Chemistry 12101 Winter 2009 Introduction to Organic Chemistry





- Slides: 61

Chemistry 121(01) Winter 2009 Introduction to Organic Chemistry and Biochemistry Instructor Dr. Upali Siriwardane (Ph. D. Ohio State) E-mail: upali@chem. latech. edu Office: 311 Carson Taylor Hall ; Phone: 318 -257 -4941; Office Hours: MTW 9: 00 am - 11: 00 am; TR 9: : 00 - !0: 00 am & 1: 00 -2: 00 pm. December 19, Test 1 (Chapters 12 -14) January 2 Test 1 (Chapters 15 -16) February 6 (Chapters 17 -19) February 27, (Chapters 20 -22) March 2, 2009, Make Up Exam: Bring Scantron Sheet 882 -E Chemistry 121, Winter 2008, LA Tech 1 -1

Chapter 14: Alcohols, Phenols and Ethers Sections 14. 1 -4. 21 Chemistry 121, Winter 2008, LA Tech 1 -2

Chapter 14: Alcohols, Phenols, and Ethers 14. 1 Bonding Characteristics of Oxygen Atoms in Organic Compounds 14. 2 Structural Characteristics of Alcohols 14. 3 Nomenclature for Alcohols 14. 4 Isomerism for Alcohols 14. 6 Physical Properties of Alcohols 14. 7 Preparation of Alcohols 14. 8 Classification of Alcohols 14. 9 Chemical Reactions of Alcohols 14. 11 Structural Characteristics of Phenols 14. 12 Nomenclature for Phenols 14. 13 Physical and Chemical Properties of Phenols 14. 15 Structural Characteristics of Ethers 14. 16 Nomenclature for Ethers 14. 18 Physical and Chemical Properties of Ethers 14. 20 Sulfur Analogs and Alcohols 14. 21 Sulfur Analogs of Ethers Menthol: A Useful Naturally Occurring Terpene Alcohol; Ethers as General Anesthetics; Marijuana: The Most Commonly Used Illicit Drug; Garlic and Onions: Odiferous Medicinal Plants Chemistry 121, Winter 2008, LA Tech 1 -3

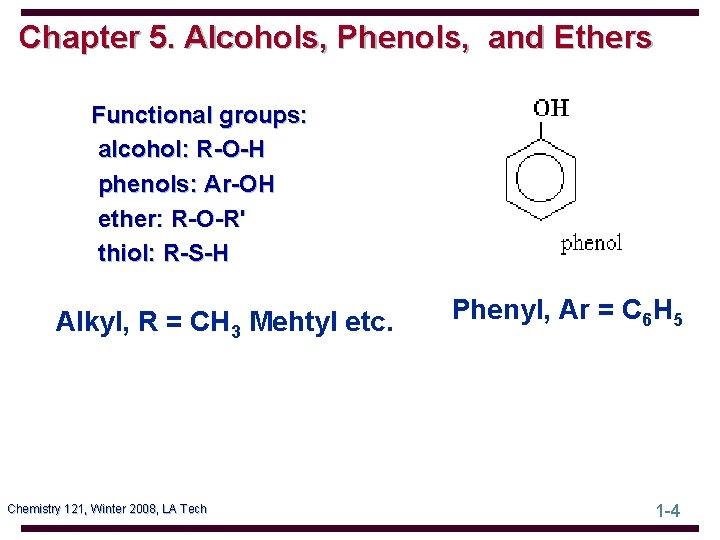
Chapter 5. Alcohols, Phenols, and Ethers Functional groups: alcohol: R-O-H phenols: Ar-OH ether: R-O-R' thiol: R-S-H Alkyl, R = CH 3 Mehtyl etc. Chemistry 121, Winter 2008, LA Tech Phenyl, Ar = C 6 H 5 1 -4

Nomenclature of compounds containing functional groups The IUPAC system deals with functional groups two different ways. Modification of the hydrocarbon name to indicate the presence of a functional group. alcohol, -OH use -ol ending. ether: CH 3 CH 2 -O-CH 3 use methoxy ethane thiol: R-S-H use -thiol ending. Chemistry 121, Winter 2008, LA Tech 1 -5

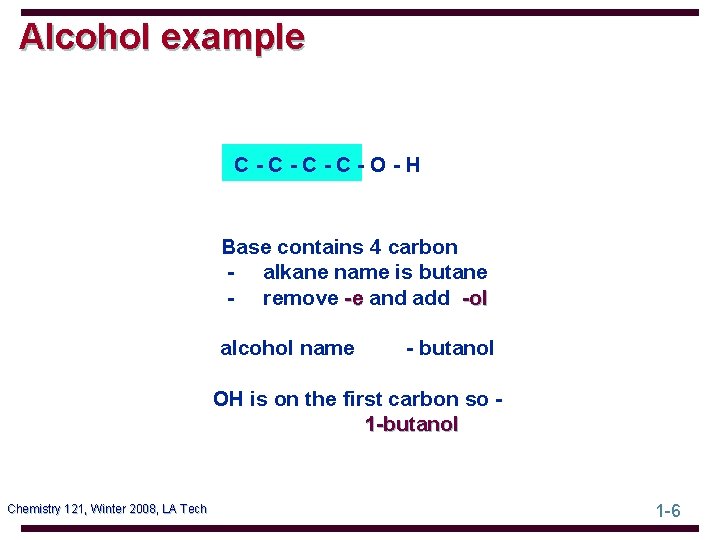
Alcohol example C-C-O-H Base contains 4 carbon - alkane name is butane - remove -e and add -ol alcohol name - butanol OH is on the first carbon so 1 -butanol Chemistry 121, Winter 2008, LA Tech 1 -6

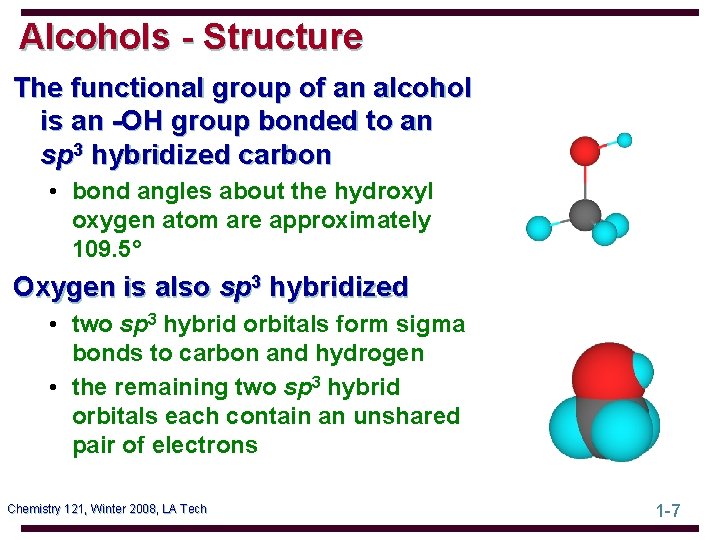
Alcohols - Structure The functional group of an alcohol is an -OH group bonded to an sp 3 hybridized carbon • bond angles about the hydroxyl oxygen atom are approximately 109. 5° Oxygen is also sp 3 hybridized • two sp 3 hybrid orbitals form sigma bonds to carbon and hydrogen • the remaining two sp 3 hybrid orbitals each contain an unshared pair of electrons Chemistry 121, Winter 2008, LA Tech 1 -7

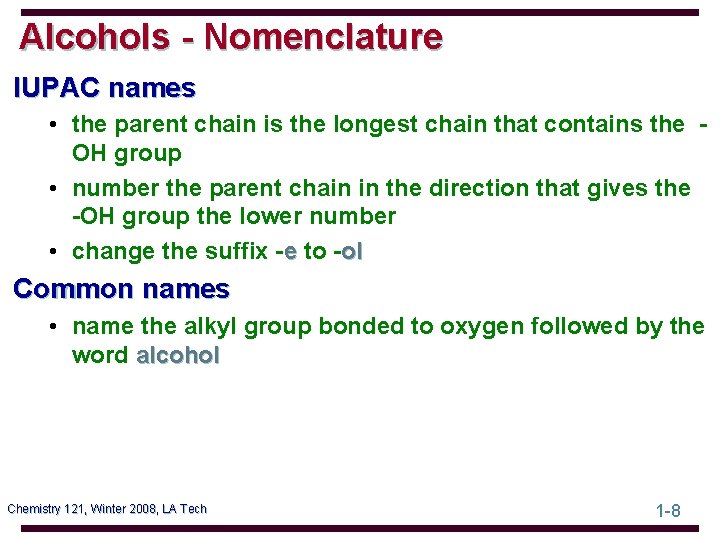
Alcohols - Nomenclature IUPAC names • the parent chain is the longest chain that contains the OH group • number the parent chain in the direction that gives the -OH group the lower number • change the suffix -e to -ol Common names • name the alkyl group bonded to oxygen followed by the word alcohol Chemistry 121, Winter 2008, LA Tech 1 -8

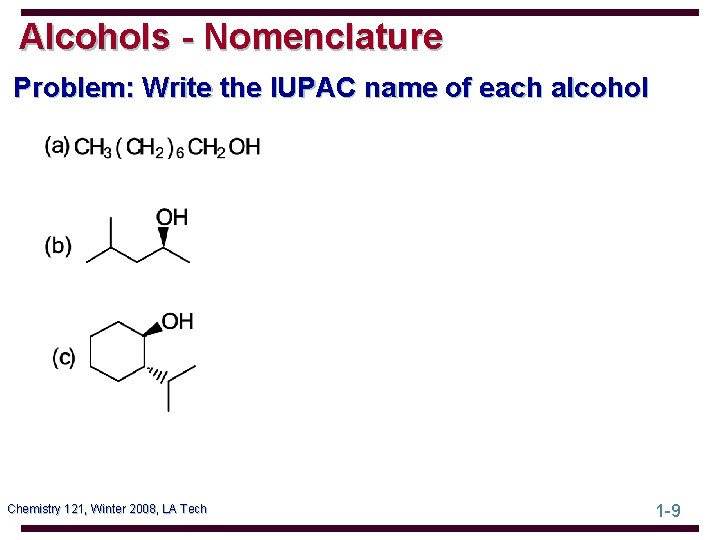
Alcohols - Nomenclature Problem: Write the IUPAC name of each alcohol Chemistry 121, Winter 2008, LA Tech 1 -9

