Chemistry The Central Science Fourteenth Edition Chapter 12





- Slides: 23

Chemistry: The Central Science Fourteenth Edition Chapter 12 Solids and Modern Materials • Copyright © 2018, 2015, 2012 Pearson Education, Inc. All Rights Reserved

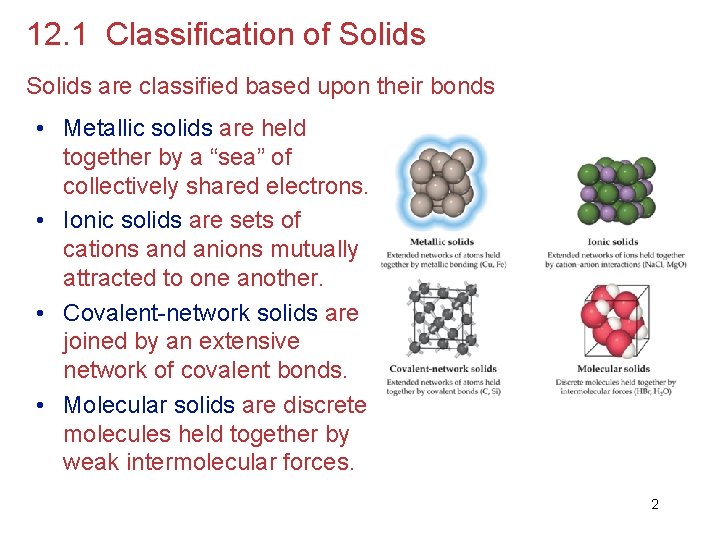
12. 1 Classification of Solids are classified based upon their bonds • Metallic solids are held together by a “sea” of collectively shared electrons. • Ionic solids are sets of cations and anions mutually attracted to one another. • Covalent-network solids are joined by an extensive network of covalent bonds. • Molecular solids are discrete molecules held together by weak intermolecular forces. 2

• Polymers contain long chains of atoms (usually carbon) held together by covalent bonds; adjacent chains are held together with weaker intermolecular bonds. Ø Normally stronger then molecular solids with a higher melting point, and more flexible than other types of solids. • Nanomaterials are solids in which the dimensions of the individual crystals have been reduced in scale in the order of 1 -100 nm. 3

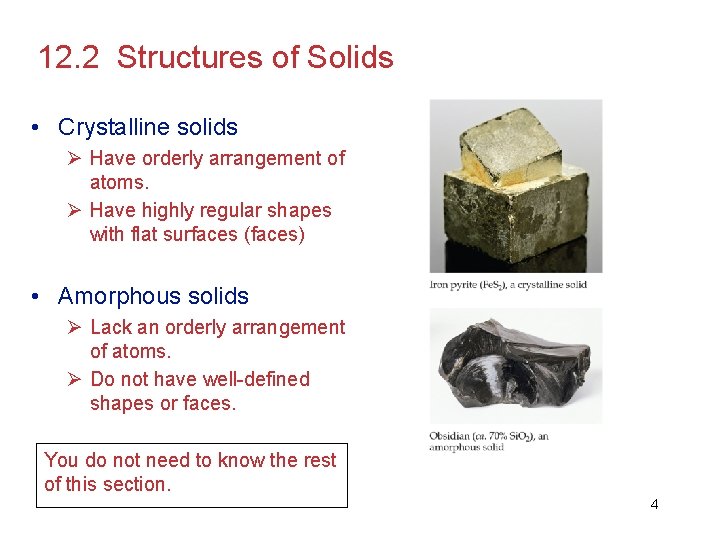
12. 2 Structures of Solids • Crystalline solids Ø Have orderly arrangement of atoms. Ø Have highly regular shapes with flat surfaces (faces) • Amorphous solids Ø Lack an orderly arrangement of atoms. Ø Do not have well-defined shapes or faces. You do not need to know the rest of this section. 4

12. 3/12. 4 Metallic Solids/Metallic Bonding • Consist entirely of metal atoms. • Atoms are not held together by covalent bonds as there are too few valence electrons to form bonds. • Rather, valence electrons are delocalized (that is, spread) throughout the entire solid to form metallic bonds between atoms. • Metals can be visualized as an array of positively charged ions immersed in a “sea” of delocalized electrons. • The shortage valence electrons and the fact that they are collectively shared make it favorable for the atoms in a metal to pack together closely. 5

