Video 6 1 Review Gas es Liquids and




























- Slides: 71

Video 6. 1 Review Gas es, Liquids, and Solids With Phase Change Dia grams

Objectives 0 By the end of this video you should be able to… 0 Identify and explain the three phases of matter. 0 Label and read phase change diagrams.

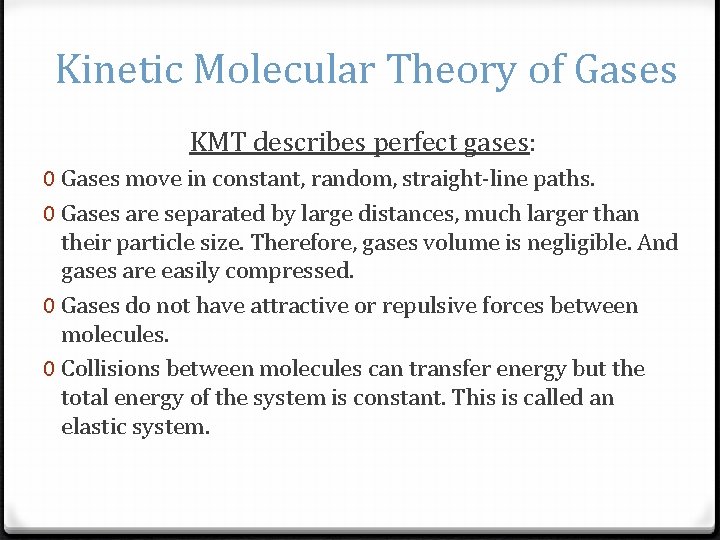
Kinetic Molecular Theory of Gases KMT describes perfect gases: 0 Gases move in constant, random, straight-line paths. 0 Gases are separated by large distances, much larger than their particle size. Therefore, gases volume is negligible. And gases are easily compressed. 0 Gases do not have attractive or repulsive forces between molecules. 0 Collisions between molecules can transfer energy but the total energy of the system is constant. This is called an elastic system.

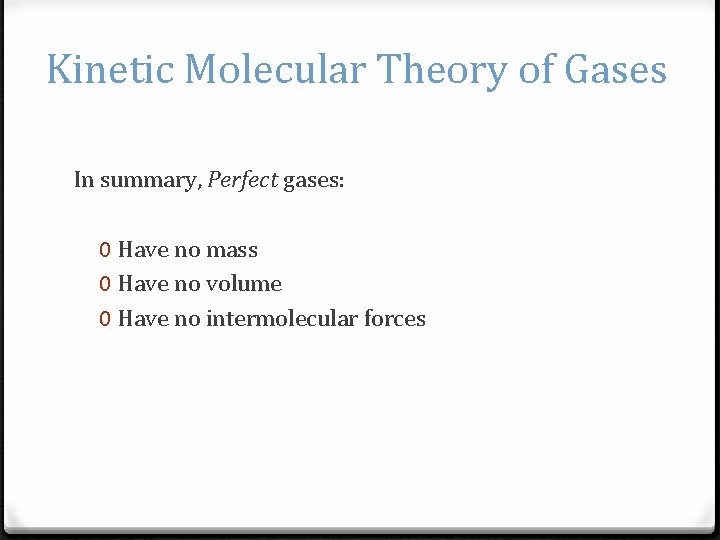
Kinetic Molecular Theory of Gases In summary, Perfect gases: 0 Have no mass 0 Have no volume 0 Have no intermolecular forces

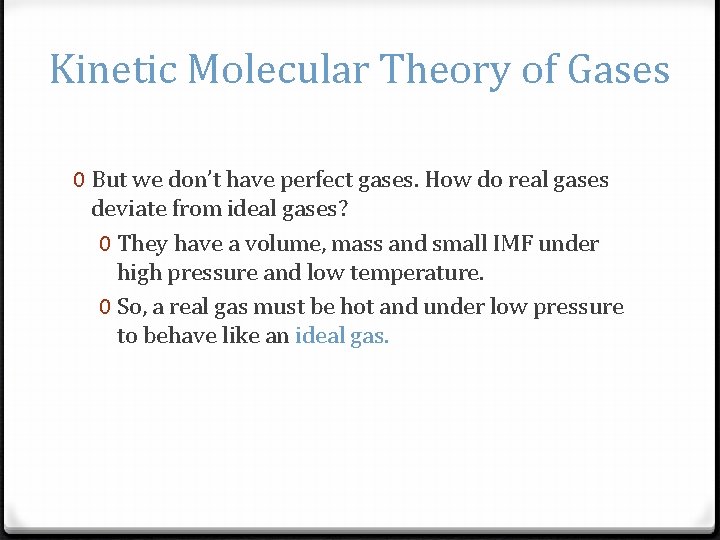
Kinetic Molecular Theory of Gases 0 But we don’t have perfect gases. How do real gases deviate from ideal gases? 0 They have a volume, mass and small IMF under high pressure and low temperature. 0 So, a real gas must be hot and under low pressure to behave like an ideal gas.

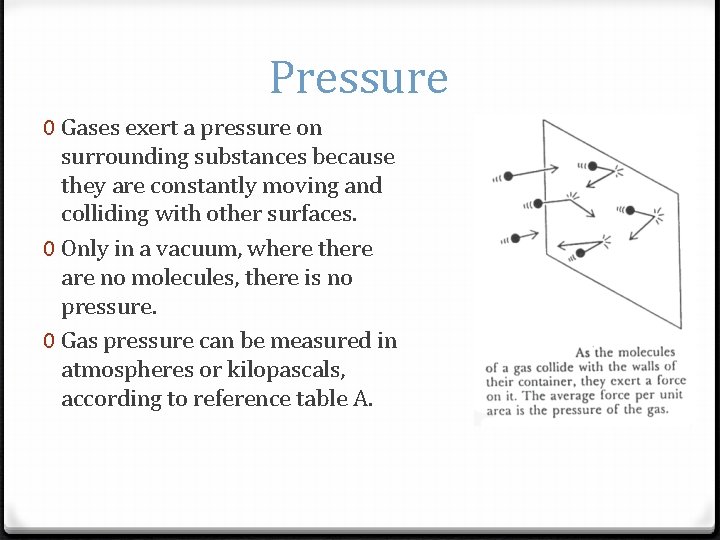
Pressure 0 Gases exert a pressure on surrounding substances because they are constantly moving and colliding with other surfaces. 0 Only in a vacuum, where there are no molecules, there is no pressure. 0 Gas pressure can be measured in atmospheres or kilopascals, according to reference table A.

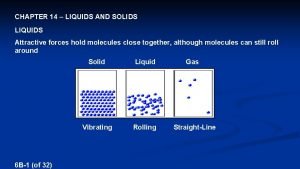
Liquids 0 No definite shape 0 Definite volume 0 Constant motion 0 No arrangement 0 Molecules are closer together than a gas

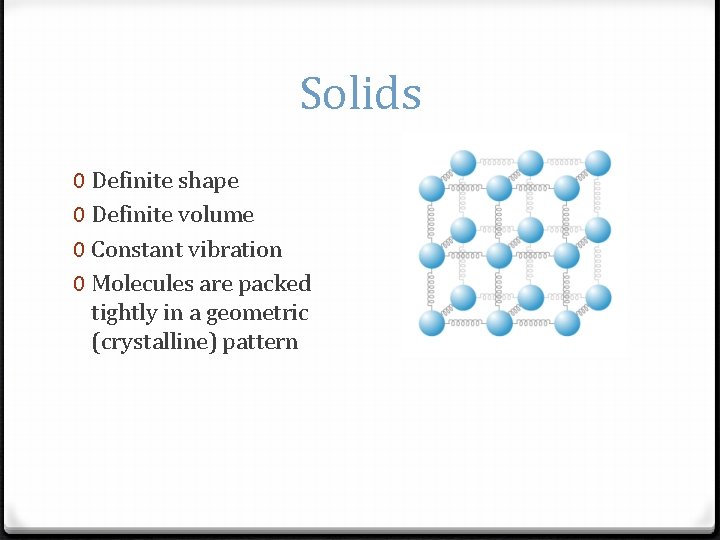
Solids 0 Definite shape 0 Definite volume 0 Constant vibration 0 Molecules are packed tightly in a geometric (crystalline) pattern

