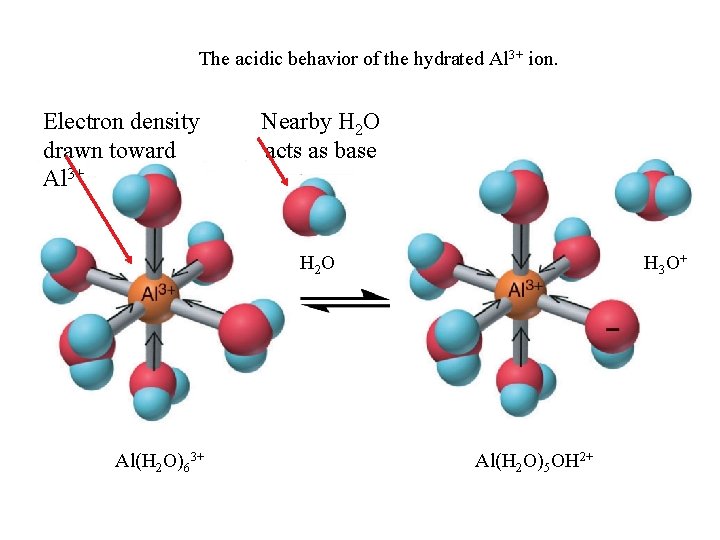
The acidic behavior of the hydrated Al 3+ ion. Electron density drawn toward Al 3+ Nearby H 2 O acts as base H 2 O Al(H 2 O)63+ H 3 O+ Al(H 2 O)5 OH 2+

Acid-Base Properties of Salts • salts are water soluble ionic compounds • salts that contain the cation of a strong base and an anion that is the conjugate base of a weak acid are basic – Na. HCO 3 solutions are basic • Na+ is the cation of the strong base Na. OH • HCO 3− is the conjugate base of the weak acid H 2 CO 3 • salts that contain cations that are the conjugate acid of a weak base and an anion of a strong acid are acidic – NH 4 Cl solutions are acidic • NH 4+ is the conjugate acid of the weak base NH 3 • Cl− is the anion of the strong acid HCl 67

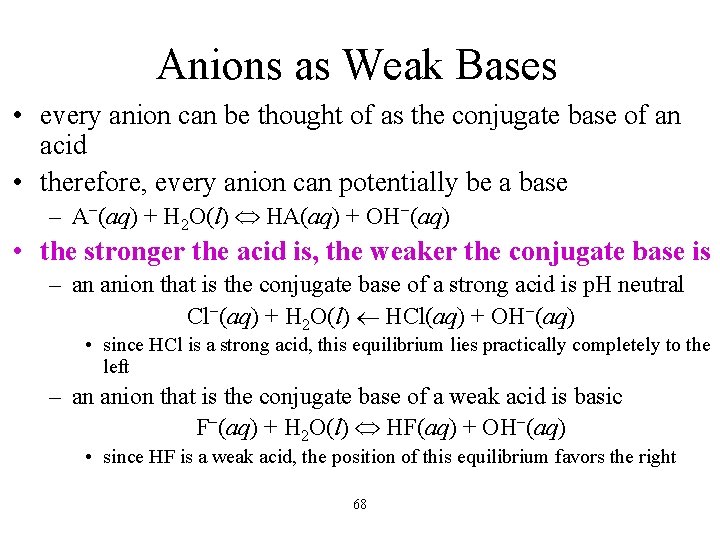
Anions as Weak Bases • every anion can be thought of as the conjugate base of an acid • therefore, every anion can potentially be a base – A−(aq) + H 2 O(l) HA(aq) + OH−(aq) • the stronger the acid is, the weaker the conjugate base is – an anion that is the conjugate base of a strong acid is p. H neutral Cl−(aq) + H 2 O(l) HCl(aq) + OH−(aq) • since HCl is a strong acid, this equilibrium lies practically completely to the left – an anion that is the conjugate base of a weak acid is basic F−(aq) + H 2 O(l) HF(aq) + OH−(aq) • since HF is a weak acid, the position of this equilibrium favors the right 68