• The chemical bonding in metals is reflected in their properties. • Metals are lustrous, have thermal and electrical conductivity, and are malleable and ductile. • These properties indicate that metal atoms are capable of slipping past one another, unlike ionic and covalent netnetwork solids that tend to be brittle. You do not need to know about the structures of metallic solids or close packing arrangements. 6

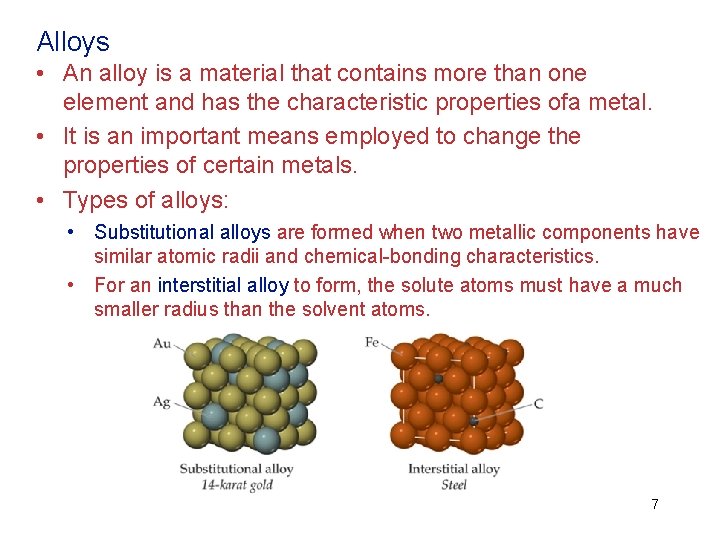
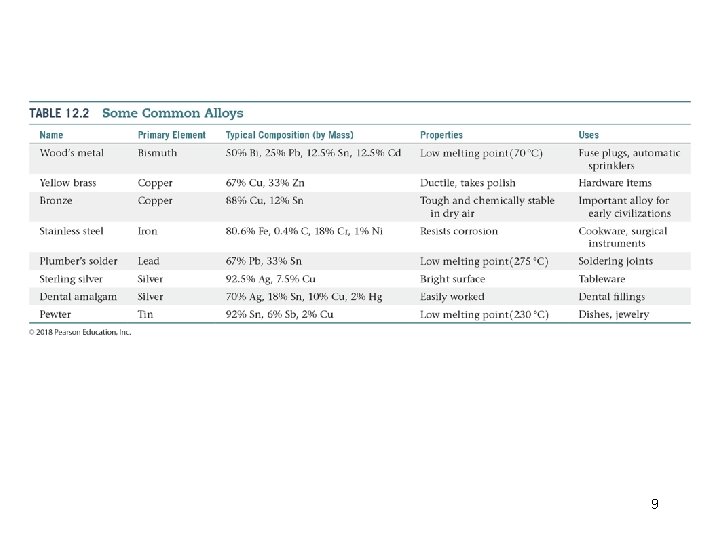
Alloys • An alloy is a material that contains more than one element and has the characteristic properties ofa metal. • It is an important means employed to change the properties of certain metals. • Types of alloys: • Substitutional alloys are formed when two metallic components have similar atomic radii and chemical-bonding characteristics. • For an interstitial alloy to form, the solute atoms must have a much smaller radius than the solvent atoms. 7

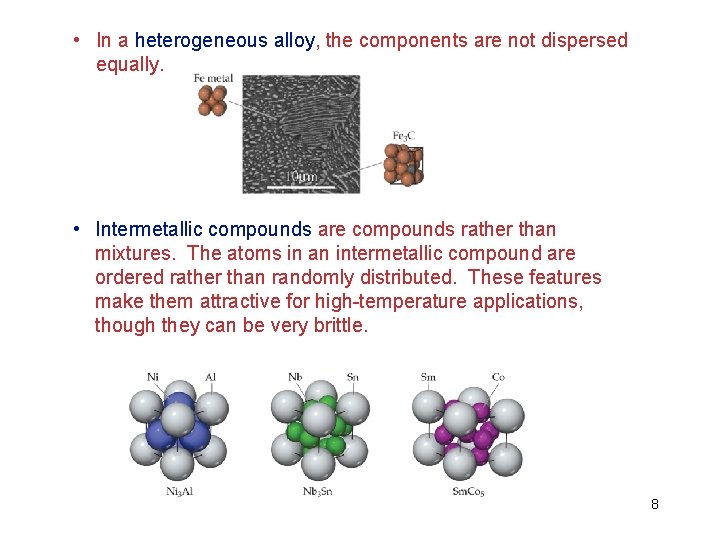
• In a heterogeneous alloy, the components are not dispersed equally. • Intermetallic compounds are compounds rather than mixtures. The atoms in an intermetallic compound are ordered rather than randomly distributed. These features make them attractive for high-temperature applications, though they can be very brittle. 8