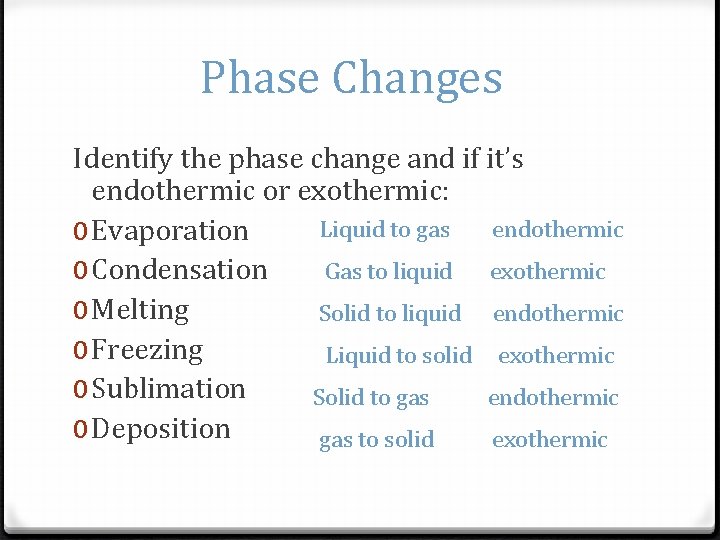
Phase Changes Identify the phase change and if it’s endothermic or exothermic: Liquid to gas endothermic 0 Evaporation Gas to liquid exothermic 0 Condensation 0 Melting Solid to liquid endothermic 0 Freezing Liquid to solid exothermic 0 Sublimation Solid to gas endothermic 0 Deposition gas to solid exothermic

Thermochemistry 0 The study of energy changes that occur in chemical reactions. 0 Kinetic energy refers to energy of motion. (Temperature) 0 Potential Energy refers to stored energy.

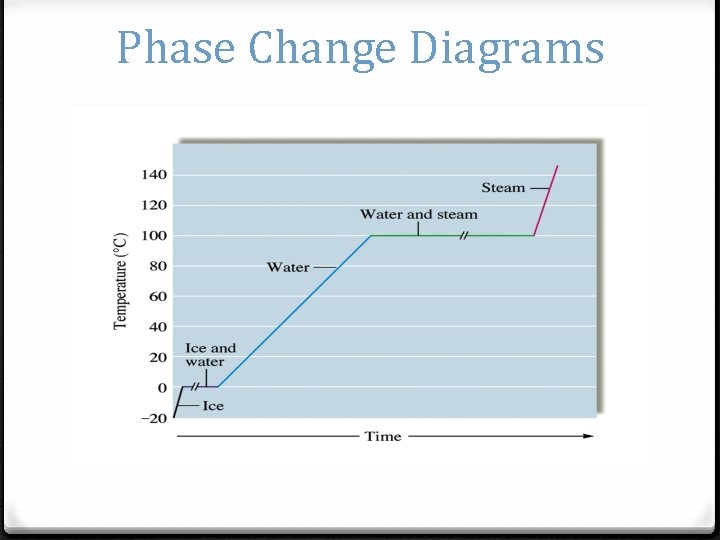
Phase Change Diagrams

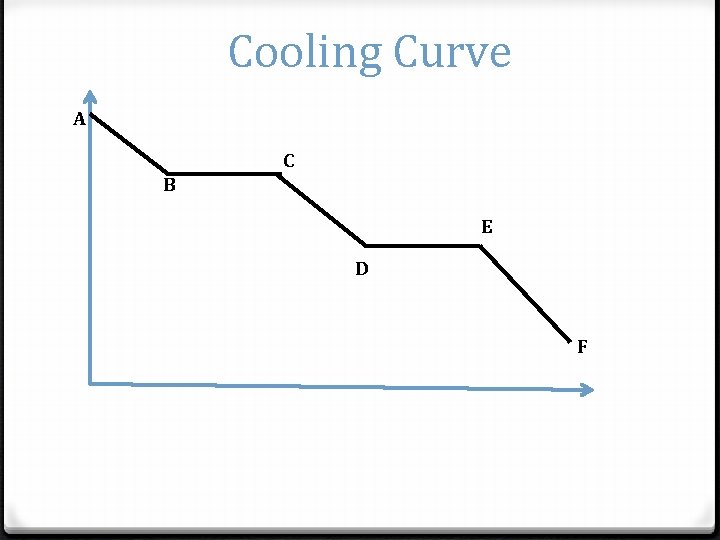
Cooling Curve A C B E D F

Objectives 0 Now you must be able to… 0 Identify and explain the three phases of matter. 0 Label and read phase change diagrams.

Video 6. 2 Q=mcΔT

Objectives 0 By the end of this video you should be able to… 0 Describe system and surroundings in terms of heat flow. 0 Describe and calculate the specific heat of a substance. 0 Calculate the heat of a reaction suing q=mcΔT

System and Surroundings 0 The system includes the molecules we want to study (here, the hydrogen and oxygen molecules). 0 The surroundings are everything else (here, the cylinder and piston).

Systems 0 An open system allows for energy and mass to change, like an open cup of coffee or beaker. 0 A closed system allows for changes in energy only, like a closed coffee cup or beaker. 0 An isolated system doesn’t allow any change in temperature or heat to occur, like a perfect calorimeter.

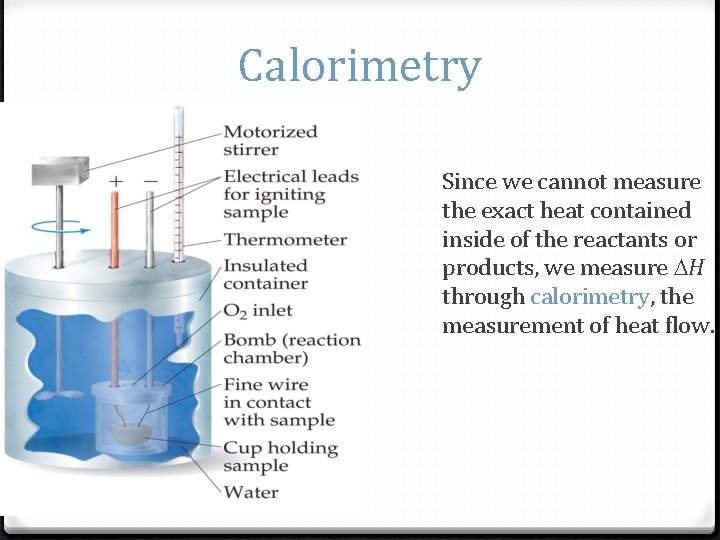
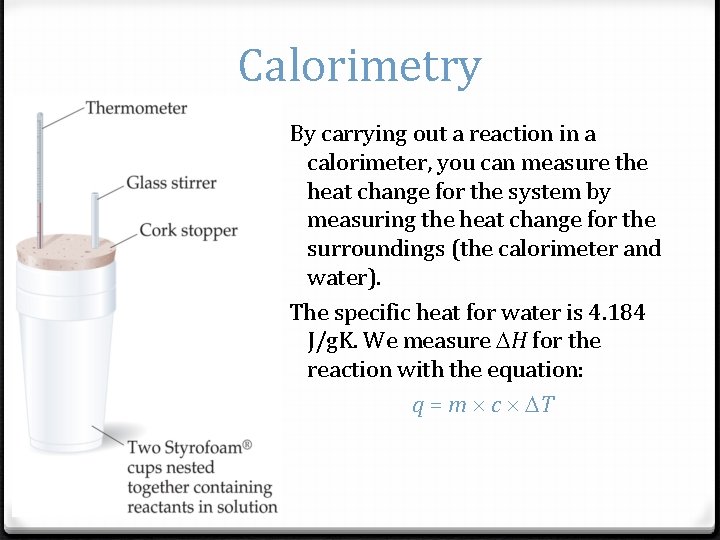
Calorimetry Since we cannot measure the exact heat contained inside of the reactants or products, we measure H through calorimetry, the measurement of heat flow.

