CHAPTER 14 LIQUIDS AND SOLIDS LIQUIDS Attractive forces






















- Slides: 37

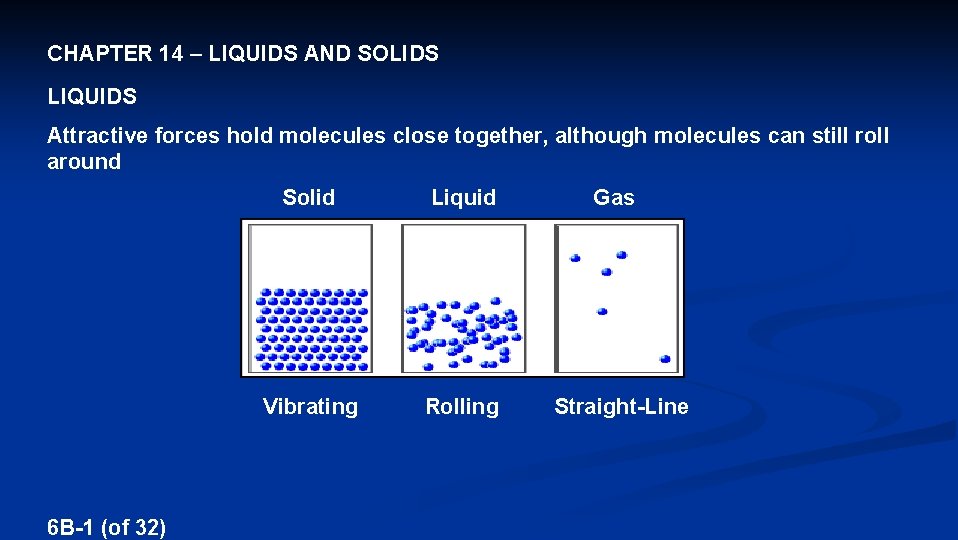
CHAPTER 14 – LIQUIDS AND SOLIDS LIQUIDS Attractive forces hold molecules close together, although molecules can still roll around 6 B-1 (of 32) Solid Liquid Vibrating Rolling Gas Straight-Line

PHASE CHANGES (1) EVAPORATION If surface molecules acquire enough kinetic energy to overcome the attractive forces, they can escape With liquid water in a closed container: (2) CONDENSATION Vapor molecules collide with the surface of the liquid, lose energy, and are captured When these 2 processes are equal, it looks like evaporation has stopped Really, evaporation and condensation are occurring at equal rates 6 B-2

DYNAMIC EQUILIBRIUM – When 2 opposing processes in the same system proceed at equal rates Evaporation: liquid → Condensation: vapor → Equilibrium: liquid ↔ liquid vapor EQUILIBRIUM VAPOR PRESSURE – The pressure exerted by a vapor when it is in equilibrium with its liquid 6 B-3

EVP depends on temperature Temp (ºC) 10 20 30 6 B-4 EVP (torr) 9 18 32

BOILING Atmospheric Pressure A bubble is full of steam that exerts water’s EVP A bubble survives when its EVP equals atmospheric pressure When bubbles can reach the top the liquid is boiling BOILING POINT – The temperature at which the EVP of the liquid equals the prevailing atmospheric pressure 6 B-5

Temp (ºC) EVP (torr) 5 10 7 9 18 32 634 760 1557 20 30 95 100 122 6 B-6

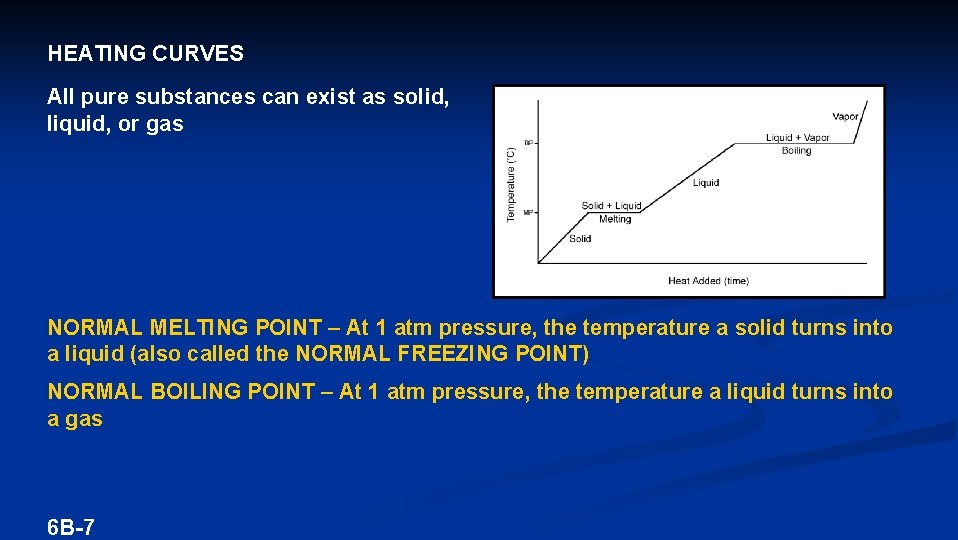
HEATING CURVES All pure substances can exist as solid, liquid, or gas NORMAL MELTING POINT – At 1 atm pressure, the temperature a solid turns into a liquid (also called the NORMAL FREEZING POINT) NORMAL BOILING POINT – At 1 atm pressure, the temperature a liquid turns into a gas 6 B-7