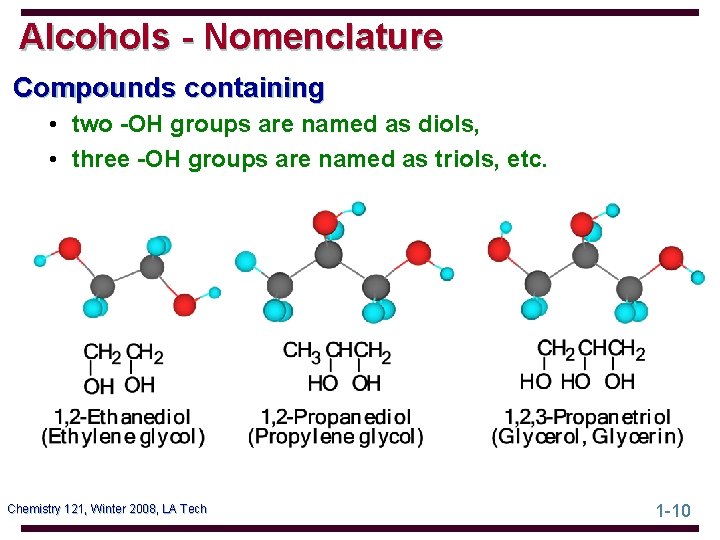
Alcohols - Nomenclature Compounds containing • two -OH groups are named as diols, • three -OH groups are named as triols, etc. Chemistry 121, Winter 2008, LA Tech 1 -10

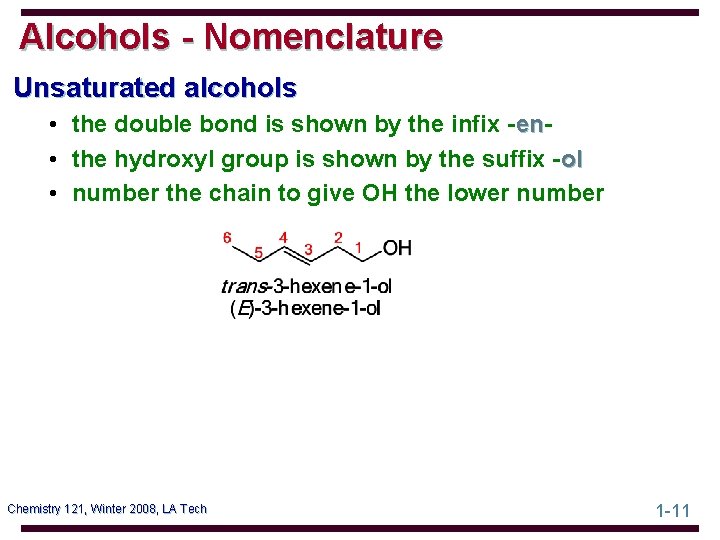
Alcohols - Nomenclature Unsaturated alcohols • the double bond is shown by the infix -enen • the hydroxyl group is shown by the suffix -ol • number the chain to give OH the lower number Chemistry 121, Winter 2008, LA Tech 1 -11

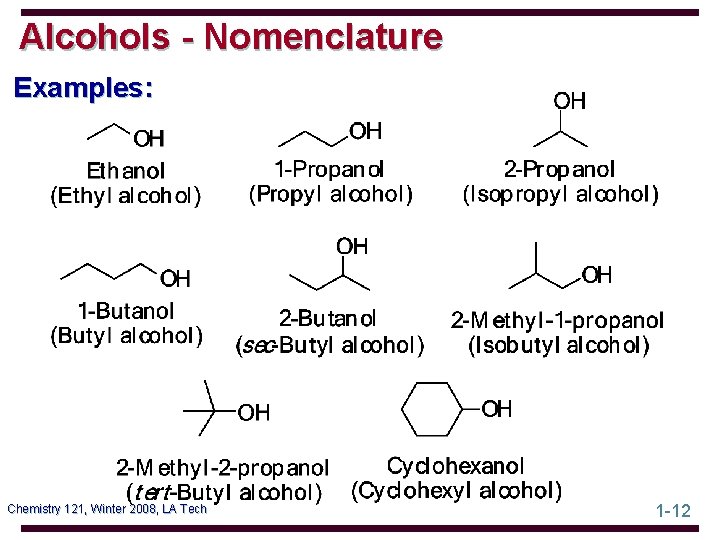
Alcohols - Nomenclature Examples: Chemistry 121, Winter 2008, LA Tech 1 -12

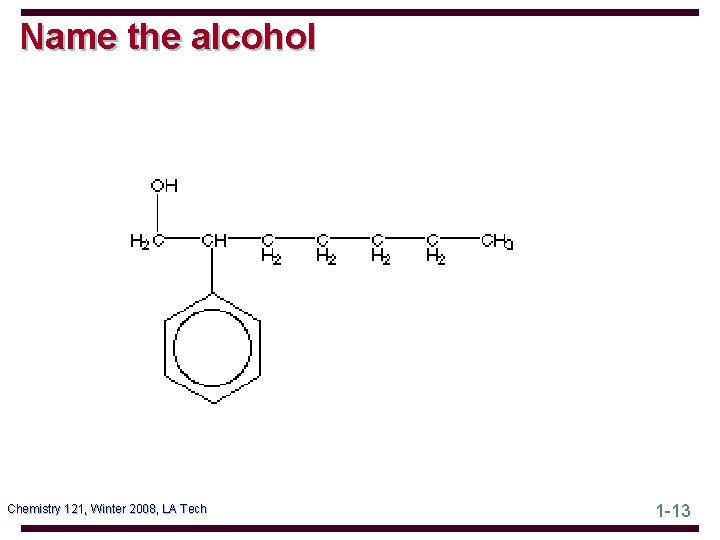
Name the alcohol Chemistry 121, Winter 2008, LA Tech 1 -13

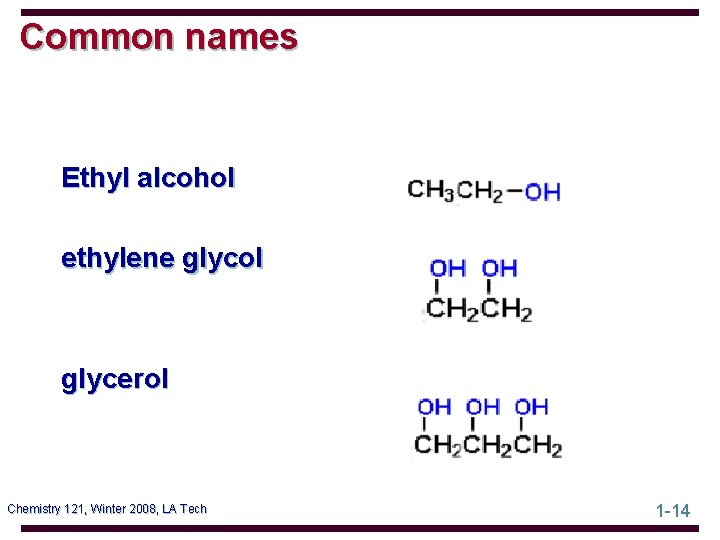
Common names Ethyl alcohol ethylene glycol glycerol Chemistry 121, Winter 2008, LA Tech 1 -14

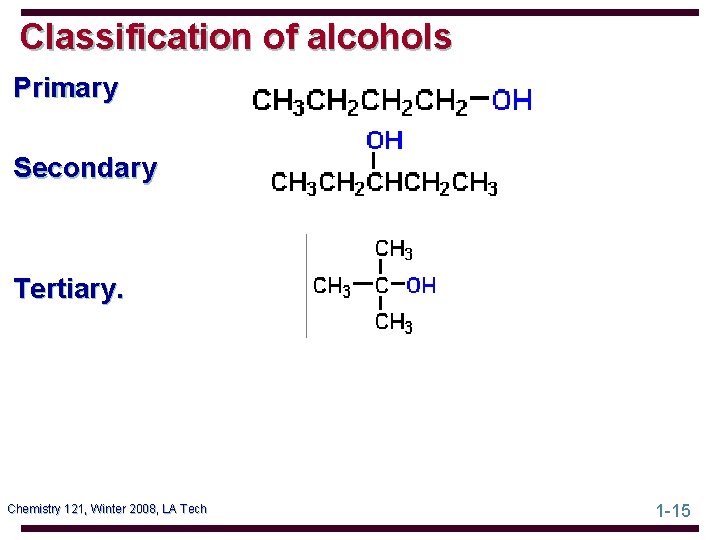
Classification of alcohols Primary Secondary Tertiary. Chemistry 121, Winter 2008, LA Tech 1 -15

Chemistry 121, Winter 2008, LA Tech 1 -16

Chemistry 121, Winter 2008, LA Tech 1 -17

Chemistry 121, Winter 2008, LA Tech 1 -18

Chemistry 121, Winter 2008, LA Tech 1 -19

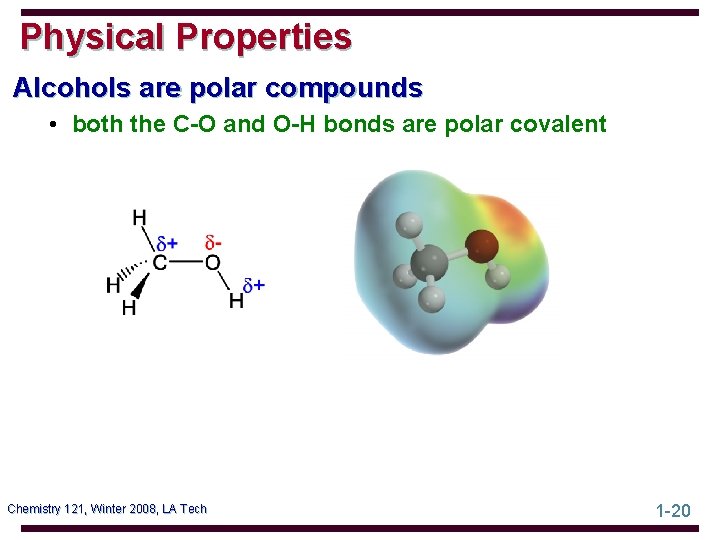
Physical Properties Alcohols are polar compounds • both the C-O and O-H bonds are polar covalent Chemistry 121, Winter 2008, LA Tech 1 -20