9

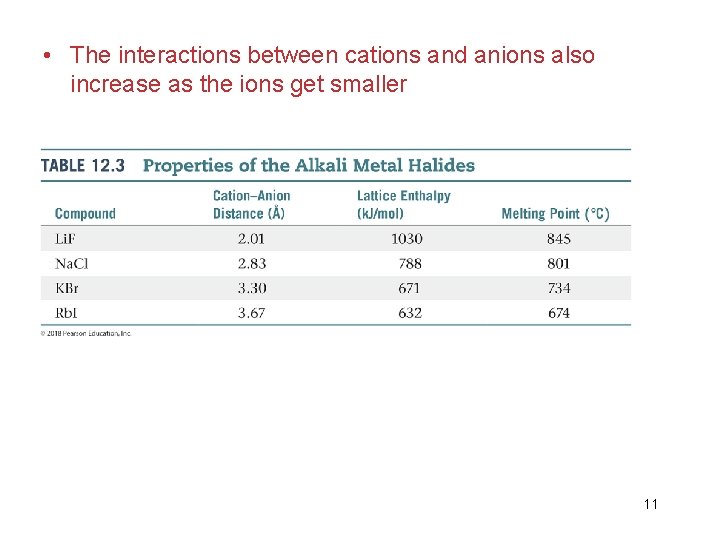
12. 5 Ionic Solids • They are held together by electrostatic attractions between cations and anions. • Ionic solids have very high melting and boiling points and are quintessential crystals. • Because the valence electrons are confined to the anions, rather than being delocalized, ionic compounds are electronic insulators. • The strength of ionic bonds depends on the charges and sizes of the ions: 10

• The interactions between cations and anions also increase as the ions get smaller 11

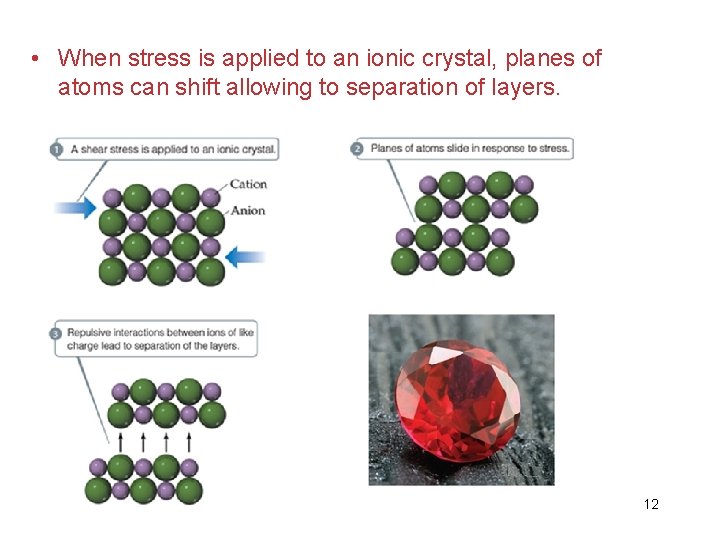
• When stress is applied to an ionic crystal, planes of atoms can shift allowing to separation of layers. 12