HEAT OF FUSION – The heat needed to melt a specific amount of a solid For ice: 334 J/g or 6. 02 k. J/mol HEAT OF VAPORIZATION – The heat needed to boil a specific amount of a liquid For water: 2, 250 J/g or 40. 6 k. J/mol 6 B-8

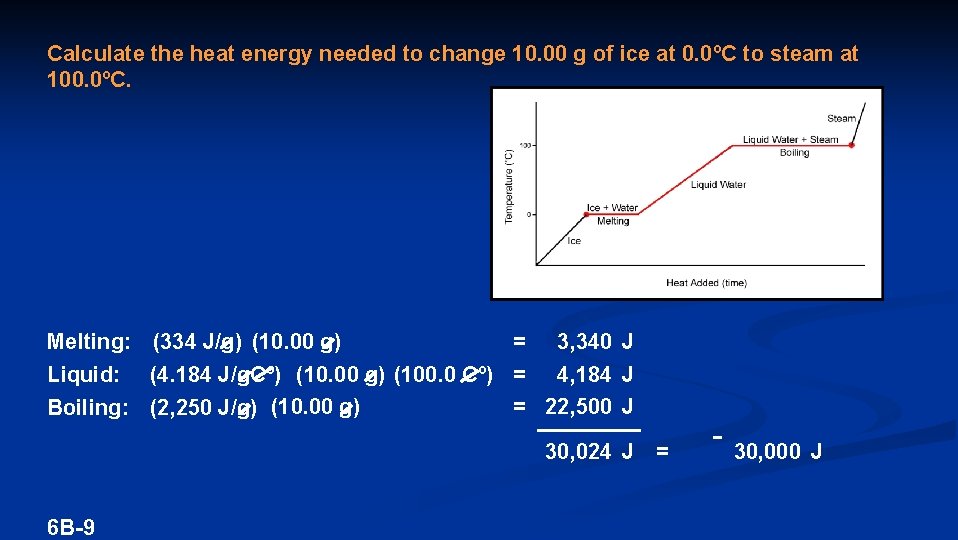
Calculate the heat energy needed to change 10. 00 g of ice at 0. 0ºC to steam at 100. 0ºC. Melting: (334 J/g) (10. 00 g) = 3, 340 J Liquid: (4. 184 J/g. Cº) (10. 00 g) (100. 0 Cº) = 4, 184 J = 22, 500 J Boiling: (2, 250 J/g) (10. 00 g) 30, 024 J = 6 B-9 30, 000 J

SUBLIMATION – A solid changing directly to a gas Iodine (I 2), Dry Ice (CO 2), Mothballs (C 10 H 8) DEPOSITION – A gas changing directly to a solid 6 B-10

