Liquids and Solids Ch 11 Comparison of Liquids


















- Slides: 29

Liquids and Solids Ch 11

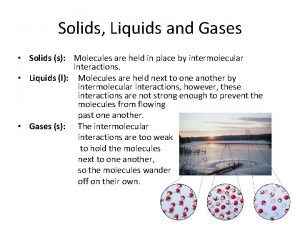
Comparison of Liquids and Solids to Gases § Liquids & solids are much more dense than gases § Inorganic liquids and solids have densities ranging from 1 – 8 g/cm 3, some up to 20 g/cm 3 § Most organic liquids & solids have densities ranging from 0. 7 – 2. 0 g/cm 3 § Gas densities are usually between 10 -2 and 10 -4 g/c, 3

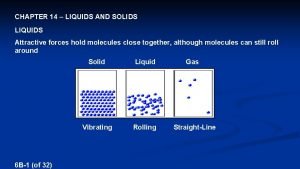
Comparison of Liquids and Solids to Gases § Gases expand to fill available space & must be kept in enclosed containers § Liquids fills any container from the bottom up to a level dictated on by the mass of the liquid present § Liquids conform to the shape of their container § Solids maintain shape without a container

Comparison of Liquids and Solids to Gases § Gases lack significant attractive forces § Liquids and Solids- significant attractive forces!

Intermolecular Forces § 3 Types to be aware of: § Dipole-Dipole Forces § London Dispersion Forces § Hydrogen Bonding

Dipole-Dipole Forces § Molecular compounds share electrons in a covalent bond, usually not equally! § e- congregate at 1 end of the molecule, giving it polarity, creating a dipole. § Polar molecules are attracted to each other. § Attractive forces are represented by the equation: § Force = (δ+)(δ-) r 2

Dipole-Dipole Forces § For gases to become a liquid – the attractive forces must overcome the KE of the moving gas molecule. § Decreasing the distance b/w molecules increases the attractive force. § Increasing P on a gas forces the molecules closer together § Cooling a gas reduces its avg KE § Force = (δ+)(δ-) r 2

Dipole-Dipole Forces § Boiling Pt (Condensation Pt) – indicator of the attractive forces b/w molecules § Measure of how much KE has to be increased so it overcomes the attractive forces in the liquid. § Low BP – low attractive forces § High BP – higher attractive forces § Highly polar molecules have higher BPs.

London Forces of Attraction § Explain how nonpolar gases develop the forces necessary to condense into liquids. § Nonpolar atoms & molecules may become momentarily polar when an unsymmetrical distribution of their e- results in instantaneous dipoles. § Sometimes called dispersion forces, instantaneous dipole forces, or induced dipole forces.

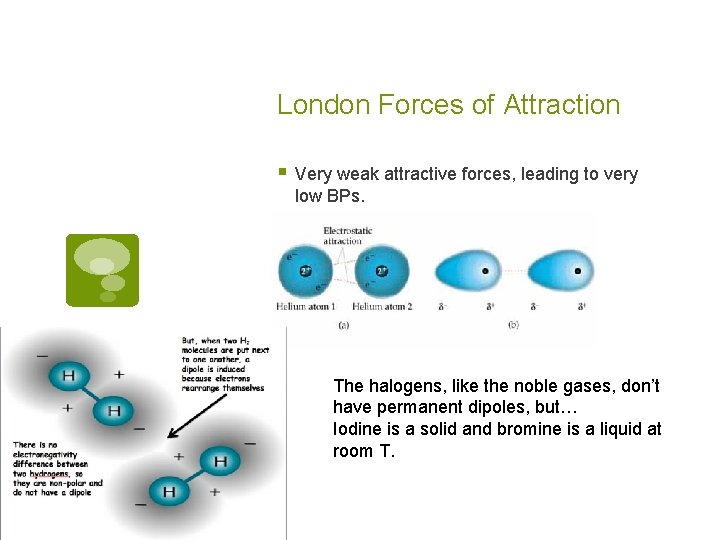
London Forces of Attraction § Very weak attractive forces, leading to very low BPs. The halogens, like the noble gases, don’t have permanent dipoles, but… Iodine is a solid and bromine is a liquid at room T.