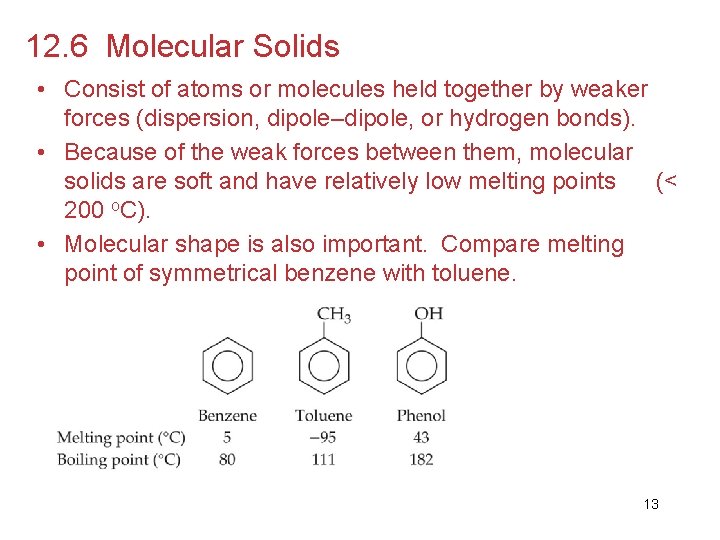
12. 6 Molecular Solids • Consist of atoms or molecules held together by weaker forces (dispersion, dipole–dipole, or hydrogen bonds). • Because of the weak forces between them, molecular solids are soft and have relatively low melting points (< 200 o. C). • Molecular shape is also important. Compare melting point of symmetrical benzene with toluene. 13

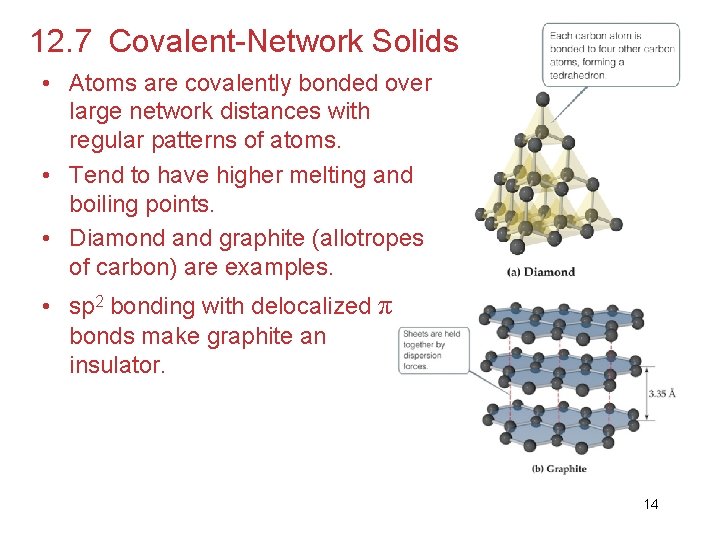
12. 7 Covalent-Network Solids • Atoms are covalently bonded over large network distances with regular patterns of atoms. • Tend to have higher melting and boiling points. • Diamond and graphite (allotropes of carbon) are examples. • sp 2 bonding with delocalized π bonds make graphite an insulator. 14

12. 8 Polymers • Polymers are molecules of high molecular weight made by joining smaller molecules, called monomers. • Plastics are polymeric solids that can be formed into various shapes. 15

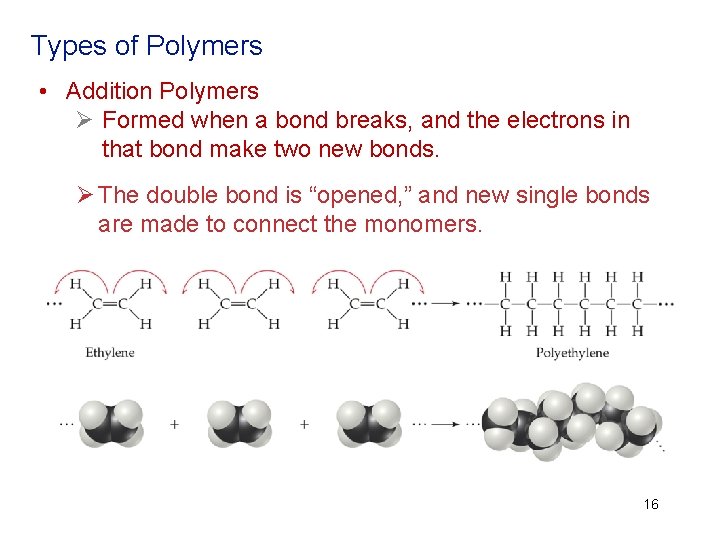
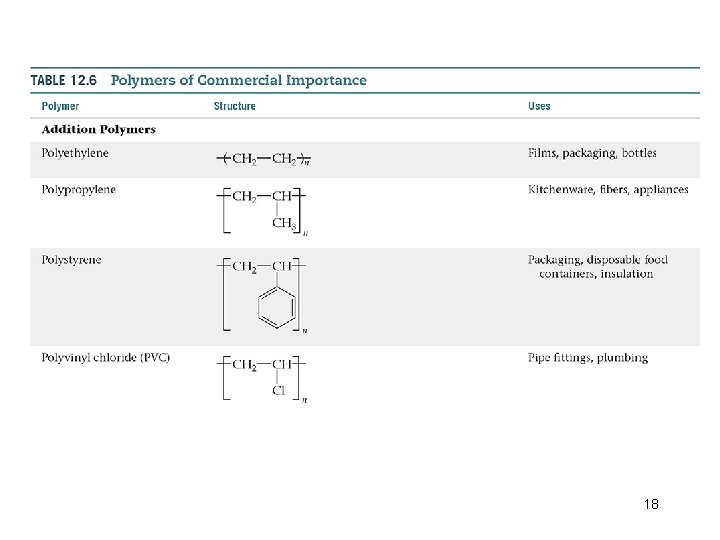
Types of Polymers • Addition Polymers Ø Formed when a bond breaks, and the electrons in that bond make two new bonds. Ø The double bond is “opened, ” and new single bonds are made to connect the monomers. Addition polymers are formed when a bond breaks, and the electrons in that bond make two new bonds. 16