Calorimetry By carrying out a reaction in a calorimeter, you can measure the heat change for the system by measuring the heat change for the surroundings (the calorimeter and water). The specific heat for water is 4. 184 J/g. K. We measure H for the reaction with the equation: q = m c T

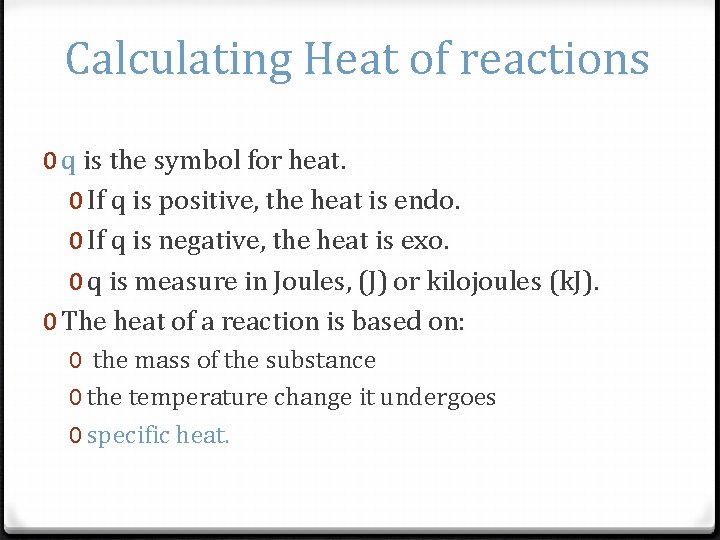
Calculating Heat of reactions 0 q is the symbol for heat. 0 If q is positive, the heat is endo. 0 If q is negative, the heat is exo. 0 q is measure in Joules, (J) or kilojoules (k. J). 0 The heat of a reaction is based on: 0 the mass of the substance 0 the temperature change it undergoes 0 specific heat.

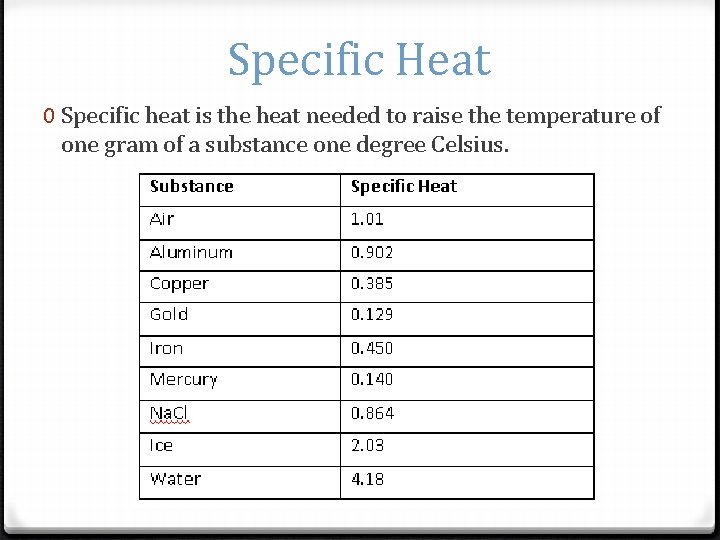
Specific Heat 0 Specific heat is the heat needed to raise the temperature of one gram of a substance one degree Celsius.

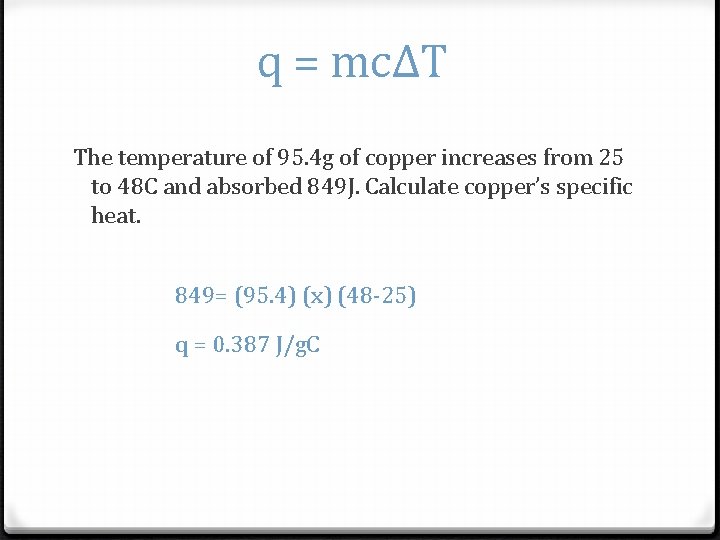
q = mcΔT The temperature of 95. 4 g of copper increases from 25 to 48 C and absorbed 849 J. Calculate copper’s specific heat. 849= (95. 4) (x) (48 -25) q = 0. 387 J/g. C

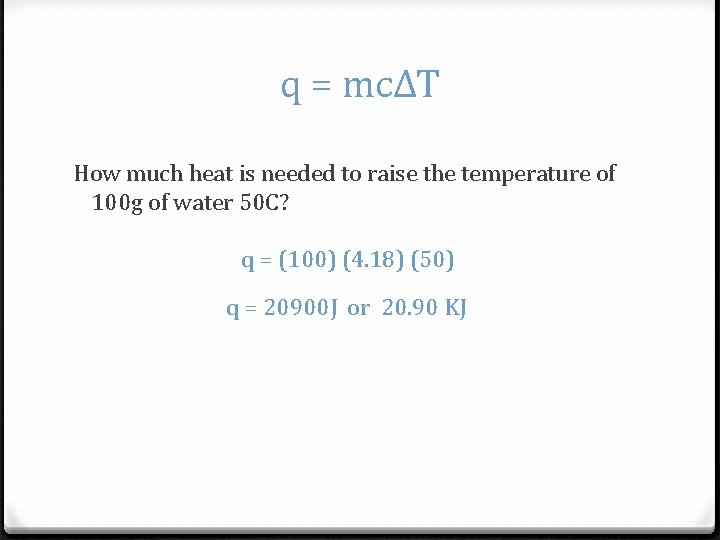
q = mcΔT How much heat is needed to raise the temperature of 100 g of water 50 C? q = (100) (4. 18) (50) q = 20900 J or 20. 90 KJ

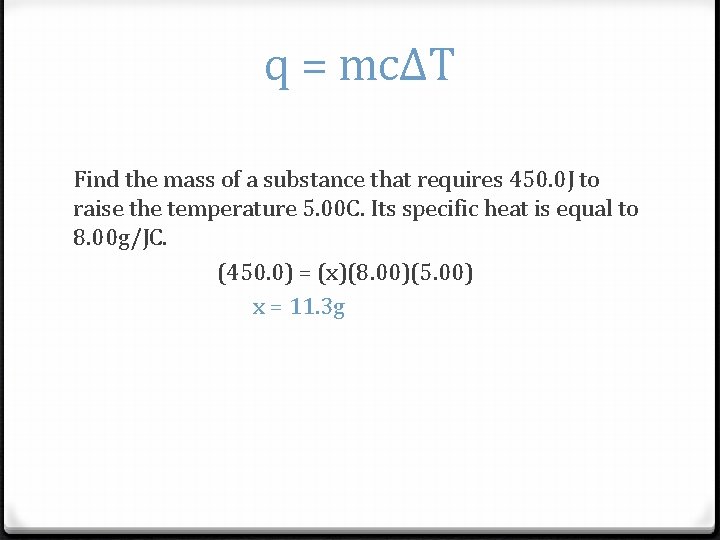
q = mcΔT Find the mass of a substance that requires 450. 0 J to raise the temperature 5. 00 C. Its specific heat is equal to 8. 00 g/JC. (450. 0) = (x)(8. 00)(5. 00) x = 11. 3 g