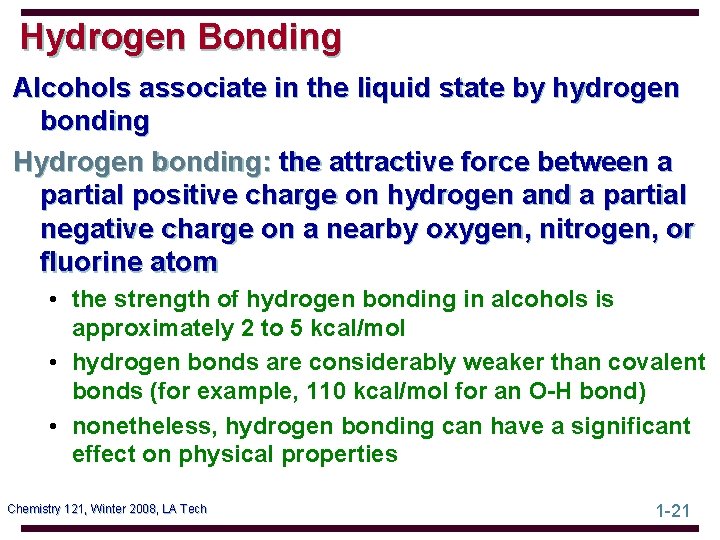
Hydrogen Bonding Alcohols associate in the liquid state by hydrogen bonding Hydrogen bonding: the attractive force between a partial positive charge on hydrogen and a partial negative charge on a nearby oxygen, nitrogen, or fluorine atom • the strength of hydrogen bonding in alcohols is approximately 2 to 5 kcal/mol • hydrogen bonds are considerably weaker than covalent bonds (for example, 110 kcal/mol for an O-H bond) • nonetheless, hydrogen bonding can have a significant effect on physical properties Chemistry 121, Winter 2008, LA Tech 1 -21

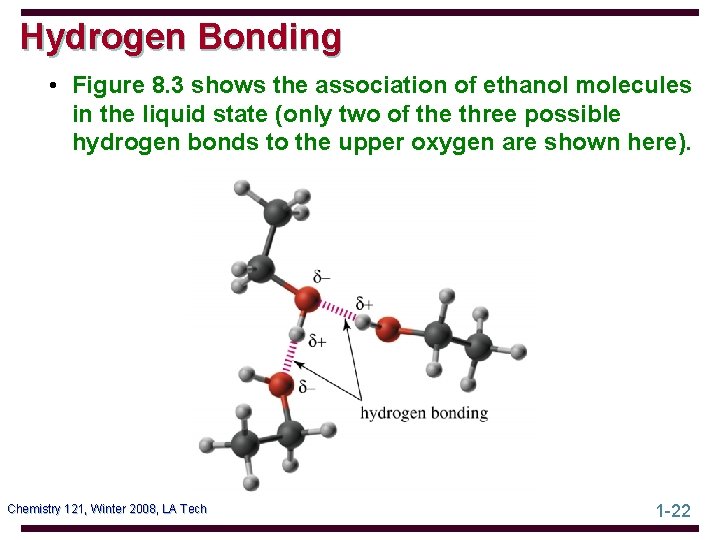
Hydrogen Bonding • Figure 8. 3 shows the association of ethanol molecules in the liquid state (only two of the three possible hydrogen bonds to the upper oxygen are shown here). Chemistry 121, Winter 2008, LA Tech 1 -22

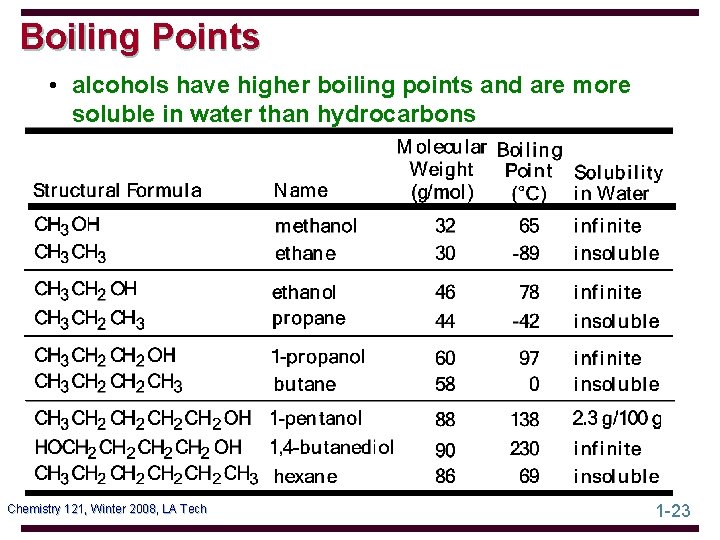
Boiling Points • alcohols have higher boiling points and are more soluble in water than hydrocarbons Chemistry 121, Winter 2008, LA Tech 1 -23

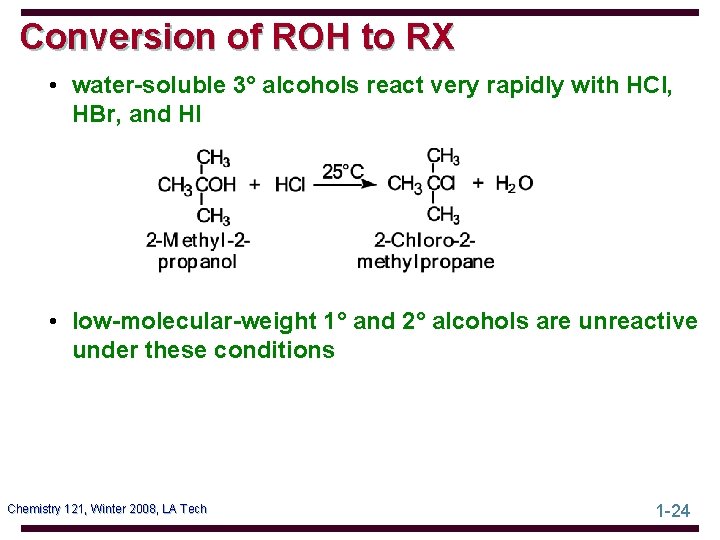
Conversion of ROH to RX • water-soluble 3° alcohols react very rapidly with HCl, HBr, and HI • low-molecular-weight 1° and 2° alcohols are unreactive under these conditions Chemistry 121, Winter 2008, LA Tech 1 -24

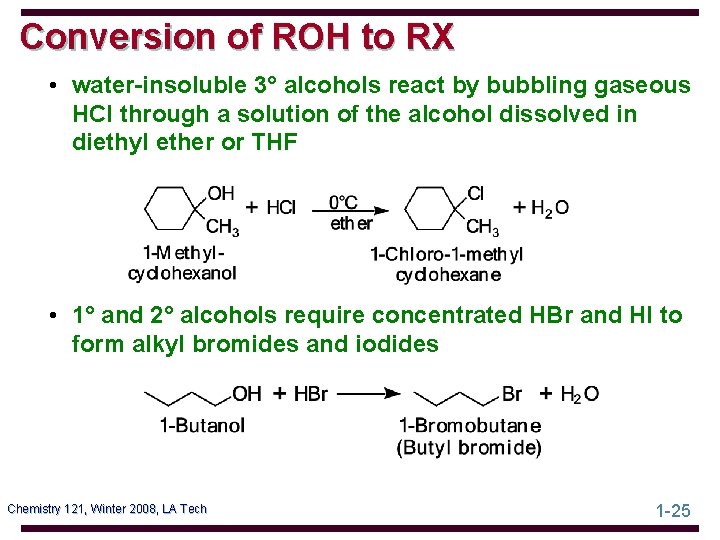
Conversion of ROH to RX • water-insoluble 3° alcohols react by bubbling gaseous HCl through a solution of the alcohol dissolved in diethyl ether or THF • 1° and 2° alcohols require concentrated HBr and HI to form alkyl bromides and iodides Chemistry 121, Winter 2008, LA Tech 1 -25

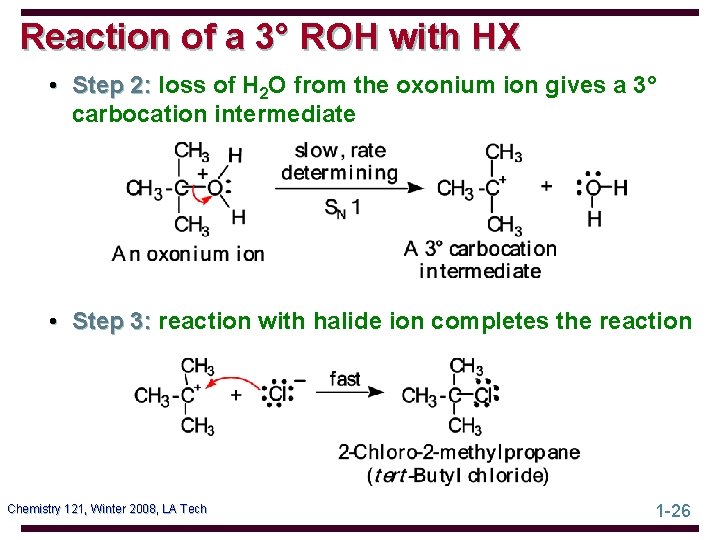
Reaction of a 3° ROH with HX • Step 2: loss of H 2 O from the oxonium ion gives a 3° carbocation intermediate • Step 3: reaction with halide ion completes the reaction Chemistry 121, Winter 2008, LA Tech 1 -26

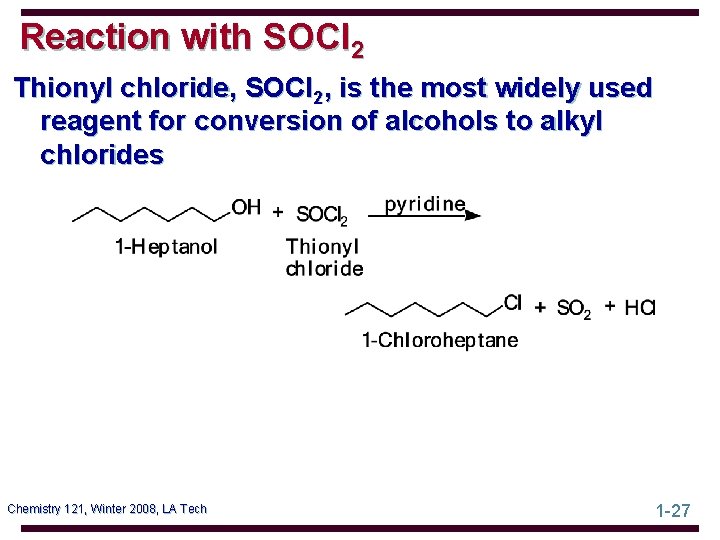
Reaction with SOCl 2 Thionyl chloride, SOCl 2, is the most widely used reagent for conversion of alcohols to alkyl chlorides Chemistry 121, Winter 2008, LA Tech 1 -27