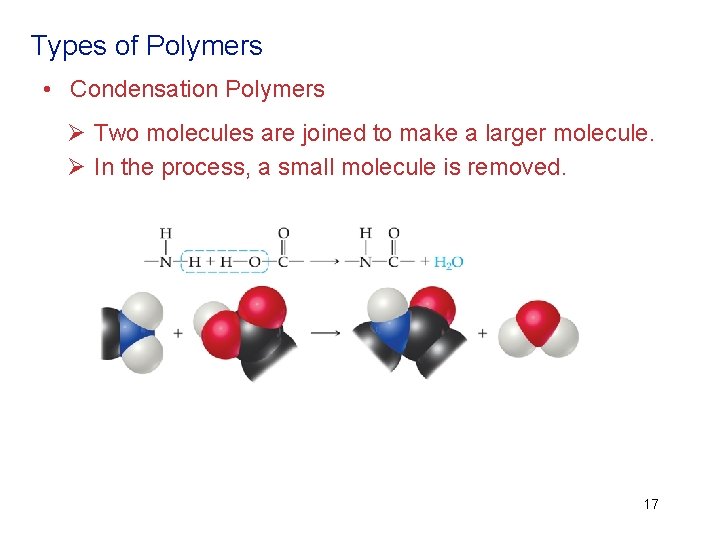
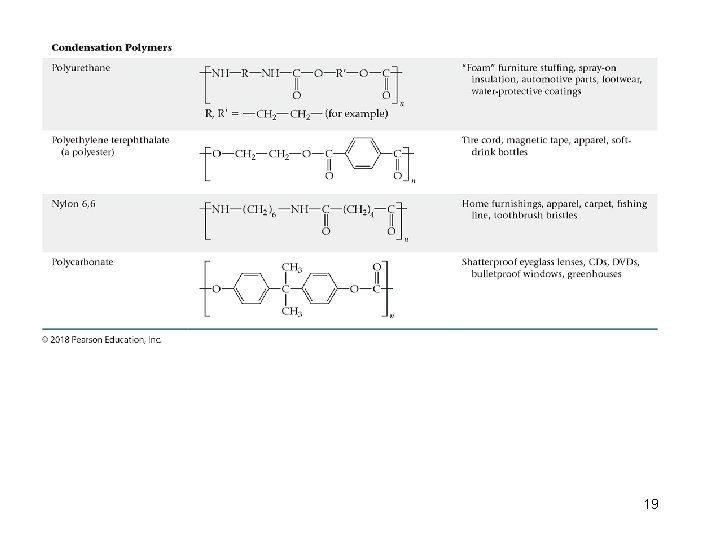
Types of Polymers • Condensation Polymers Ø Two molecules are joined to make a larger molecule. Ø In the process, a small molecule is removed. 17