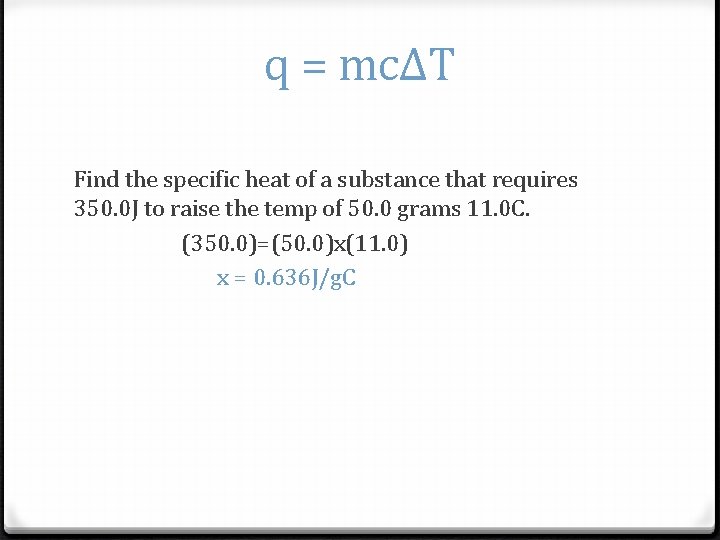
q = mcΔT Find the specific heat of a substance that requires 350. 0 J to raise the temp of 50. 0 grams 11. 0 C. (350. 0)=(50. 0)x(11. 0) x = 0. 636 J/g. C

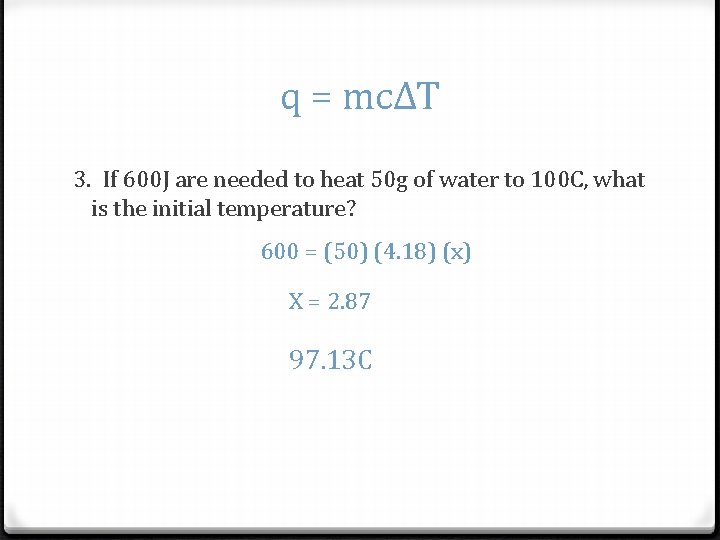
q = mcΔT 3. If 600 J are needed to heat 50 g of water to 100 C, what is the initial temperature? 600 = (50) (4. 18) (x) X = 2. 87 97. 13 C

Objectives 0 Now you must be able to… 0 Describe system and surroundings in terms of heat flow. 0 Describe and calculate the specific heat of a substance. 0 Calculate the heat of a reaction suing q=mcΔT

Video 6. 3 Table I

Objectives 0 By the end of this video you should be able to… 0 Determine if a reaction is endothermic or exothermic using Table I. 0 Fid the heat of a reaction using Table I.

Table I 0 Exothermic reactions release heat and have negative values. The products are more stable than the reactants were. 0 Example: When Carbon and Oxygen react they release 393. 5 k. J of heat per mole reacted. 0 Endothermic reactions absorb heat and have positive values. The products are less stable than the reactants were. 0 Example: When Nitrogen and Oxygen react they absorb 182. 6 k. J of heat per mole.

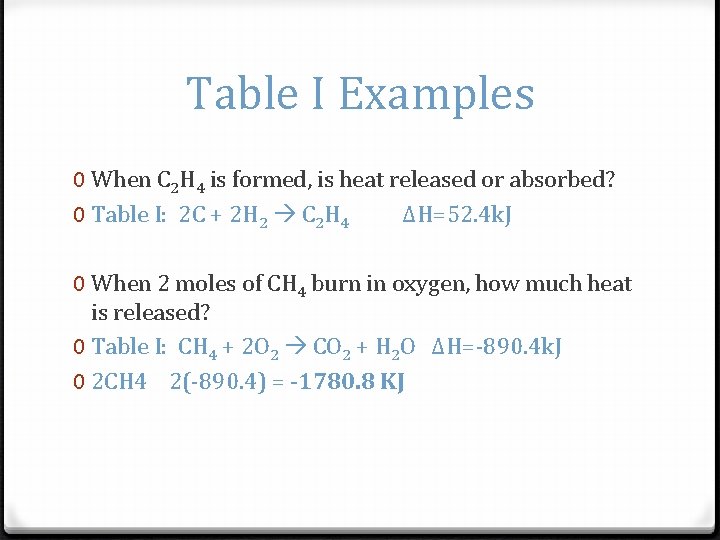
Table I Examples 0 When C 2 H 4 is formed, is heat released or absorbed? 0 Table I: 2 C + 2 H 2 C 2 H 4 ΔH=52. 4 k. J 0 When 2 moles of CH 4 burn in oxygen, how much heat is released? 0 Table I: CH 4 + 2 O 2 CO 2 + H 2 O ΔH=-890. 4 k. J 0 2 CH 4 2(-890. 4) = -1780. 8 KJ

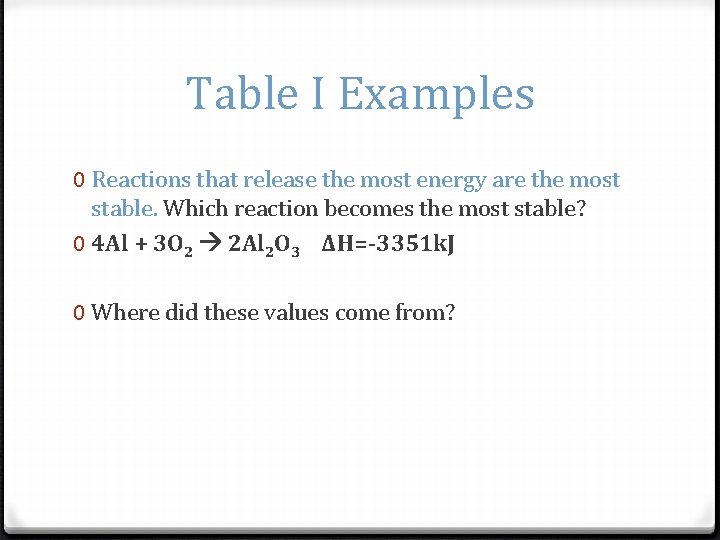
Table I Examples 0 Reactions that release the most energy are the most stable. Which reaction becomes the most stable? 0 4 Al + 3 O 2 2 Al 2 O 3 ΔH=-3351 k. J 0 Where did these values come from?

Objectives 0 By the end of this video you should be able to… 0 Determine if a reaction is endothermic or exothermic using Table I. 0 Fid the heat of a reaction using Table I.

Video 6. 4 q=m. H q=m H f v

Objectives 0 By the end of this video you should be able to… 0 Calculate the heat during a phase change.

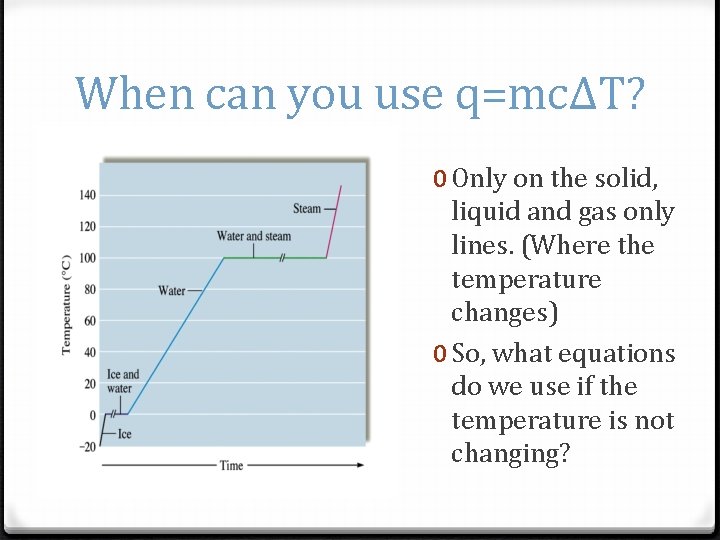
When can you use q=mcΔT? 0 Only on the solid, liquid and gas only lines. (Where the temperature changes) 0 So, what equations do we use if the temperature is not changing?