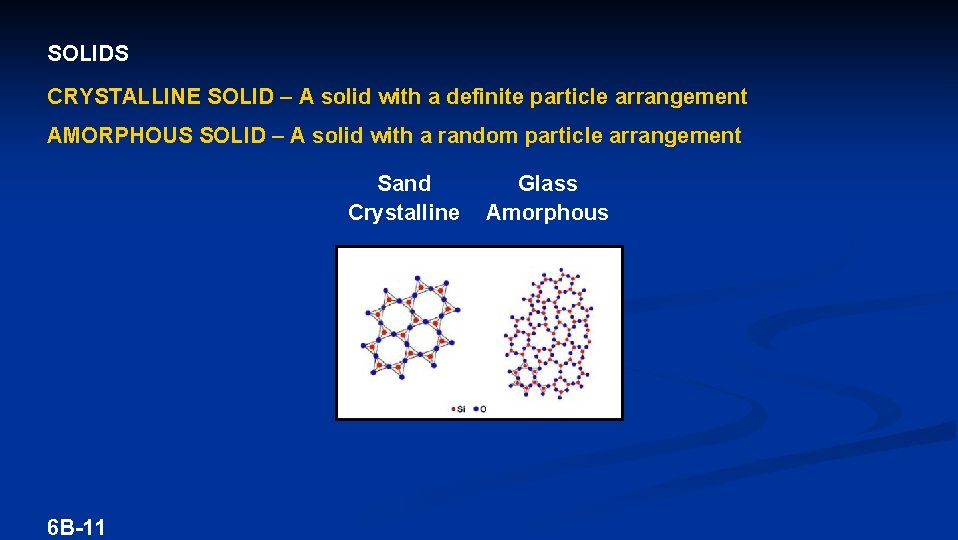
SOLIDS CRYSTALLINE SOLID – A solid with a definite particle arrangement AMORPHOUS SOLID – A solid with a random particle arrangement Sand Crystalline 6 B-11 Glass Amorphous

STRUCTURE OF SOLID MATTER Matter can be classified by the particles that make up the lattice points when it exists as a crystalline solid: (1) Molecules (2) Atoms (3) Ions 6 B-12

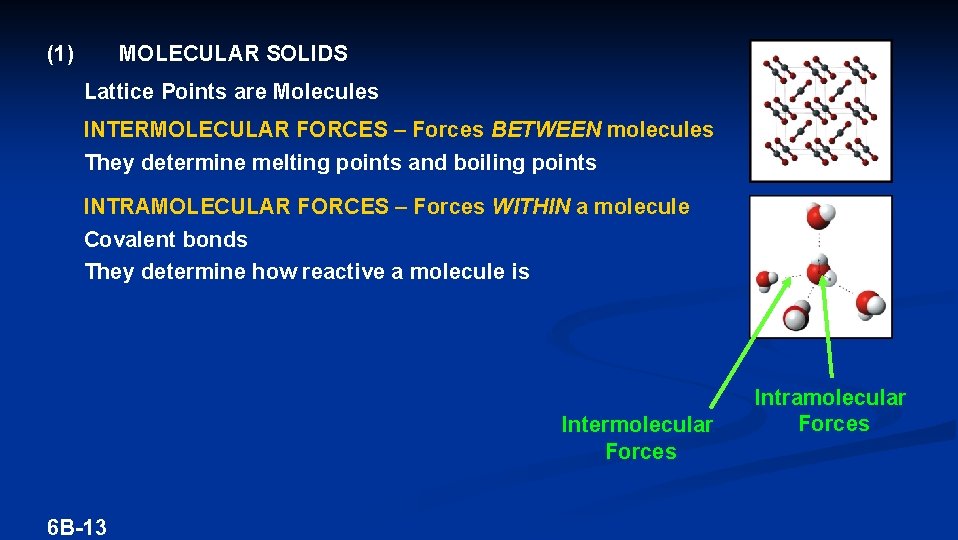
(1) MOLECULAR SOLIDS Lattice Points are Molecules INTERMOLECULAR FORCES – Forces BETWEEN molecules They determine melting points and boiling points INTRAMOLECULAR FORCES – Forces WITHIN a molecule Covalent bonds They determine how reactive a molecule is Intermolecular Forces 6 B-13 Intramolecular Forces

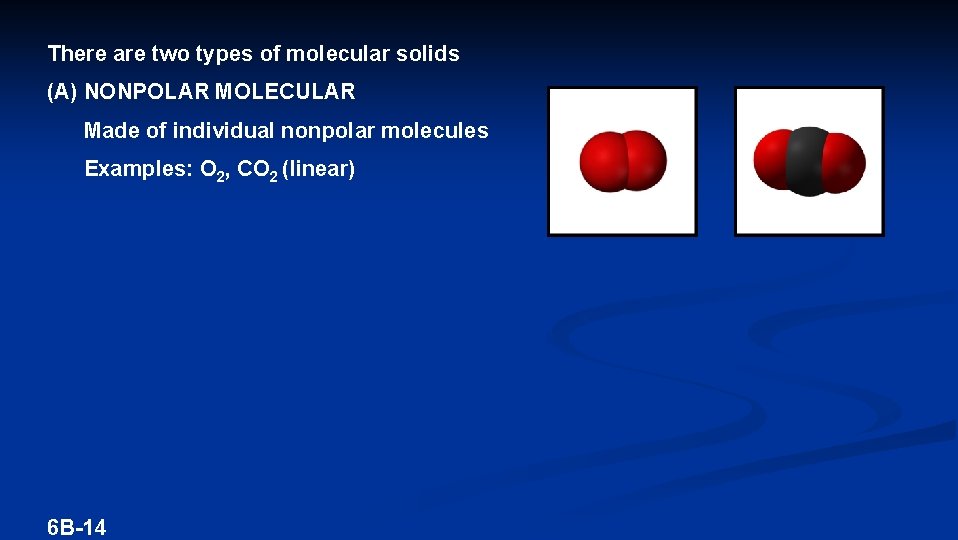
There are two types of molecular solids (A) NONPOLAR MOLECULAR Made of individual nonpolar molecules Examples: O 2, CO 2 (linear) 6 B-14