18

19

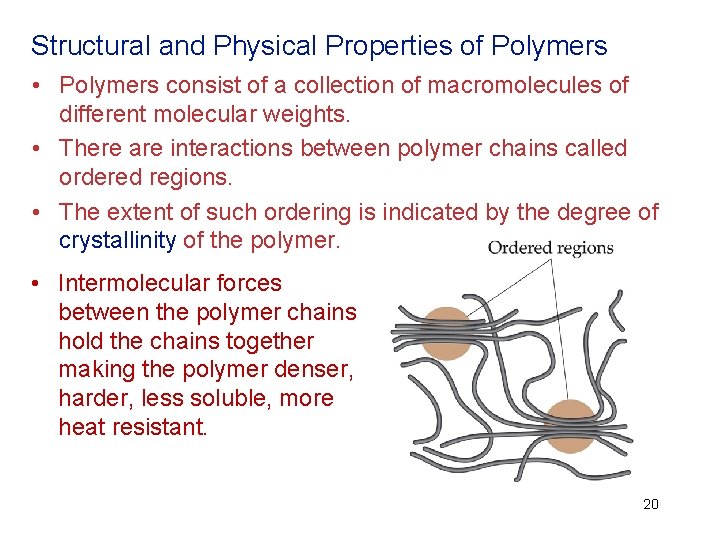
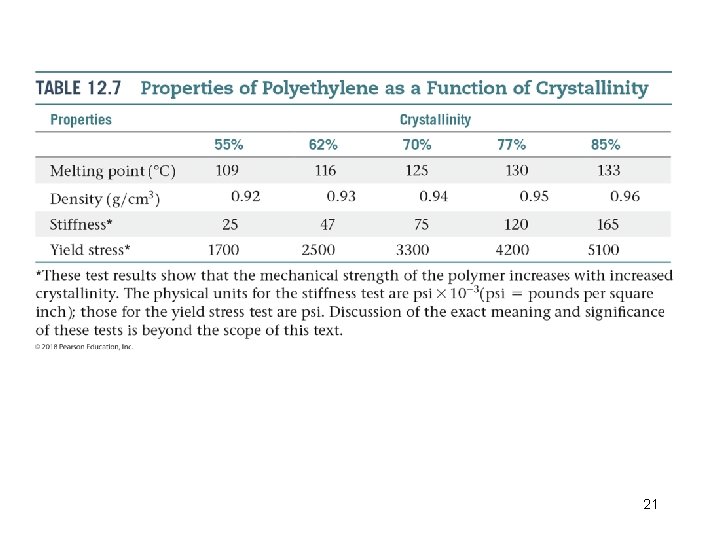
Structural and Physical Properties of Polymers • Polymers consist of a collection of macromolecules of different molecular weights. • There are interactions between polymer chains called ordered regions. • The extent of such ordering is indicated by the degree of crystallinity of the polymer. • Intermolecular forces between the polymer chains hold the chains together making the polymer denser, harder, less soluble, more heat resistant. 20

21

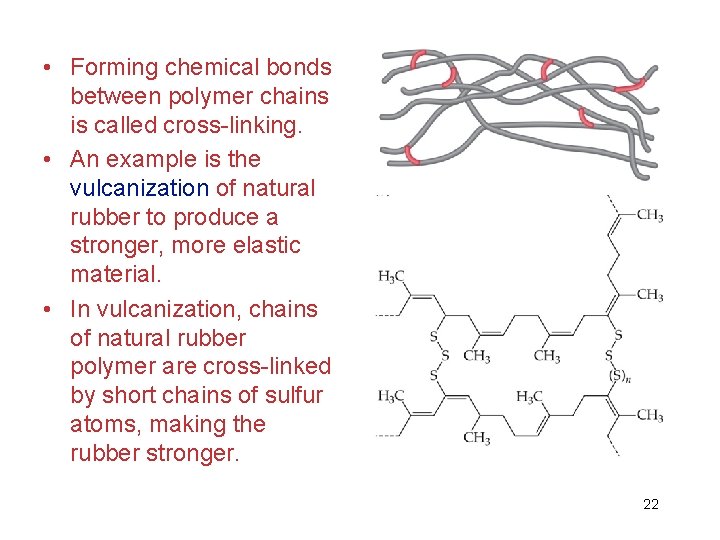
• Forming chemical bonds between polymer chains is called cross-linking. • An example is the vulcanization of natural rubber to produce a stronger, more elastic material. • In vulcanization, chains of natural rubber polymer are cross-linked by short chains of sulfur atoms, making the rubber stronger. 22

Copyright • Copyright © 2018, 2015, 2012 Pearson Education, Inc. All Rights Reserved 23