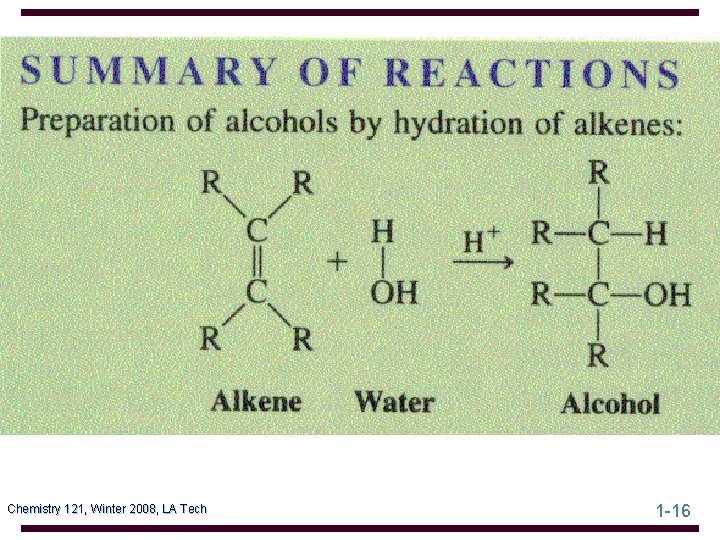
Dehydration of Alcohols An alcohol can be converted to an alkene by elimination of H and OH from adjacent carbons (a -elimination) • 1° alcohols must be heated at high temperature in the presence of an acid catalyst, such as H 2 SO 4 or H 3 PO 4 • 2° alcohols undergo dehydration at somewhat lower temperatures • 3° alcohols often require temperatures only at or slightly above room temperature Chemistry 121, Winter 2008, LA Tech 1 -28

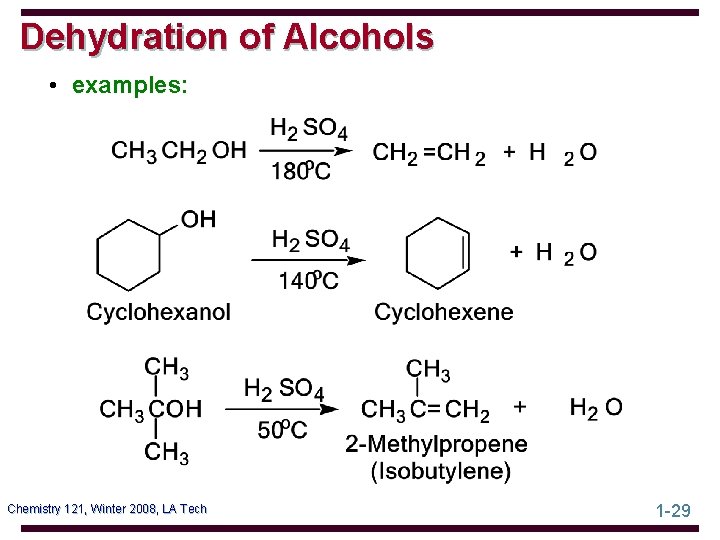
Dehydration of Alcohols • examples: Chemistry 121, Winter 2008, LA Tech 1 -29

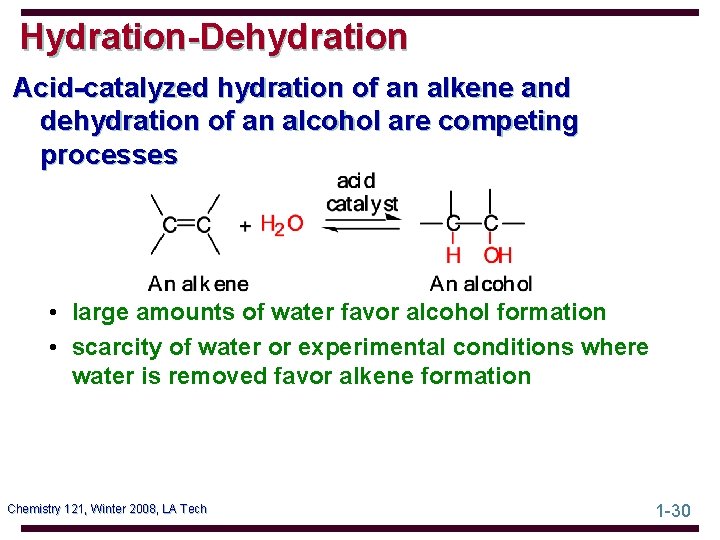
Hydration-Dehydration Acid-catalyzed hydration of an alkene and dehydration of an alcohol are competing processes • large amounts of water favor alcohol formation • scarcity of water or experimental conditions where water is removed favor alkene formation Chemistry 121, Winter 2008, LA Tech 1 -30

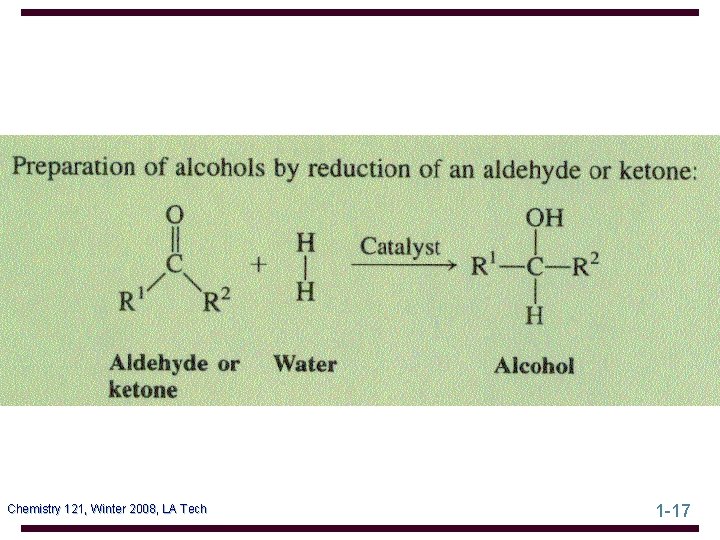
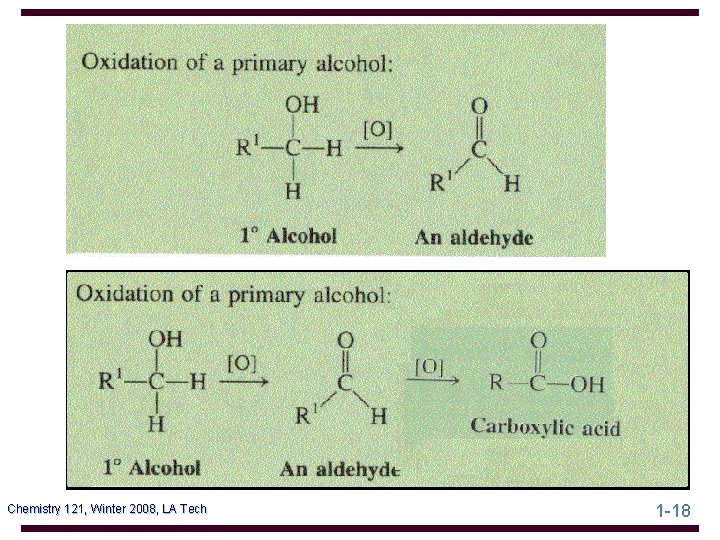
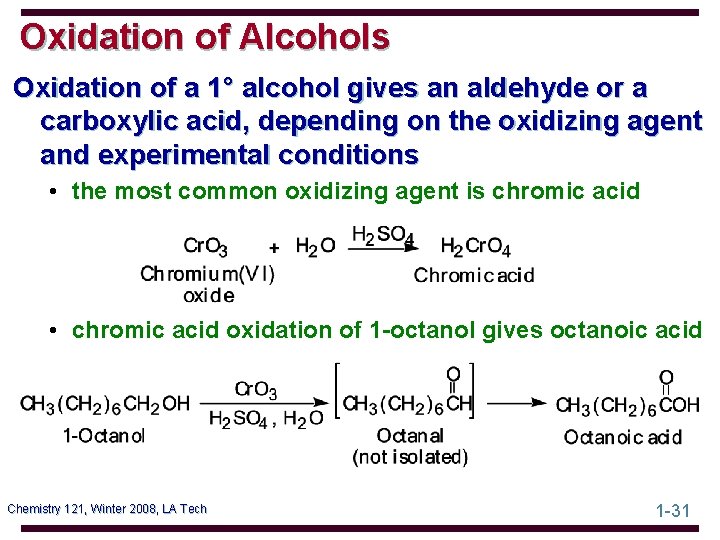
Oxidation of Alcohols Oxidation of a 1° alcohol gives an aldehyde or a carboxylic acid, depending on the oxidizing agent and experimental conditions • the most common oxidizing agent is chromic acid • chromic acid oxidation of 1 -octanol gives octanoic acid Chemistry 121, Winter 2008, LA Tech 1 -31

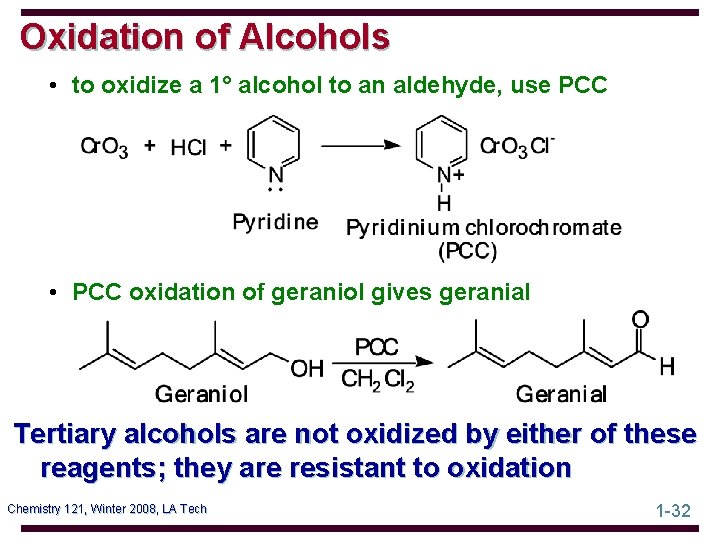
Oxidation of Alcohols • to oxidize a 1° alcohol to an aldehyde, use PCC • PCC oxidation of geraniol gives geranial Tertiary alcohols are not oxidized by either of these reagents; they are resistant to oxidation Chemistry 121, Winter 2008, LA Tech 1 -32