Intermolecular Forces: LONDON DISPERSION FORCES (or VAN DER WAALS FORCES) – The attraction of electrons in one molecule to the nuclei in a neighboring molecule due to the synchronizing of electrons 6 B-15 δ+ δ-

LDF’s are weak attractive forces, so nonpolar molecular solids have low melting points LDF’s increase in strength as the number of electrons per molecule increase Substance Cl 2 Br 2 l 2 6 B-16 MP (ºC) -102 -7 114

Although they are composed of atoms, Noble Gases behave as nonpolar molecular matter 6 B-17

(B) POLAR MOLECULAR Made of individual polar molecules Examples: HCl, SO 2 (bent) 6 B-18

Intermolecular Forces: London Dispersion Forces, and DIPOLE-DIPOLE ATTRACTIONS – The attraction of the positive end of one polar molecule to the negative end of another 6 B-19 δ+ δ+ δ- δ-

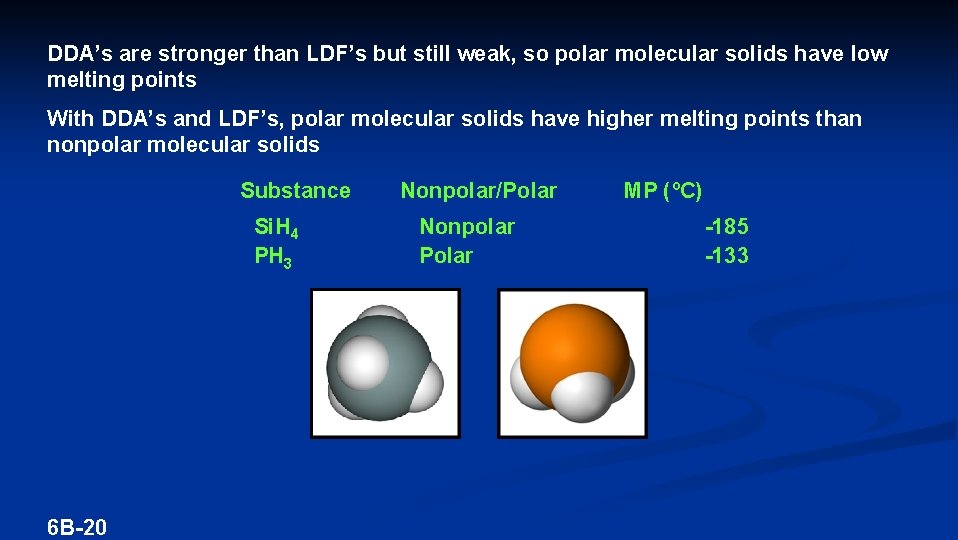
DDA’s are stronger than LDF’s but still weak, so polar molecular solids have low melting points With DDA’s and LDF’s, polar molecular solids have higher melting points than nonpolar molecular solids Substance Si. H 4 PH 3 6 B-20 Nonpolar/Polar Nonpolar Polar MP (ºC) -185 -133