SALT SOLUTIONS 1. Salts derived from strong bases and strong acids have p. H = 7 Na. Cl Ca(NO 3)2 2. Salts derived from strong bases and weak acids have p. H > 7 Na. Cl. O Ba(C 2 H 3 O 2)2 3. Salts derived from weak bases and strong acids have p. H < 7 NH 4 Cl Al(NO 3)3 4. Salts derived from weak base and weak acids, p. H is dependent on extent NH 4 CN Fe 2(CO 3)3 NH 4 C 2 H 3 O 2

1. SA/SB: Why neutral? 2. SB/WA Why basic? neither cation nor anion hydrolyzes anion = strong CB hydrolyzes to produce OH- , so Basic 3. WB/SA: Why Acidic? 4. NH 4 CN Ka Slide 65 5. Fe. CO 3 cation = strong CA hydrolyzes to produce H+ so Acidic CN- = Kb = 2 x 10 -5 NH 4+ = Ka = 5. 6 x 10 -10 CN- hydrolyzes more than NH 4+ p. H > 7 basic more basic than acidic Fe 3+ = 6 x 10 -3 Ka CO 32 - = Ka 1 = 4. 5 x 10 -7 Ka 2 = 4. 7 x 10 -11 Kb 1 = 2. 2 x 10 -8 Kb 2 = 2. 13 x 10 -4

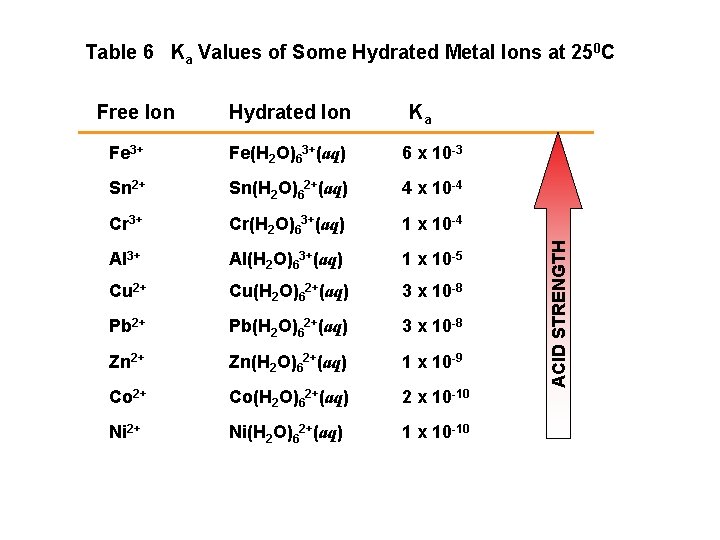
Table 6 Ka Values of Some Hydrated Metal Ions at 250 C Hydrated Ion Ka Fe 3+ Fe(H 2 O)63+(aq) 6 x 10 -3 Sn 2+ Sn(H 2 O)62+(aq) 4 x 10 -4 Cr 3+ Cr(H 2 O)63+(aq) 1 x 10 -4 Al 3+ Al(H 2 O)63+(aq) 1 x 10 -5 Cu 2+ Cu(H 2 O)62+(aq) 3 x 10 -8 Pb 2+ Pb(H 2 O)62+(aq) 3 x 10 -8 Zn 2+ Zn(H 2 O)62+(aq) 1 x 10 -9 Co 2+ Co(H 2 O)62+(aq) 2 x 10 -10 Ni 2+ Ni(H 2 O)62+(aq) 1 x 10 -10 ACID STRENGTH Free Ion

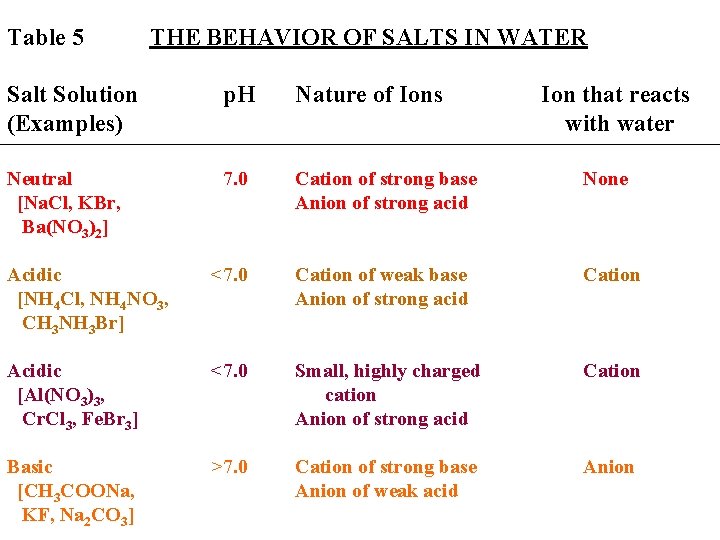
Table 5 THE BEHAVIOR OF SALTS IN WATER Salt Solution (Examples) p. H Nature of Ions Ion that reacts with water Neutral [Na. Cl, KBr, Ba(NO 3)2] 7. 0 Cation of strong base Anion of strong acid None Acidic [NH 4 Cl, NH 4 NO 3, CH 3 NH 3 Br] <7. 0 Cation of weak base Anion of strong acid Cation Acidic [Al(NO 3)3, Cr. Cl 3, Fe. Br 3] <7. 0 Small, highly charged cation Anion of strong acid Cation Basic [CH 3 COONa, KF, Na 2 CO 3] >7. 0 Cation of strong base Anion of weak acid Anion