Table T 0 Heat of vaporization: heat needed to change a substance from gas to liquid or liquid to gas. q=m. Hv 0 Heat of fusion: heat needed to change a substance from solid to liquid or liquid to solid. q=m. Hf 0 If the IMF is strong, the heats of vaporization and fusion is high.

q=m. Hv 1. Calculate the number of joules needed to vaporize 423 g of H 2 O. q = (423) (2260) 955, 980 J or 955. 98 KJ

q=m. Hf 0 How much heat is needed to melt ice at 0 C if the sample weighs 255 g? q = (255) (334) 85, 170 J or 85. 17 KJ

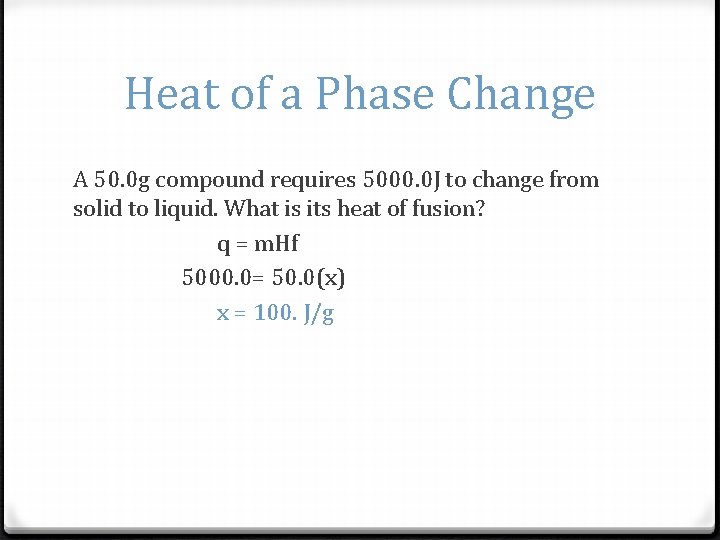
Heat of a Phase Change A 50. 0 g compound requires 5000. 0 J to change from solid to liquid. What is its heat of fusion? q = m. Hf 5000. 0= 50. 0(x) x = 100. J/g

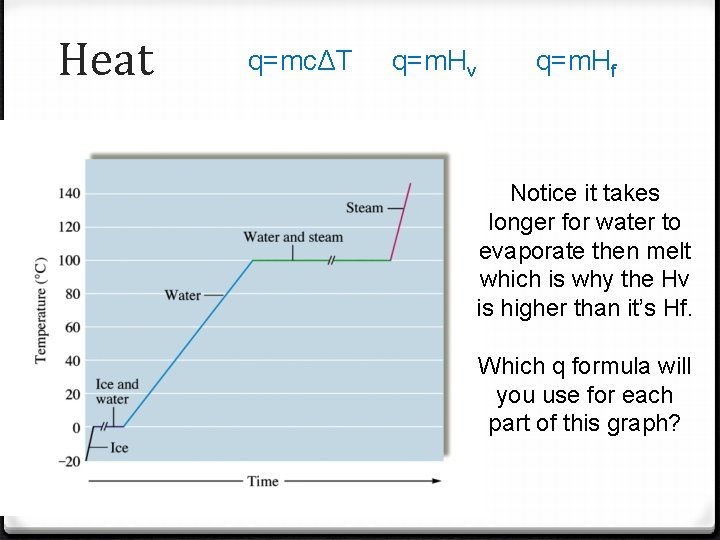
Heat q=mcΔT q=m. Hv q=m. Hf Notice it takes longer for water to evaporate then melt which is why the Hv is higher than it’s Hf. Which q formula will you use for each part of this graph?

Objectives 0 Now you must be able to… 0 Calculate the heat during a phase change.

Video 6. 5 Heat of Form ation and H ess’ Law

Objectives 0 By the end of this video you should be able to… 0 Calculate heats of reactions using heats of formation values. 0 Calculate heats of reactions using Hess’ Law.

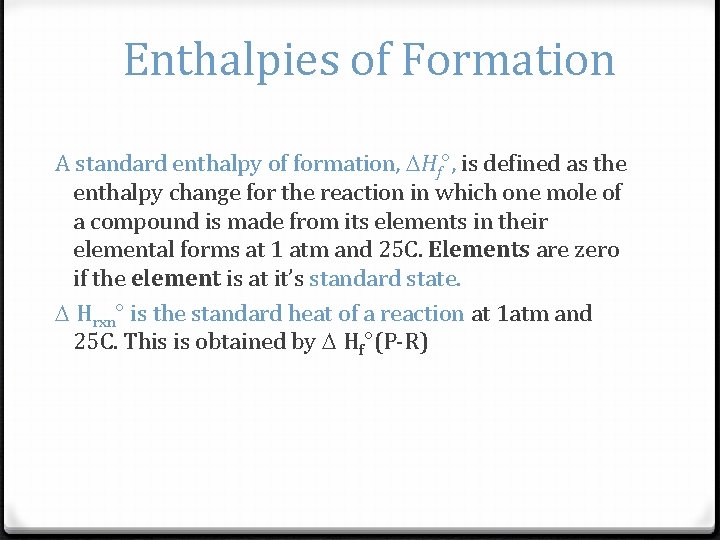
Enthalpies of Formation A standard enthalpy of formation, Hf°, is defined as the enthalpy change for the reaction in which one mole of a compound is made from its elements in their elemental forms at 1 atm and 25 C. Elements are zero if the element is at it’s standard state. Hrxn° is the standard heat of a reaction at 1 atm and 25 C. This is obtained by Hf°(P-R)

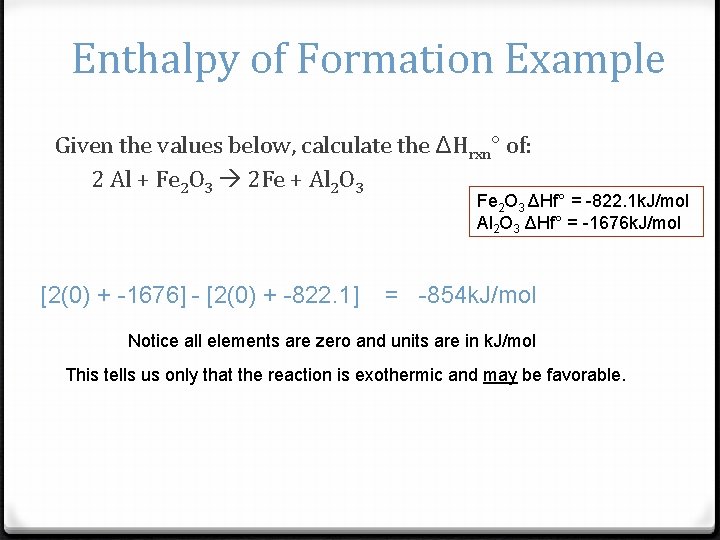
Enthalpy of Formation Example Given the values below, calculate the ΔHrxn° of: 2 Al + Fe 2 O 3 2 Fe + Al 2 O 3 Fe 2 O 3 ΔHf° = -822. 1 k. J/mol Al 2 O 3 ΔHf° = -1676 k. J/mol [2(0) + -1676] - [2(0) + -822. 1] = -854 k. J/mol Notice all elements are zero and units are in k. J/mol This tells us only that the reaction is exothermic and may be favorable.