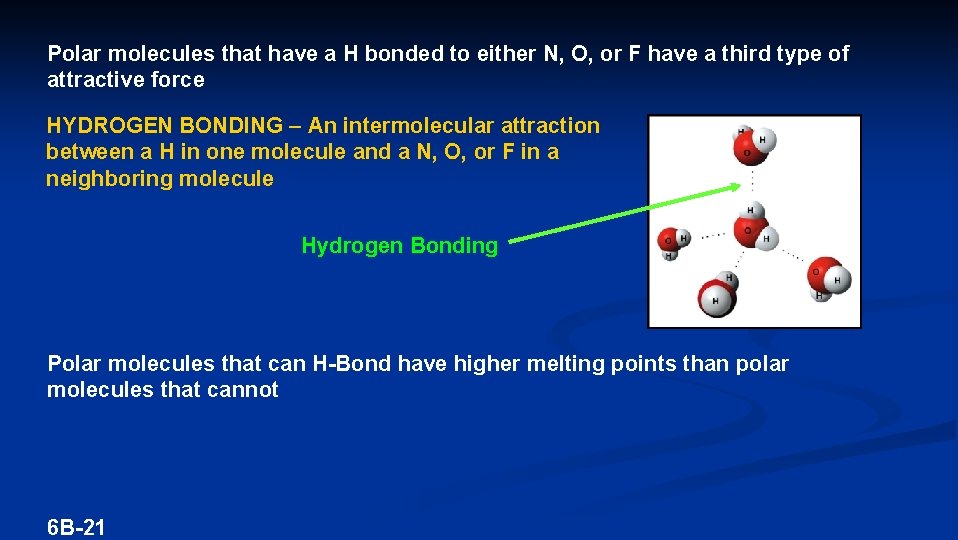
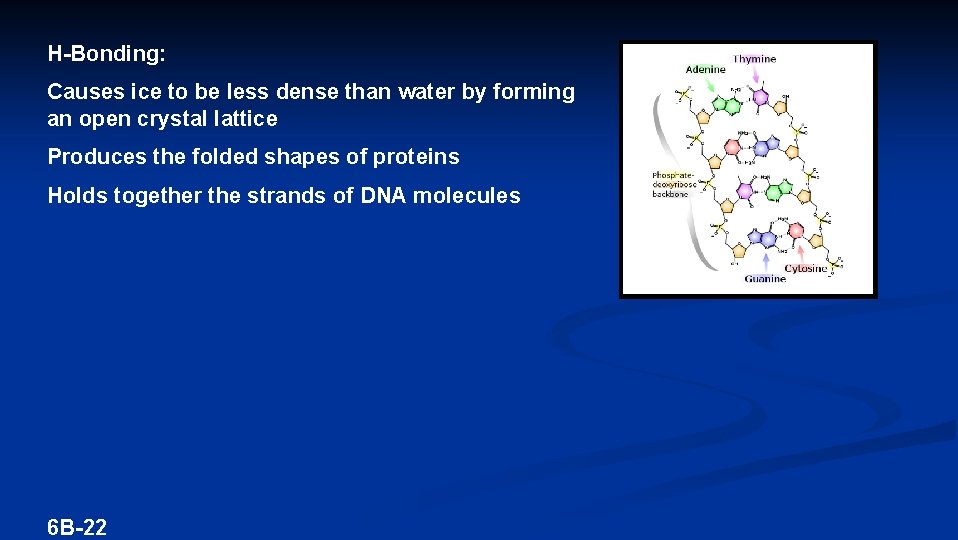
Polar molecules that have a H bonded to either N, O, or F have a third type of attractive force HYDROGEN BONDING – An intermolecular attraction between a H in one molecule and a N, O, or F in a neighboring molecule Hydrogen Bonding Polar molecules that can H-Bond have higher melting points than polar molecules that cannot 6 B-21

H-Bonding: Causes ice to be less dense than water by forming an open crystal lattice Produces the folded shapes of proteins Holds together the strands of DNA molecules 6 B-22

(2) ATOMIC SOLIDS Lattice points are atoms There are two types of atomic solids 6 B-23

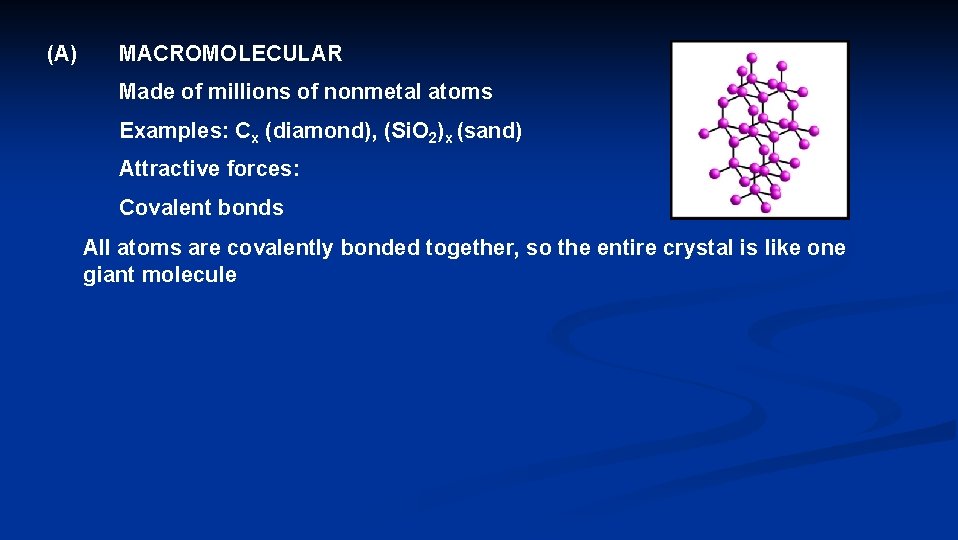
(A) MACROMOLECULAR Made of millions of nonmetal atoms Examples: Cx (diamond), (Si. O 2)x (sand) Attractive forces: Covalent bonds All atoms are covalently bonded together, so the entire crystal is like one giant molecule