Ex 15. 16 - Determine whether a solution of the following salts is acidic, basic, or neutral a) Sr. Cl 2 b) Al. Br 3 c) CH 3 NO 3 73

Ex 15. 16 - Determine whether a solution of the following salts is acidic, basic, or neutral d) Na. CHO 2 e) NH 4 F 74

Lewis Acid–Base Chemistry and Hydrated Metal Cations • The hydrated metal cations are primary examples of electron pair donor/acceptor chemistry. • Higher charges result in stronger water to metal bonds, making them stronger acids.

ACID/BASE PROPERTIES OF SALT SOLUTIONS HYDROLYSIS Ions react with water to generate either H+ or OH- A- + H 2 O HA + OHPractice Problems on Hydrolysis: Q. Predict whether Na 2 HPO 4 will form an acidic or basic solution. Q. Predict whether K 2 HC 7 H 5 O 7 will form an acidic or basic solution.

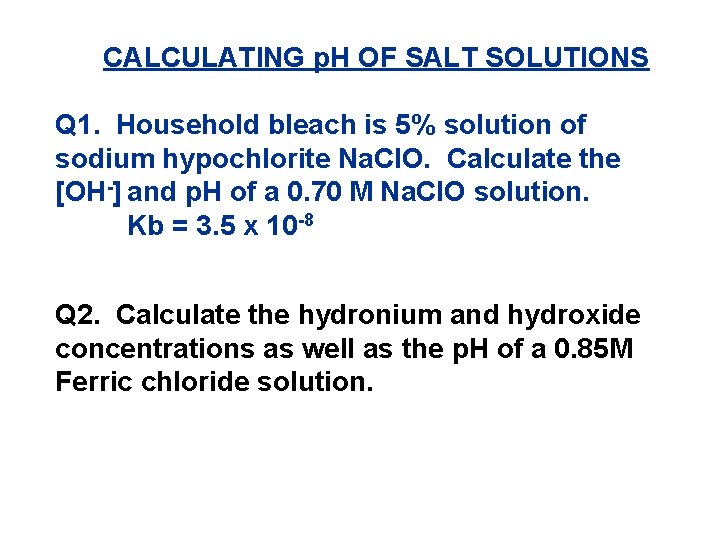
CALCULATING p. H OF SALT SOLUTIONS Q 1. Household bleach is 5% solution of sodium hypochlorite Na. Cl. O. Calculate the [OH-] and p. H of a 0. 70 M Na. Cl. O solution. Kb = 3. 5 x 10 -8 Q 2. Calculate the hydronium and hydroxide concentrations as well as the p. H of a 0. 85 M Ferric chloride solution.

Workshop AB #7 on p. H of salts 1) Determine the p. H of a 0. 62 M NH 4 NO 3 solution at 25°C. The Kb for NH 3 is 1. 76 × 10 -5. 2) Calculate the p. H of a 0. 800 M Na. CH 3 CO 2 solution. Ka for acetic acid, CH 3 CO 2 H, is 1. 8 × 10 -5. 3) Calculate the p. H of a 1. 60 M KBr. O solution. Ka for hypobromous acid, HBr. O, is 2. 0 × 10 -9. 28) Calculate the p. H of a 1. 60 M CH 3 NH 3 Cl solution. Kb for methylamine, CH 3 NH 2, is 3. 7 × 10 -4.