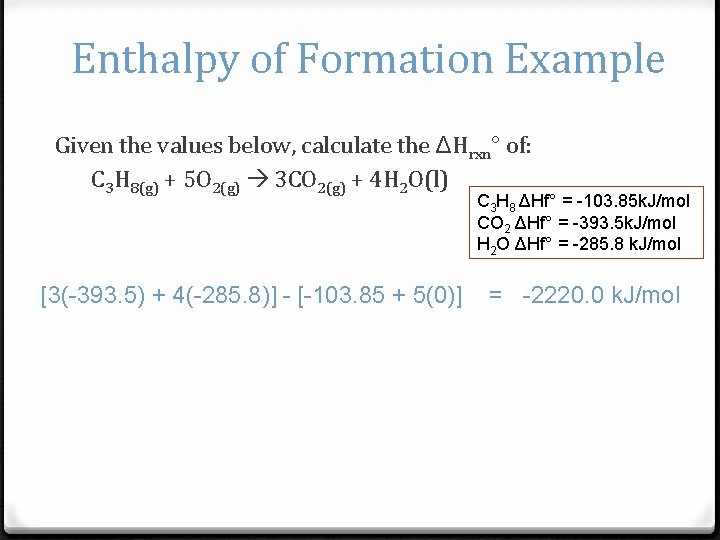
Enthalpy of Formation Example Given the values below, calculate the ΔHrxn° of: C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O(l) C 3 H 8 ΔHf° = -103. 85 k. J/mol CO 2 ΔHf° = -393. 5 k. J/mol H 2 O ΔHf° = -285. 8 k. J/mol [3(-393. 5) + 4(-285. 8)] - [-103. 85 + 5(0)] = -2220. 0 k. J/mol

Hess’s Law 0 H° is well known for many reactions, and it is inconvenient to measure H° for every reaction in which we are interested. 0 However, we can estimate H° using other H° values that are published. 0 Hess’s law states that “If a reaction is carried out in a series of steps, H° for the overall reaction will be equal to the sum of the enthalpy changes for the individual steps. ”

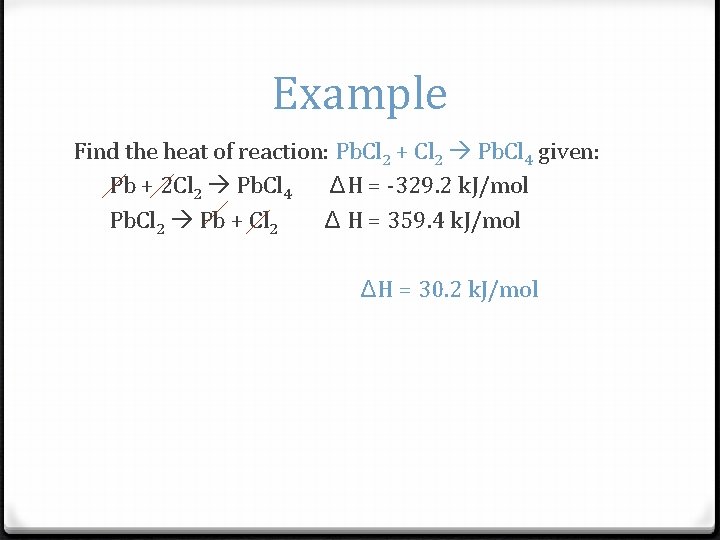
Example Find the heat of reaction: Pb. Cl 2 + Cl 2 Pb. Cl 4 given: Pb + 2 Cl 2 Pb. Cl 4 ΔH = -329. 2 k. J/mol Pb. Cl 2 Pb + Cl 2 Δ H = 359. 4 k. J/mol ΔH = 30. 2 k. J/mol

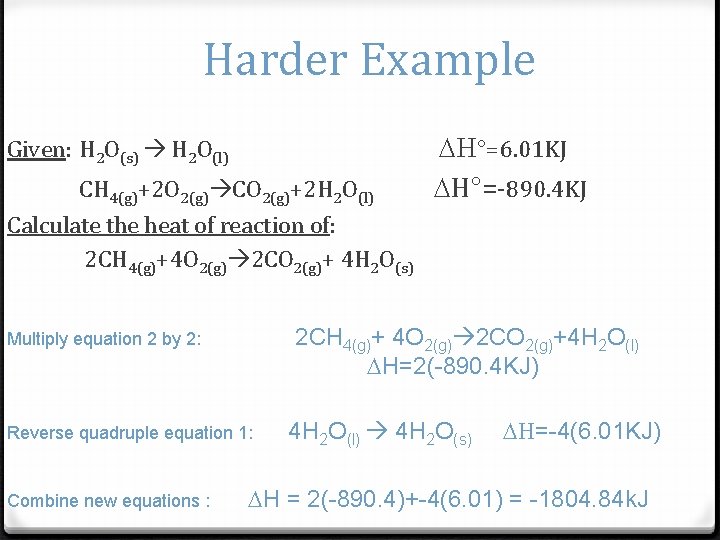
Harder Example Given: H 2 O(s) H 2 O(l) CH 4(g)+2 O 2(g) CO 2(g)+2 H 2 O(l) Calculate the heat of reaction of: 2 CH 4(g)+4 O 2(g) 2 CO 2(g)+ 4 H 2 O(s) 2 CH 4(g)+ 4 O 2(g) 2 CO 2(g)+4 H 2 O(l) H=2(-890. 4 KJ) Multiply equation 2 by 2: Reverse quadruple equation 1: Combine new equations : H°=6. 01 KJ H°=-890. 4 KJ 4 H 2 O(l) 4 H 2 O(s) H=-4(6. 01 KJ) H = 2(-890. 4)+-4(6. 01) = -1804. 84 k. J

Objectives 0 Now you must be able to… 0 Calculate heats of reactions using heats of formation values. 0 Calculate heats of reactions using Hess’ Law.

Video 6. 6 Entropy

Objectives 0 By the end of this video you should be able to… 0 Define spontaneous reactions and entropy. 0 Determine which species have higher entropy values.

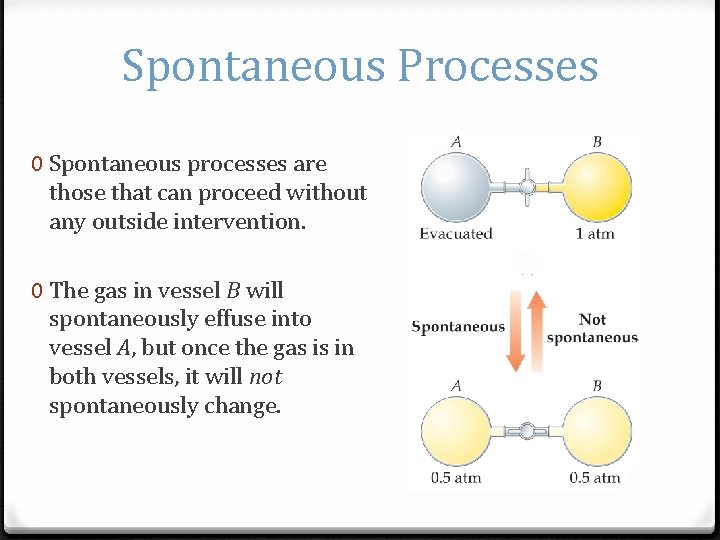
Spontaneous Processes 0 Spontaneous processes are those that can proceed without any outside intervention. 0 The gas in vessel B will spontaneously effuse into vessel A, but once the gas is in both vessels, it will not spontaneously change.

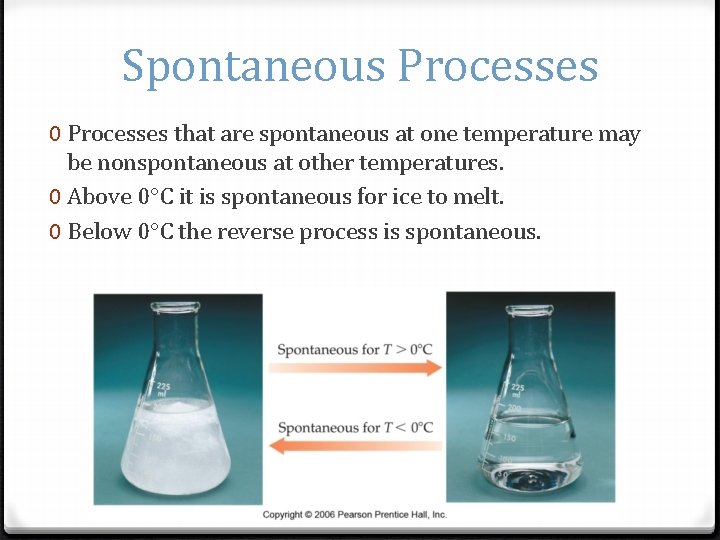
Spontaneous Processes 0 Processes that are spontaneous at one temperature may be nonspontaneous at other temperatures. 0 Above 0 C it is spontaneous for ice to melt. 0 Below 0 C the reverse process is spontaneous.

Spontaneous Processes 0 Spontaneity depends on two important issues: 0 Entropy can be thought of as a measure of the randomness of a system. 0 Enthalpy is the heat change of a reaction.