Covalent Bonds are strong, so nonmetallic networks have high melting points Substance Cx (Si. O 2)x 6 B-25 MP (ºC) 3550 1723

(B) METALLIC Made of millions of metal atoms Examples: Fe, Au, bronze (Cu + Sn), brass (Cu + Zn) Attractive forces: Metallic Bonds 6 B-26

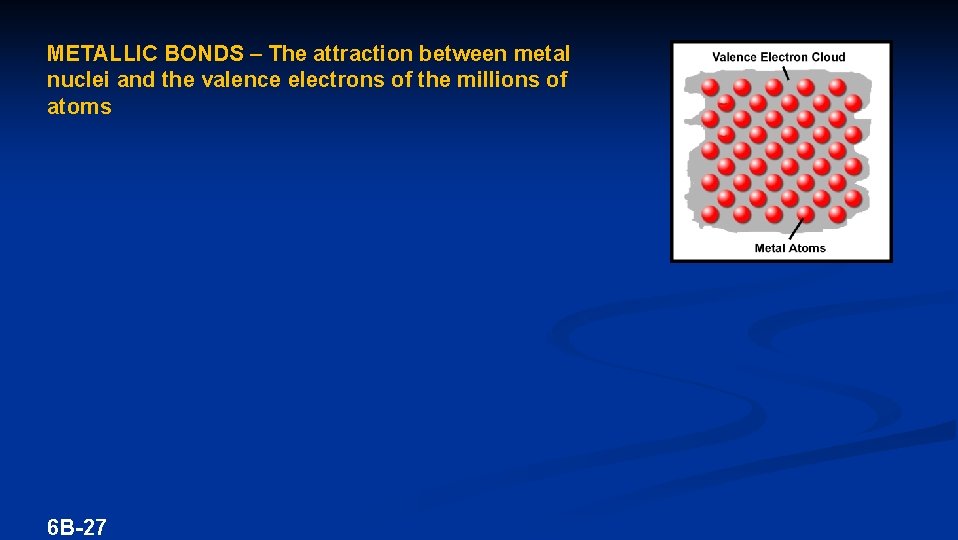
METALLIC BONDS – The attraction between metal nuclei and the valence electrons of the millions of atoms 6 B-27

Metallic Bonds are strong, so metallic networks have high melting points Substance Cu Au 6 B-28 MP (ºC) 1085 1065

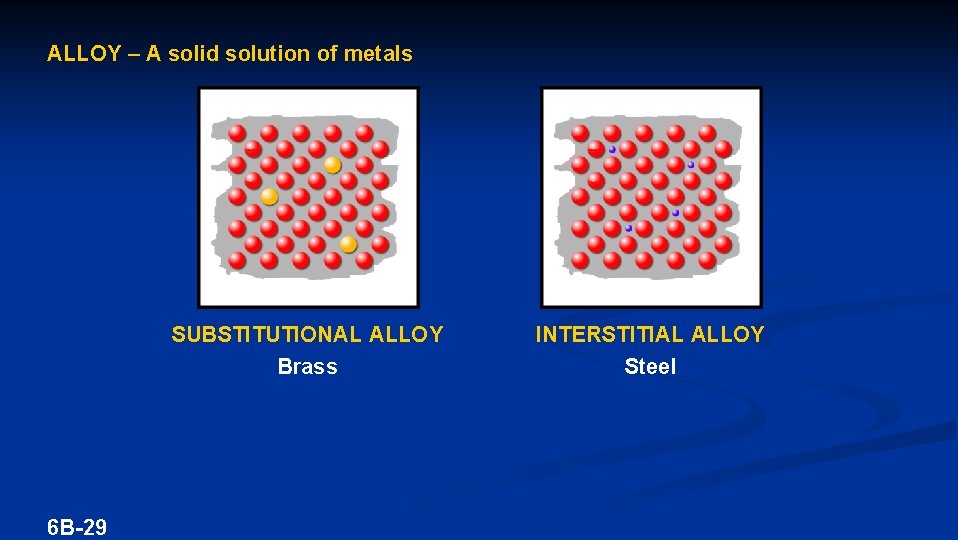
ALLOY – A solid solution of metals SUBSTITUTIONAL ALLOY Brass 6 B-29 INTERSTITIAL ALLOY Steel