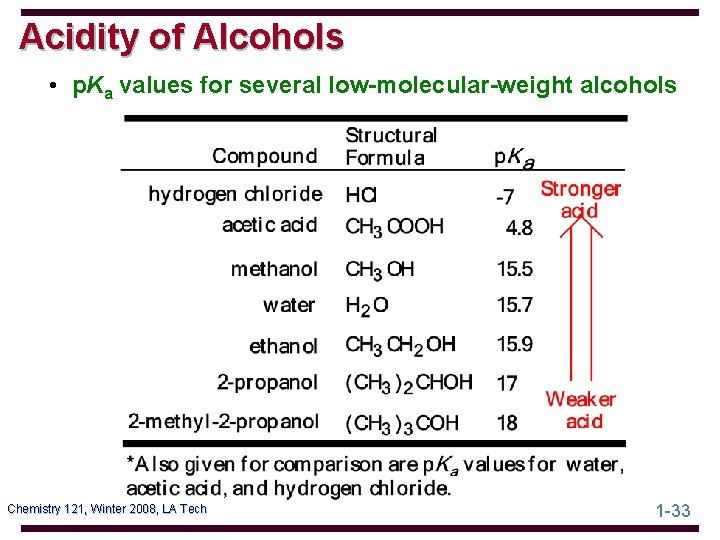
Acidity of Alcohols • p. Ka values for several low-molecular-weight alcohols Chemistry 121, Winter 2008, LA Tech 1 -33

Reaction with Active Metals Alcohols react with Li, Na, K, and other active metals to liberate hydrogen gas and form metal alkoxides • Na is oxidized to Na+ and H+ is reduced to H 2 • alkoxides are somewhat stronger bases that OH • alkoxides can be used as nucleophiles in nucleophilic substitution reactions • they can also be used as bases in -elimination reactions Chemistry 121, Winter 2008, LA Tech 1 -34

Conversion of ROH to RX Conversion of an alcohol to an alkyl halide involves substitution of halogen for -OH at a saturated carbon • the most common reagents for this purpose are the halogen acids, HX, and thionyl chloride, SOCl 2 Chemistry 121, Winter 2008, LA Tech 1 -35

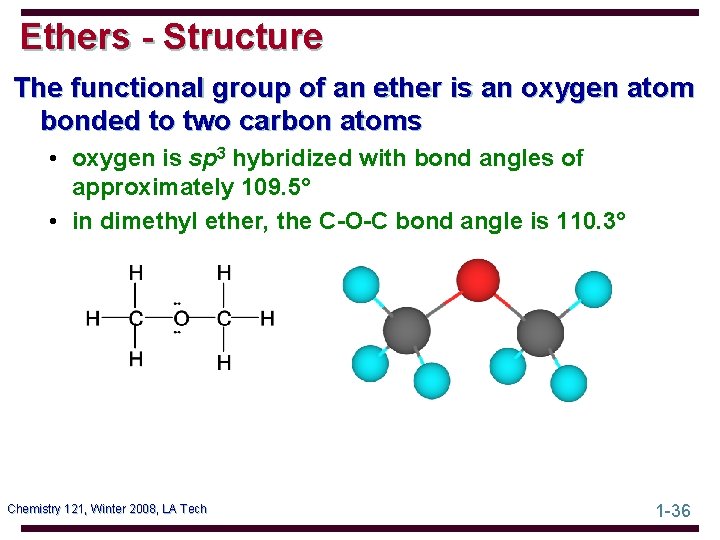
Ethers - Structure The functional group of an ether is an oxygen atom bonded to two carbon atoms • oxygen is sp 3 hybridized with bond angles of approximately 109. 5° • in dimethyl ether, the C-O-C bond angle is 110. 3° Chemistry 121, Winter 2008, LA Tech 1 -36

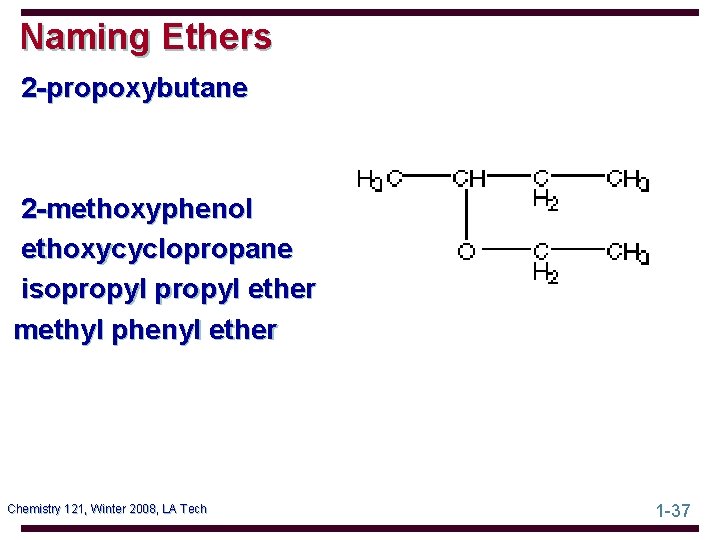
Naming Ethers 2 -propoxybutane 2 -methoxyphenol ethoxycyclopropane isopropyl ether methyl phenyl ether Chemistry 121, Winter 2008, LA Tech 1 -37

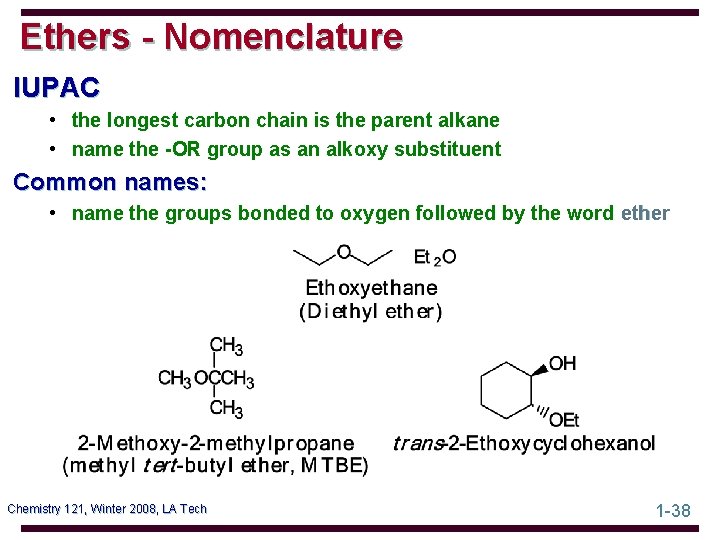
Ethers - Nomenclature IUPAC • the longest carbon chain is the parent alkane • name the -OR group as an alkoxy substituent Common names: • name the groups bonded to oxygen followed by the word ether Chemistry 121, Winter 2008, LA Tech 1 -38

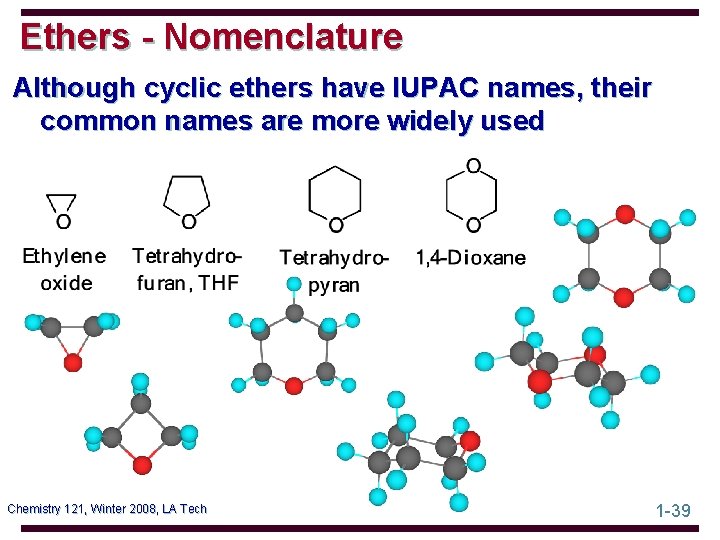
Ethers - Nomenclature Although cyclic ethers have IUPAC names, their common names are more widely used Chemistry 121, Winter 2008, LA Tech 1 -39

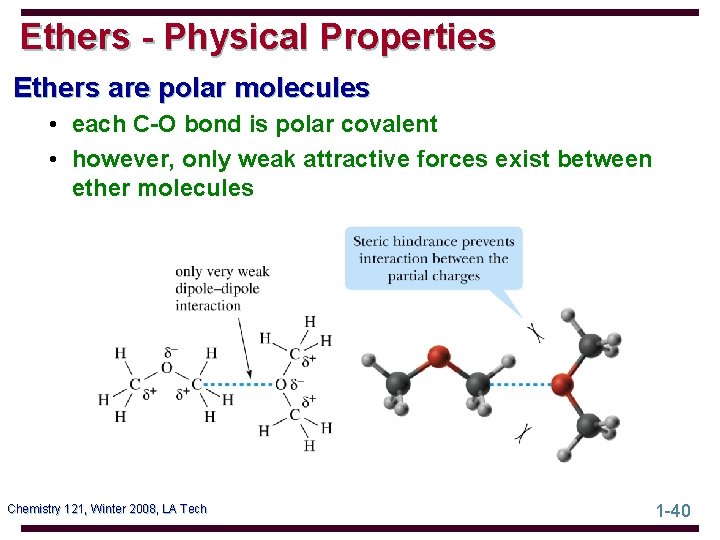
Ethers - Physical Properties Ethers are polar molecules • each C-O bond is polar covalent • however, only weak attractive forces exist between ether molecules Chemistry 121, Winter 2008, LA Tech 1 -40

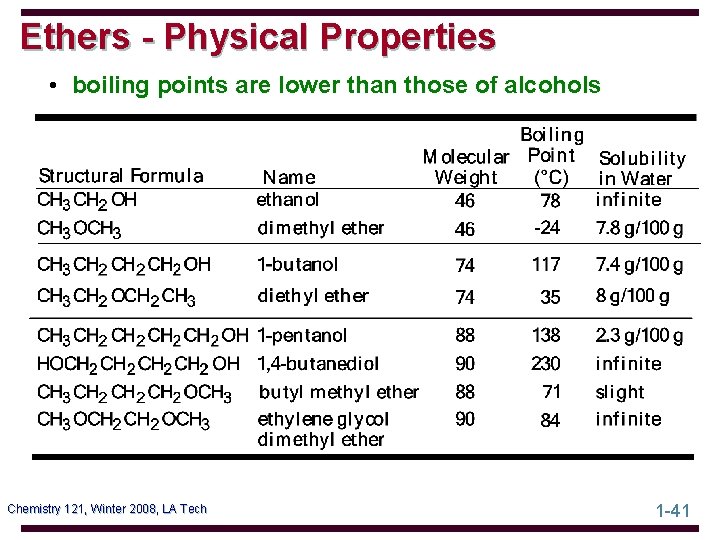
Ethers - Physical Properties • boiling points are lower than those of alcohols Chemistry 121, Winter 2008, LA Tech 1 -41