Second Law of Thermodynamics 0 The second law of thermodynamics states that the entropy of the universe increases for spontaneous processes.

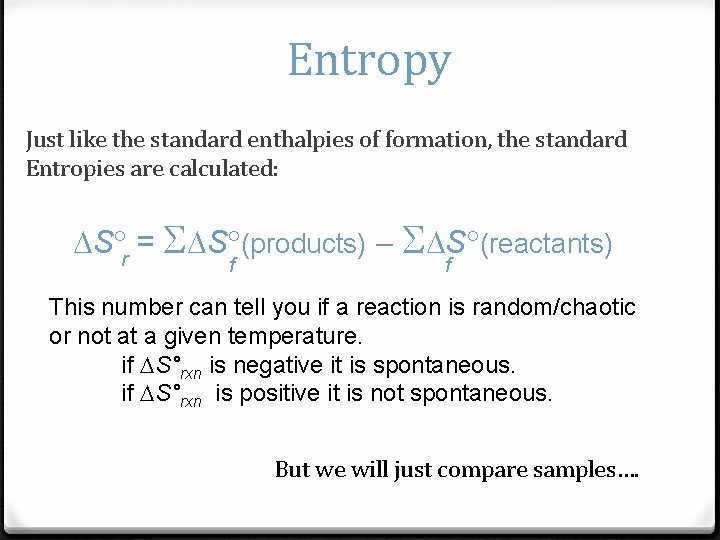
Entropy Just like the standard enthalpies of formation, the standard Entropies are calculated: S r = S S (products) S S (reactants) f f This number can tell you if a reaction is random/chaotic or not at a given temperature. if S°rxn is negative it is spontaneous. if S°rxn is positive it is not spontaneous. But we will just compare samples….

Entropy is dependent on: 0 Temperature (phase changes) S(g) > S(l) > S(s) 0 The number of independently moving molecules Ssolute < Ssolution 0 The change in phase usually has a larger affect than the number of molecules. 0 Polarity provides order to the particles because they align themselves like poles of magnets. Entropy decreases when polar molecules form.

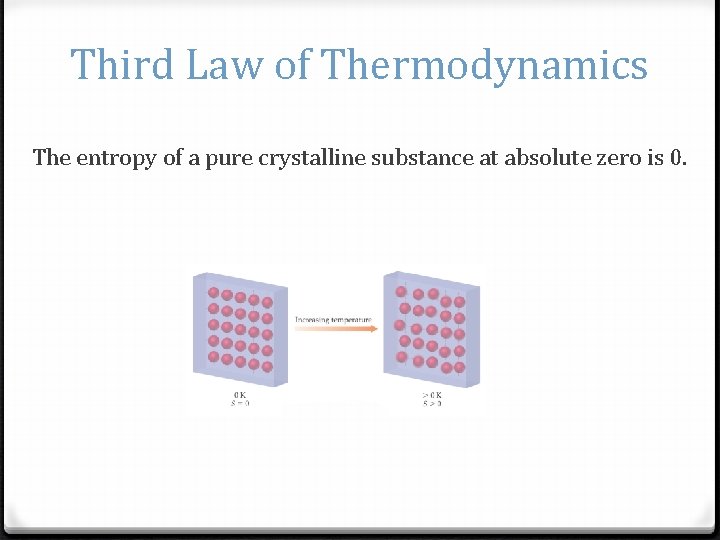
Third Law of Thermodynamics The entropy of a pure crystalline substance at absolute zero is 0.

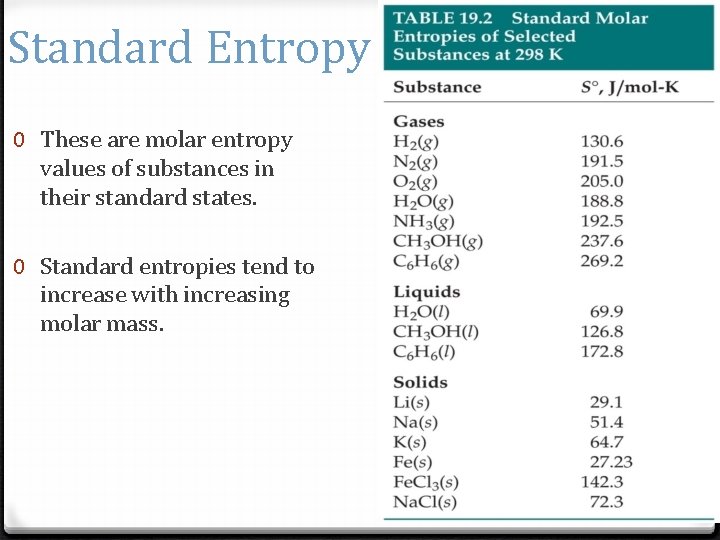
Standard Entropy 0 These are molar entropy values of substances in their standard states. 0 Standard entropies tend to increase with increasing molar mass.

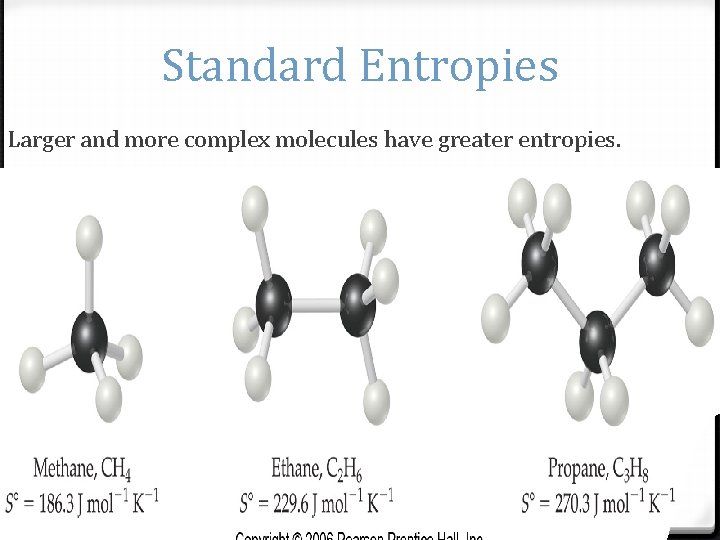
Standard Entropies Larger and more complex molecules have greater entropies.

Examples 0 Which has a greater entropy? 0 Na. Cl(s) or Na. Cl(aq) 0 CO 2(s) or CO 2(g) 0 KI(aq) at 45 C or 100 C 0 Is the entropy positive (increasing) or negative? 0 2 H 2(g) + O 2(g) 2 H 2 O(l) 0 NH 4 Cl(s) NH 3(g) + HCl(g)

Objectives 0 Now you must be able to… 0 Define spontaneous reactions and entropy. 0 Determine which species have higher entropy values.

Video 6. 7 Gibbs Free E nergy

Objectives 0 By the end of this video you should be able to… 0 Use Gibbs Free Energy to determine if a reaction is spontaneous.

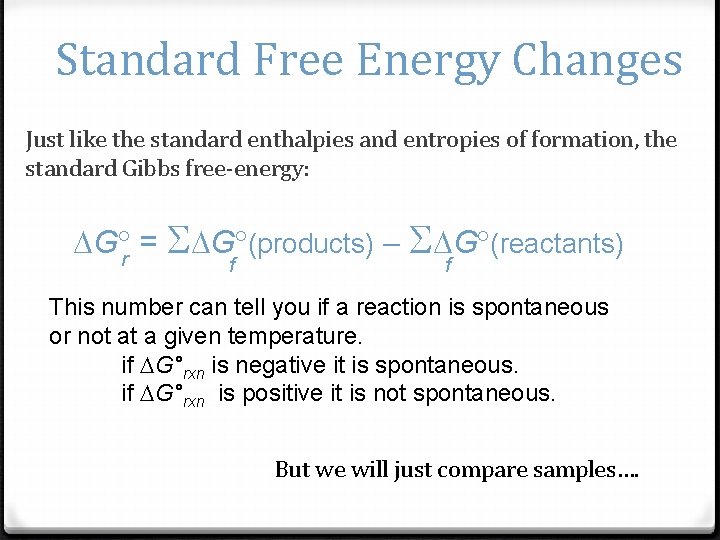
Standard Free Energy Changes Just like the standard enthalpies and entropies of formation, the standard Gibbs free-energy: G r = S G (products) S G (reactants) f f This number can tell you if a reaction is spontaneous or not at a given temperature. if G°rxn is negative it is spontaneous. if G°rxn is positive it is not spontaneous. But we will just compare samples….