(3) IONIC SOLIDS Lattice points are positive and negative ions Made of millions of positive and negative ions Examples: Na. Cl, Cu. SO 4 Attractive forces: Ionic Bonds 6 B-30

Ionic Bonds are strong, so ionic substances have high melting points Substance Na. Cl 6 B-31 MP (ºC) 801

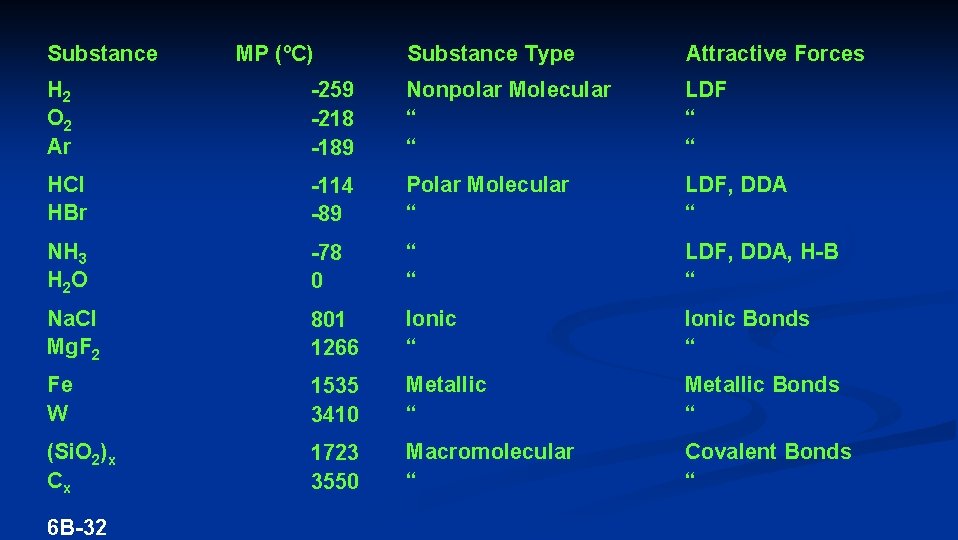
Substance MP (ºC) Substance Type Attractive Forces H 2 O 2 Ar -259 -218 -189 Nonpolar Molecular “ “ LDF “ “ HCl HBr -114 -89 Polar Molecular “ LDF, DDA “ NH 3 H 2 O -78 0 “ “ LDF, DDA, H-B “ Na. Cl Mg. F 2 801 1266 Ionic “ Ionic Bonds “ Fe W 1535 3410 Metallic “ Metallic Bonds “ (Si. O 2)x Cx 1723 3550 Macromolecular “ Covalent Bonds “ 6 B-32