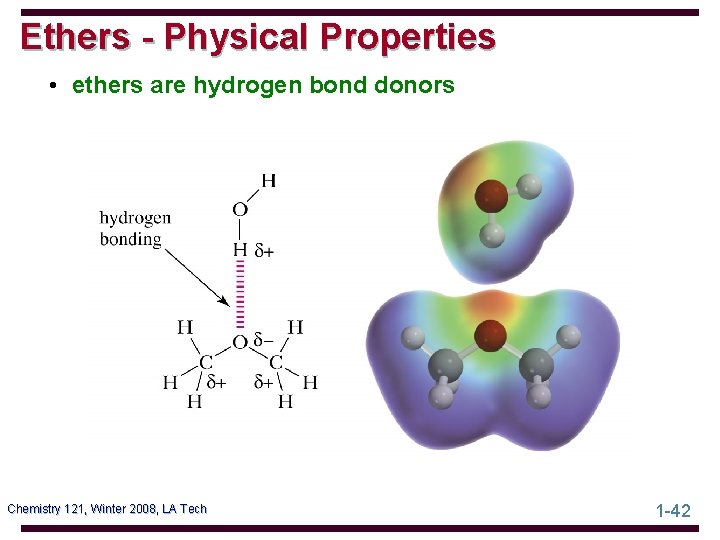
Ethers - Physical Properties • ethers are hydrogen bond donors Chemistry 121, Winter 2008, LA Tech 1 -42

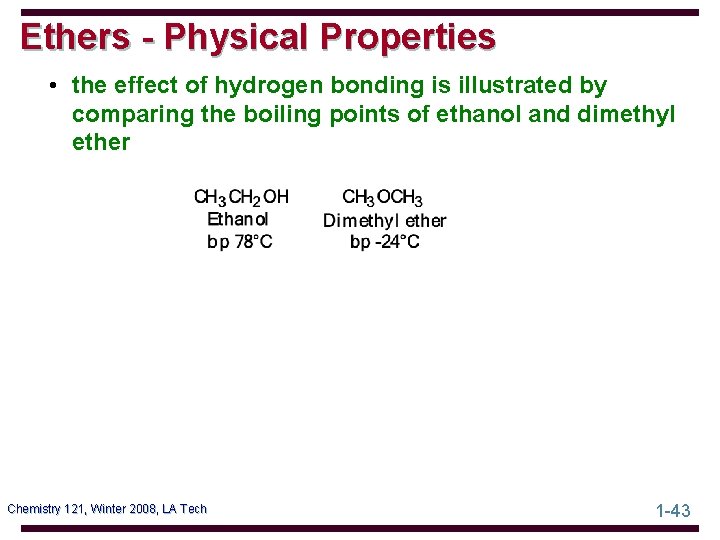
Ethers - Physical Properties • the effect of hydrogen bonding is illustrated by comparing the boiling points of ethanol and dimethyl ether Chemistry 121, Winter 2008, LA Tech 1 -43

Reactions of Ethers resemble hydrocarbons in their resistance to chemical reaction • they do not react with strong oxidizing agents such as chromic acid, H 2 Cr. O 4 • they are not affected by most acids and bases at moderate temperatures Because of their good solvent properties and general inertness to chemical reaction, ethers are excellent solvents in which to carry out organic reactions Chemistry 121, Winter 2008, LA Tech 1 -44

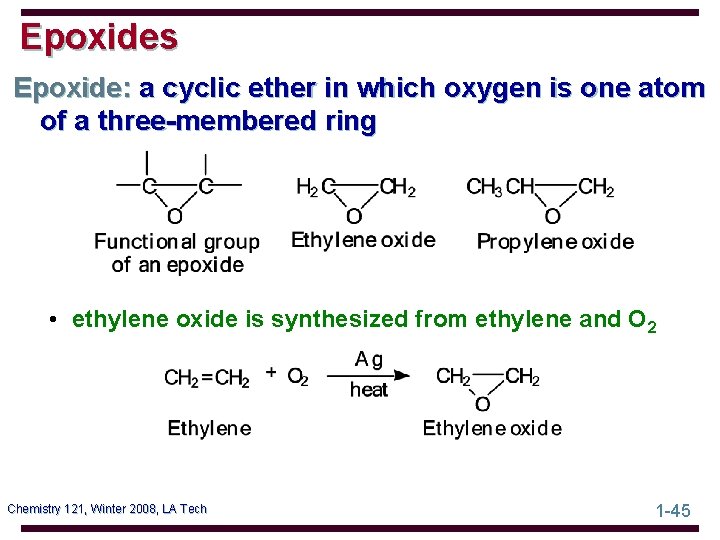
Epoxides Epoxide: a cyclic ether in which oxygen is one atom of a three-membered ring • ethylene oxide is synthesized from ethylene and O 2 Chemistry 121, Winter 2008, LA Tech 1 -45

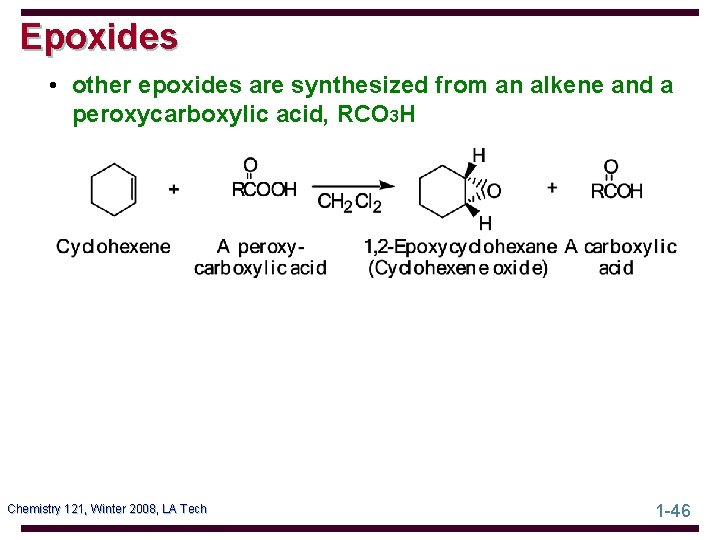
Epoxides • other epoxides are synthesized from an alkene and a peroxycarboxylic acid, RCO 3 H Chemistry 121, Winter 2008, LA Tech 1 -46

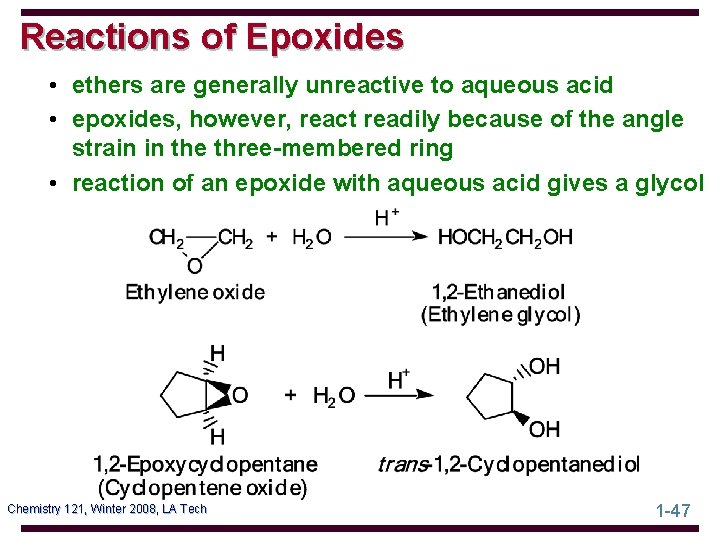
Reactions of Epoxides • ethers are generally unreactive to aqueous acid • epoxides, however, react readily because of the angle strain in the three-membered ring • reaction of an epoxide with aqueous acid gives a glycol Chemistry 121, Winter 2008, LA Tech 1 -47

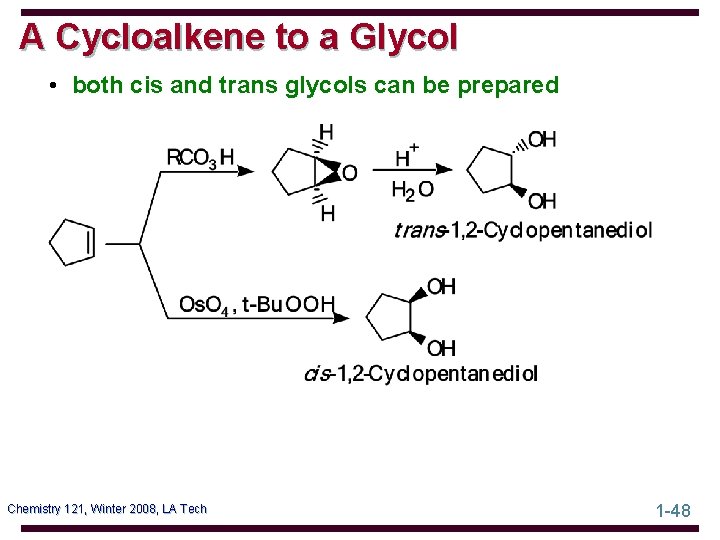
A Cycloalkene to a Glycol • both cis and trans glycols can be prepared Chemistry 121, Winter 2008, LA Tech 1 -48

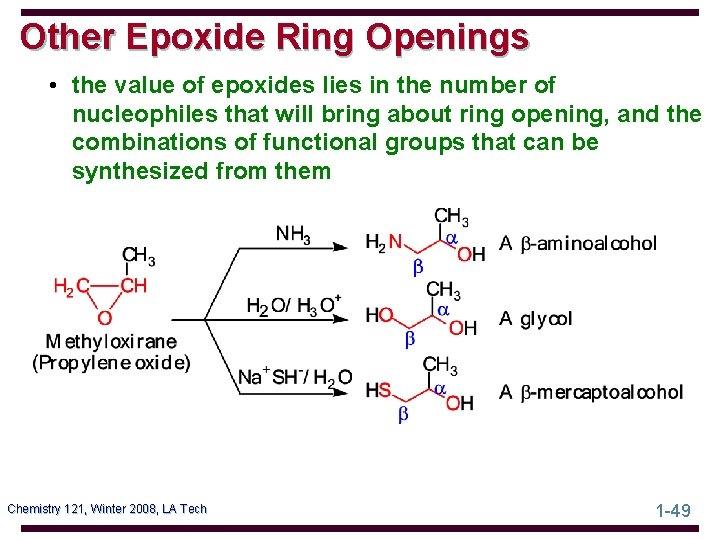
Other Epoxide Ring Openings • the value of epoxides lies in the number of nucleophiles that will bring about ring opening, and the combinations of functional groups that can be synthesized from them Chemistry 121, Winter 2008, LA Tech 1 -49