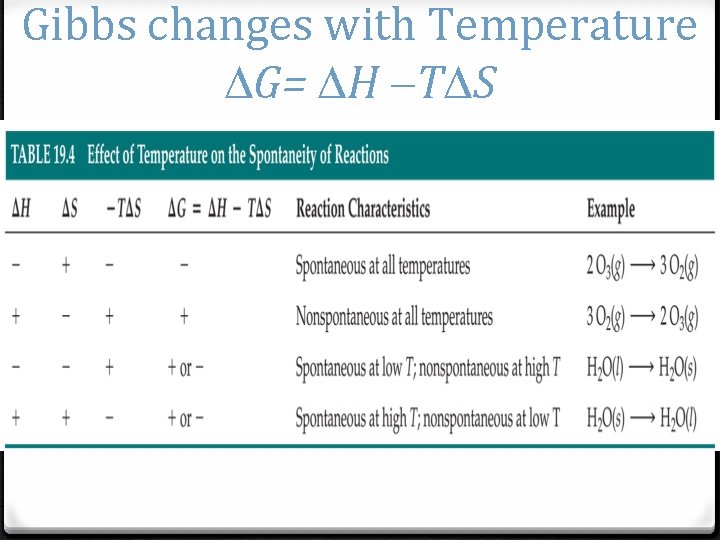
Gibbs changes with Temperature G= H T S

Examples Will the following reactions be spontaneous? 0 A reaction that is exothermic and produces many moles of gas. 0 A reaction that is endothermic and produces solids. 0 A reaction that is cold to the touch and condenses. 0 A reaction that is hot to the touch and forms smoke.

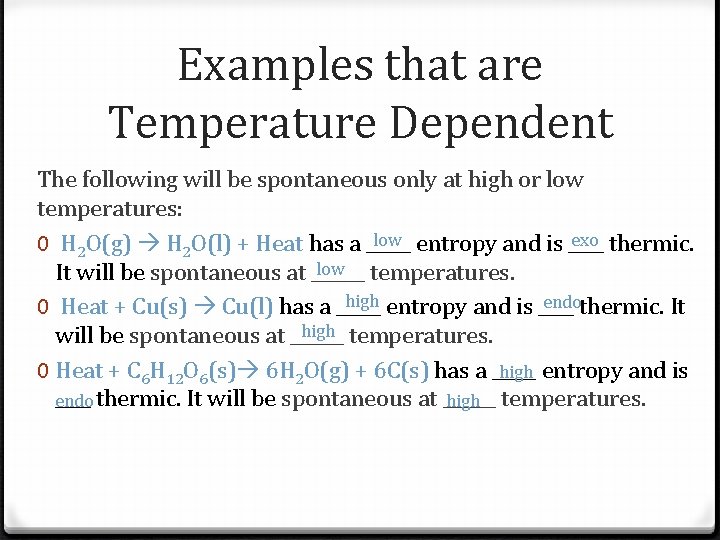
Examples that are Temperature Dependent The following will be spontaneous only at high or low temperatures: low entropy and is ____ exo thermic. 0 H 2 O(g) H 2 O(l) + Heat has a _____ low temperatures. It will be spontaneous at ______ high entropy and is ____ endothermic. It 0 Heat + Cu(s) Cu(l) has a _____ high temperatures. will be spontaneous at ______ 0 Heat + C 6 H 12 O 6(s) 6 H 2 O(g) + 6 C(s) has a _____ high entropy and is ____ thermic. It will be spontaneous at ______ endo high temperatures.

Objectives 0 Now you must be able to… 0 Use Gibbs Free Energy to determine if a reaction is spontaneous.
 Yandex search video
Yandex search video Yahoo search video
Yahoo search video Tw yahoo mail
Tw yahoo mail The frame size of a video refers to the video’s
The frame size of a video refers to the video’s Differences between ideal gas and real gas
Differences between ideal gas and real gas Difference between ideal gas and real gas
Difference between ideal gas and real gas Market revolution apush
Market revolution apush Pseudo reduced specific volume
Pseudo reduced specific volume Imaginary gas
Imaginary gas Ideal gas vs perfect gas
Ideal gas vs perfect gas Reason of bhopal gas tragedy
Reason of bhopal gas tragedy Gas leaked in bhopal gas tragedy
Gas leaked in bhopal gas tragedy Volume molare
Volume molare Flue gas desulfurisation gas filter
Flue gas desulfurisation gas filter Poisonous gas leaked in bhopal gas tragedy
Poisonous gas leaked in bhopal gas tragedy Kinetika kimia
Kinetika kimia Gas exchange key events in gas exchange
Gas exchange key events in gas exchange Expansion of solids liquids and gases examples
Expansion of solids liquids and gases examples Nourishing element
Nourishing element Buoyancyability
Buoyancyability Chapter 14 solids liquids and gases
Chapter 14 solids liquids and gases Molecules of solid liquid and gas
Molecules of solid liquid and gas A yolk and cream mixture that is used to thicken liquids *
A yolk and cream mixture that is used to thicken liquids * Venn diagram for solids liquids and gases
Venn diagram for solids liquids and gases Mass of solid liquid and gas
Mass of solid liquid and gas Molecular theory of gases and liquids
Molecular theory of gases and liquids Commercial liquid dielectrics
Commercial liquid dielectrics Example of solid liquid and gas
Example of solid liquid and gas Adhesive force
Adhesive force Miscible and immiscible liquids worksheet
Miscible and immiscible liquids worksheet Liquids and solids menu
Liquids and solids menu The science duo physical and chemical changes
The science duo physical and chemical changes Chapter 14 solids liquids and gases worksheet answers
Chapter 14 solids liquids and gases worksheet answers Regional metamorphism
Regional metamorphism Kesler science.com
Kesler science.com Particle movement in solids liquids and gases
Particle movement in solids liquids and gases How does sound travel through solids liquids and gases
How does sound travel through solids liquids and gases Matter and its composition
Matter and its composition Motion of particles in solids, liquids and gases
Motion of particles in solids, liquids and gases Chapter 11 - states of matter: liquids and solids
Chapter 11 - states of matter: liquids and solids Hvac unit 31 test answers
Hvac unit 31 test answers Unit 41 troubleshooting
Unit 41 troubleshooting Gas balancing review
Gas balancing review Chapter review motion part a vocabulary review answer key
Chapter review motion part a vocabulary review answer key Uncontrollable spending ap gov
Uncontrollable spending ap gov Narrative review vs systematic review
Narrative review vs systematic review Narrative review vs systematic review
Narrative review vs systematic review Narrative review vs systematic review
Narrative review vs systematic review Properties of liquid in matter
Properties of liquid in matter Is oh solid liquid or gas
Is oh solid liquid or gas Ionic liquids ppt
Ionic liquids ppt Fire extinguisher basics
Fire extinguisher basics Word transcription
Word transcription Elixir dosage form definition
Elixir dosage form definition 7 basic baking ingredients
7 basic baking ingredients A completely submerged object always displaces its own
A completely submerged object always displaces its own Why is a gas easier to compress than a liquid or a solid?
Why is a gas easier to compress than a liquid or a solid? Compressible and incompressible fluids
Compressible and incompressible fluids Viscosity of liquids
Viscosity of liquids Miscible
Miscible Why are liquids incompressible
Why are liquids incompressible Split tube thief is used in sampling of
Split tube thief is used in sampling of What elements are liquids
What elements are liquids Kinetic molecular theory of liquids
Kinetic molecular theory of liquids Theory of filtration
Theory of filtration Types of liquids
Types of liquids Plant growth with different liquids
Plant growth with different liquids How dense are you
How dense are you Water countable or uncountable noun
Water countable or uncountable noun 14. separating mixtures of liquids.
14. separating mixtures of liquids. In one sense water is the commonest of liquids
In one sense water is the commonest of liquids Thickened liquids chart
Thickened liquids chart