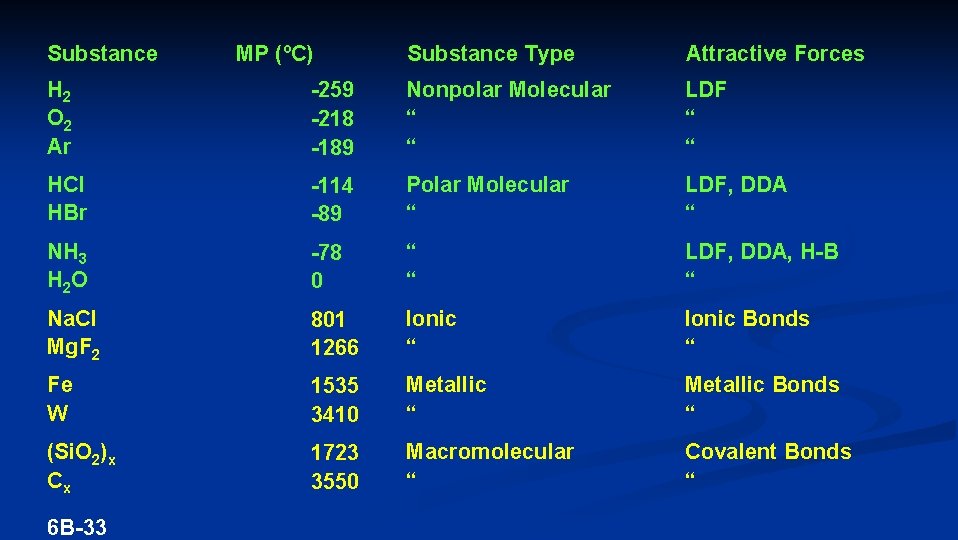
Substance MP (ºC) Substance Type Attractive Forces H 2 O 2 Ar -259 -218 -189 Nonpolar Molecular “ “ LDF “ “ HCl HBr -114 -89 Polar Molecular “ LDF, DDA “ NH 3 H 2 O -78 0 “ “ LDF, DDA, H-B “ Na. Cl Mg. F 2 801 1266 Ionic “ Ionic Bonds “ Fe W 1535 3410 Metallic “ Metallic Bonds “ (Si. O 2)x Cx 1723 3550 Macromolecular “ Covalent Bonds “ 6 B-33

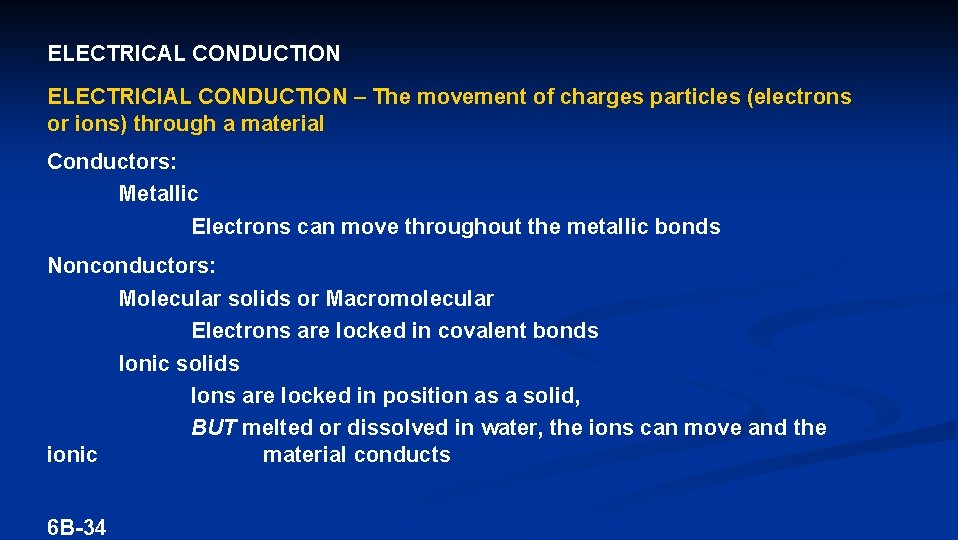
ELECTRICAL CONDUCTION ELECTRICIAL CONDUCTION – The movement of charges particles (electrons or ions) through a material Conductors: Metallic Electrons can move throughout the metallic bonds Nonconductors: Molecular solids or Macromolecular Electrons are locked in covalent bonds Ionic solids Ions are locked in position as a solid, BUT melted or dissolved in water, the ions can move and the ionic material conducts 6 B-34

REVIEW FOR TEST Pressure (mm Hg, torr, atm) Temperature (ºC, K) Standard Temperature and Pressure (STP) Boyle’s Law Charles’ Law Avogadro’s Law Char-Boyled Law Ideal Gas Law 6 B-35

REVIEW FOR TEST Partial Pressure Water Vapor Pressure of Gases Collected in Lab Over Mercury Over Water Gas Volumes in Chemical Reactions Dynamic Equilibrium Melting, Boiling, Sublimation Heat of Fusion, Heat of Vaporization, Specific Heat Capacity Heating Curves, Heat Calculations 6 B-36

REVIEW FOR TEST Crystalline, Amorphous Solids Intramolecular Forces, Intermolecular Forces London Dispersion Forces, Dipole-Dipole Attractions, Hydrogen Bonding Attractive Forces, MP’s, Conductivity of Molecular Solids (Nonpolar Molecular and Polar Molecular) Atomic Solids (Nonmetallic Networks and Metallic Networks) Ionic Solids Alloys (Substitutional, Interstitial) 6 B-37