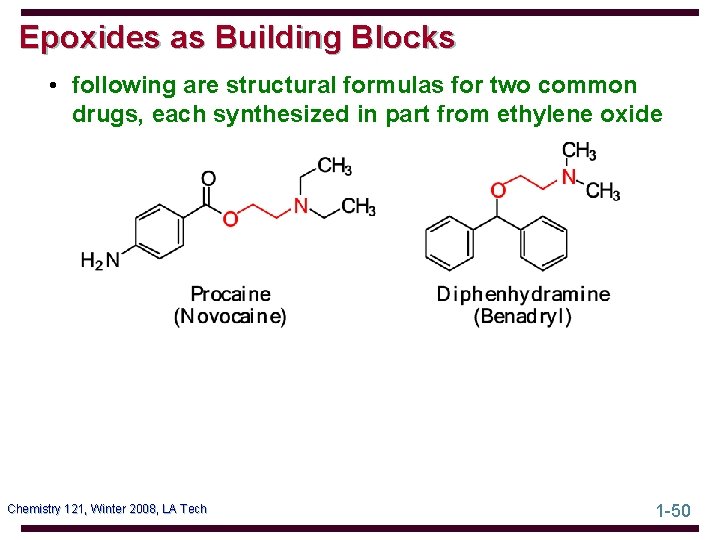
Epoxides as Building Blocks • following are structural formulas for two common drugs, each synthesized in part from ethylene oxide Chemistry 121, Winter 2008, LA Tech 1 -50

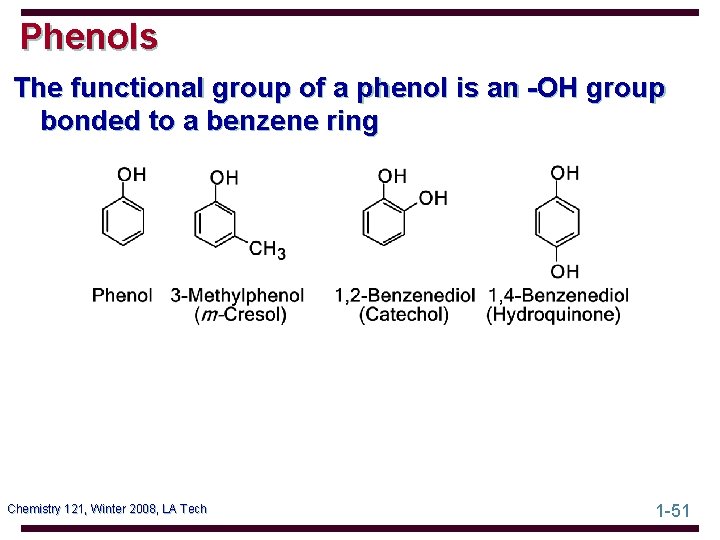
Phenols The functional group of a phenol is an -OH group bonded to a benzene ring Chemistry 121, Winter 2008, LA Tech 1 -51

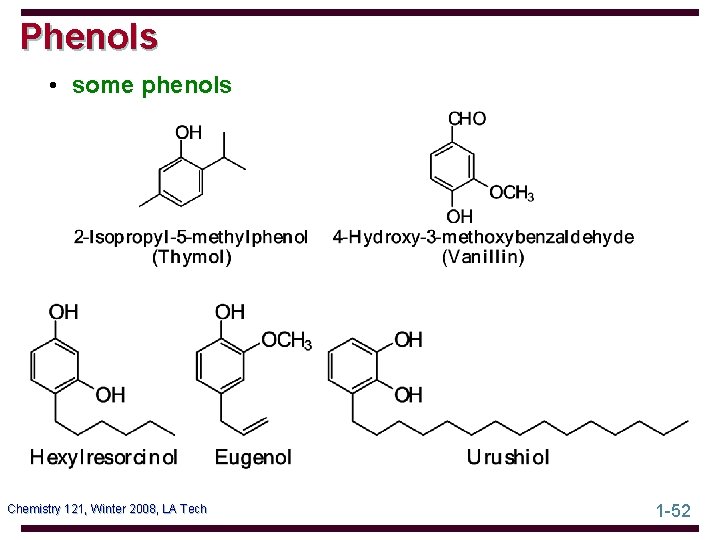
Phenols • some phenols Chemistry 121, Winter 2008, LA Tech 1 -52

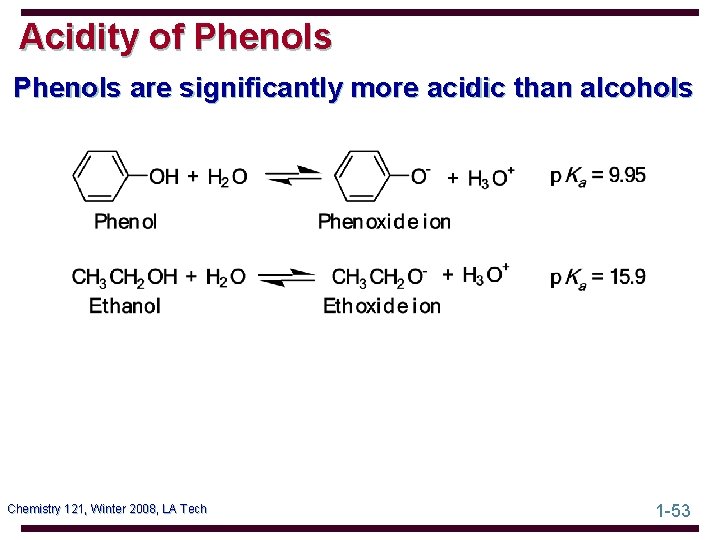
Acidity of Phenols are significantly more acidic than alcohols Chemistry 121, Winter 2008, LA Tech 1 -53

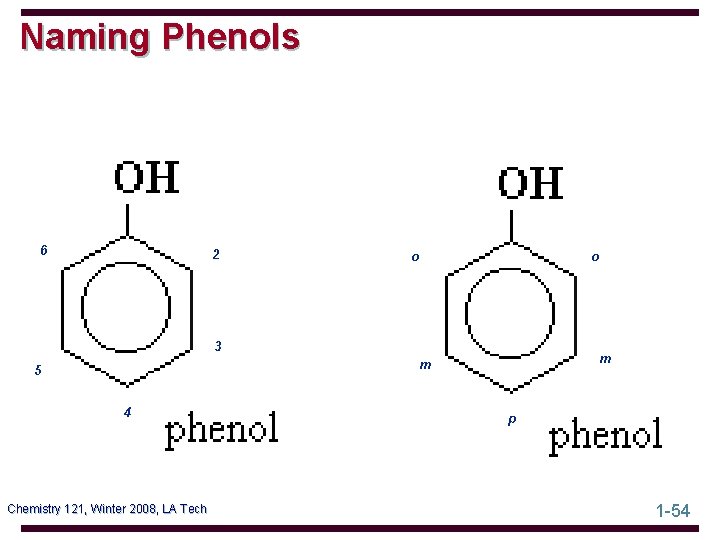
Naming Phenols 6 2 o o 3 m m 5 4 Chemistry 121, Winter 2008, LA Tech p 1 -54

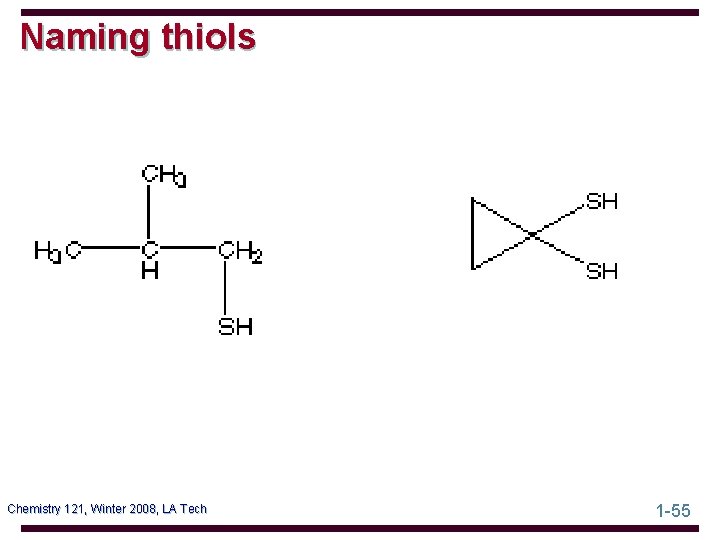
Naming thiols Chemistry 121, Winter 2008, LA Tech 1 -55

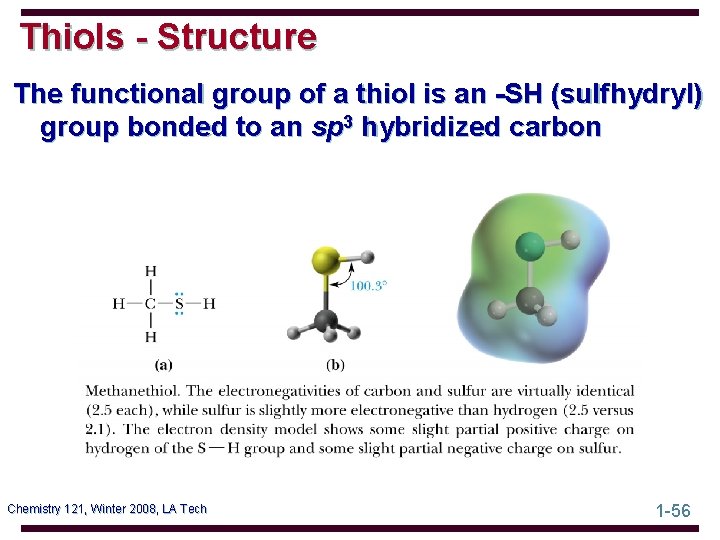
Thiols - Structure The functional group of a thiol is an -SH (sulfhydryl) group bonded to an sp 3 hybridized carbon Chemistry 121, Winter 2008, LA Tech 1 -56

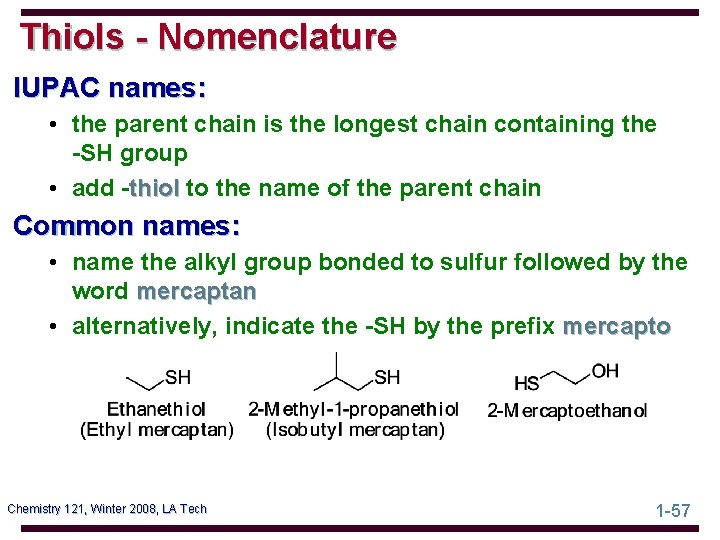
Thiols - Nomenclature IUPAC names: • the parent chain is the longest chain containing the -SH group • add -thiol to the name of the parent chain Common names: • name the alkyl group bonded to sulfur followed by the word mercaptan • alternatively, indicate the -SH by the prefix mercapto Chemistry 121, Winter 2008, LA Tech 1 -57

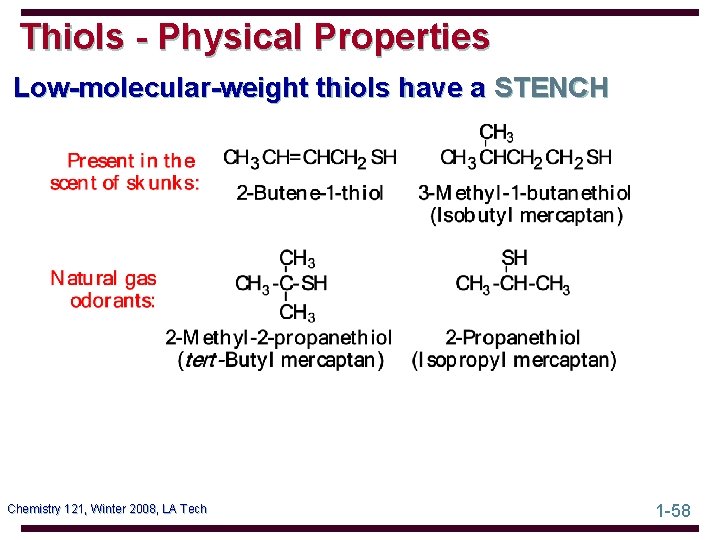
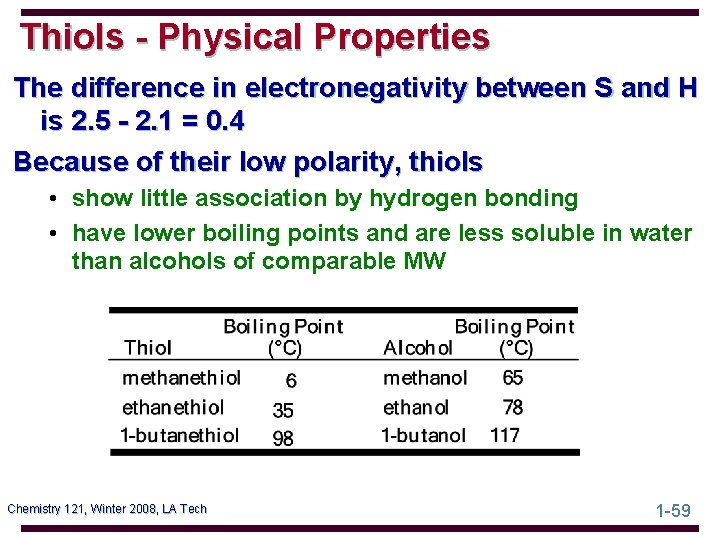
Thiols - Physical Properties Low-molecular-weight thiols have a STENCH Chemistry 121, Winter 2008, LA Tech 1 -58

Thiols - Physical Properties The difference in electronegativity between S and H is 2. 5 - 2. 1 = 0. 4 Because of their low polarity, thiols • show little association by hydrogen bonding • have lower boiling points and are less soluble in water than alcohols of comparable MW Chemistry 121, Winter 2008, LA Tech 1 -59

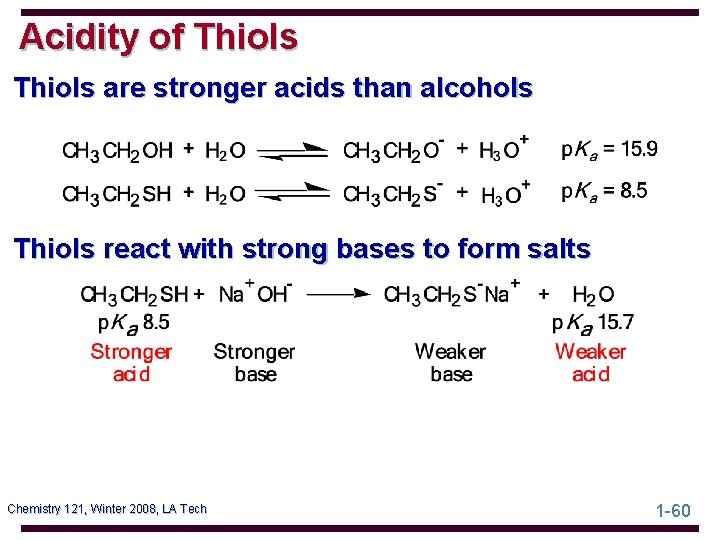
Acidity of Thiols are stronger acids than alcohols Thiols react with strong bases to form salts Chemistry 121, Winter 2008, LA Tech 1 -60

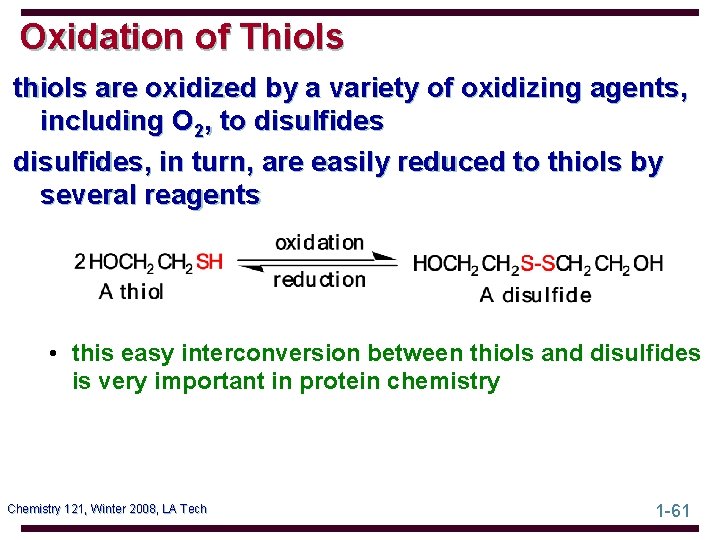
Oxidation of Thiols thiols are oxidized by a variety of oxidizing agents, including O 2, to disulfides, in turn, are easily reduced to thiols by several reagents • this easy interconversion between thiols and disulfides is very important in protein chemistry Chemistry 121, Winter 2008, LA Tech 1 -61
 Ib chemistry organic chemistry
Ib chemistry organic chemistry Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Organic vs inorganic compounds
Organic vs inorganic compounds Winter kommt winter kommt flocken fallen nieder
Winter kommt winter kommt flocken fallen nieder Winter kommt winter kommt flocken fallen nieder lied
Winter kommt winter kommt flocken fallen nieder lied Es ist kalt es ist kalt flocken fallen nieder
Es ist kalt es ist kalt flocken fallen nieder Chemistry january 2018 answers
Chemistry january 2018 answers Numbering carbon chains
Numbering carbon chains Soap organic chemistry
Soap organic chemistry Ester organic chemistry
Ester organic chemistry Homologous series formula
Homologous series formula Transition state energy diagram
Transition state energy diagram Ee organic chemistry
Ee organic chemistry Ario organic chemistry
Ario organic chemistry Thermodynamic vs kinetic control
Thermodynamic vs kinetic control Organic chemistry david klein 3rd edition
Organic chemistry david klein 3rd edition Is alkane an organic compound
Is alkane an organic compound Leveling effect organic chemistry
Leveling effect organic chemistry Organic chemistry iupac name
Organic chemistry iupac name Objective lab report example
Objective lab report example Www.masterorganicchemistry.com
Www.masterorganicchemistry.com Grade 10 organic chemistry
Grade 10 organic chemistry Cyclo organic chemistry
Cyclo organic chemistry Organic chemistry wade
Organic chemistry wade Math eth prop bute
Math eth prop bute Chemistry cracking
Chemistry cracking Meth eth prop but
Meth eth prop but Organic chemistry myanmar
Organic chemistry myanmar Hhcchh
Hhcchh M+2 mass spec
M+2 mass spec Hono organic chemistry
Hono organic chemistry Vicinal dihalide
Vicinal dihalide Topic 11 organic chemistry
Topic 11 organic chemistry Chapter 22 review organic chemistry section 1 answers
Chapter 22 review organic chemistry section 1 answers Organic chemistry reaction pathways
Organic chemistry reaction pathways Organic chemistry nomenclature
Organic chemistry nomenclature What is organic chemistry like
What is organic chemistry like Organic vs inorganic molecules
Organic vs inorganic molecules Organic chemistry vocabulary
Organic chemistry vocabulary Organic chemistry (3rd) edition chapter 1 problem 20s
Organic chemistry (3rd) edition chapter 1 problem 20s Extraction of caffeine from vivarin tablets lab report
Extraction of caffeine from vivarin tablets lab report A level chemistry ocr organic synthesis
A level chemistry ocr organic synthesis Compound lipids definition
Compound lipids definition Ario+
Ario+ How to calculate percent yield
How to calculate percent yield Polarimetry organic chemistry
Polarimetry organic chemistry Organic chemistry third edition david klein
Organic chemistry third edition david klein Radicals
Radicals Hammonds postulate
Hammonds postulate Klein
Klein Organic chemistry chapter 9
Organic chemistry chapter 9 Chemistry ethics case studies
Chemistry ethics case studies Chapter 7 chemistry review
Chapter 7 chemistry review Chapter 3 organic chemistry
Chapter 3 organic chemistry Organic chemistry chapter 1 problem 59pp
Organic chemistry chapter 1 problem 59pp Hammond's postulate organic chemistry
Hammond's postulate organic chemistry Structure and bonding in organic chemistry
Structure and bonding in organic chemistry Conformation of sugars ppt
Conformation of sugars ppt Resonance in benzyl carbocation
Resonance in benzyl carbocation Organic chemistry
Organic chemistry Organic chemistry
Organic chemistry Organic chemistry
Organic chemistry