London Forces of Attraction § What’s up with I 2 and Br 2? § Polarizability of e- clouds! § The ease with which the e- cloud around an atom or molecule can be deformed into a dipole. § Small atoms/molecules have e- clouds held tightly to nucleus- low polarizability. § Large atoms/molecules, w/ loosely held e- have high polarizability

London Forces of Attraction § These forces can explain the behavior or many molecules. § The more e- in a molecule, the more opportunity to form instantaneous dipolesso… increase in attractive forces means higher BPs. Aklanes – Cn. H 2 n+2 Called normal alkanes, nalkanes, because the vary in a regular way. (homologous series)

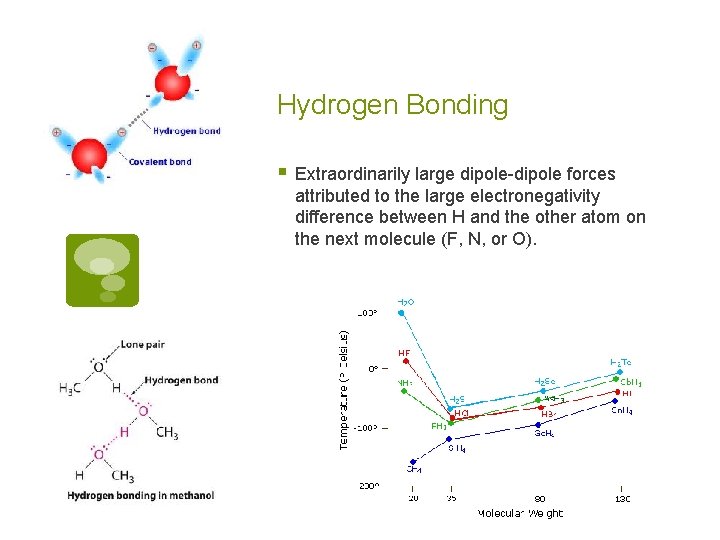
Hydrogen Bonding § Extraordinarily large dipole-dipole forces attributed to the large electronegativity difference between H and the other atom on the next molecule (F, N, or O).

Physical Properties of Liquids § Surface tension § Viscosity § Evaporation § Vapor Pressure § Boiling Pt. § Heat of Vaporizaiton

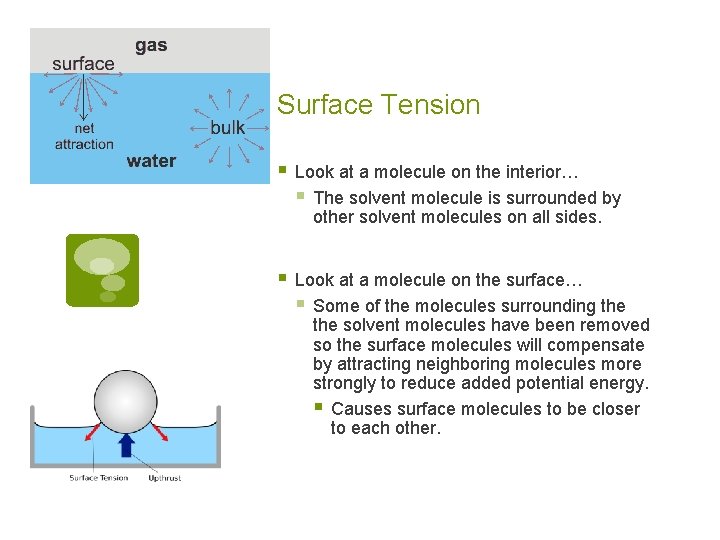
Surface Tension § Caused by an increase in the attractive forces b/w molecules at the surface of a liquid compared to the forces b/w molecules in the center (bulk) of the liquid. § Causes fluids to minimize their surface area… § Small droplets form spheres

Surface Tension § Look at a molecule on the interior… § The solvent molecule is surrounded by other solvent molecules on all sides. § Look at a molecule on the surface… § Some of the molecules surrounding the solvent molecules have been removed so the surface molecules will compensate by attracting neighboring molecules more strongly to reduce added potential energy. § Causes surface molecules to be closer to each other.

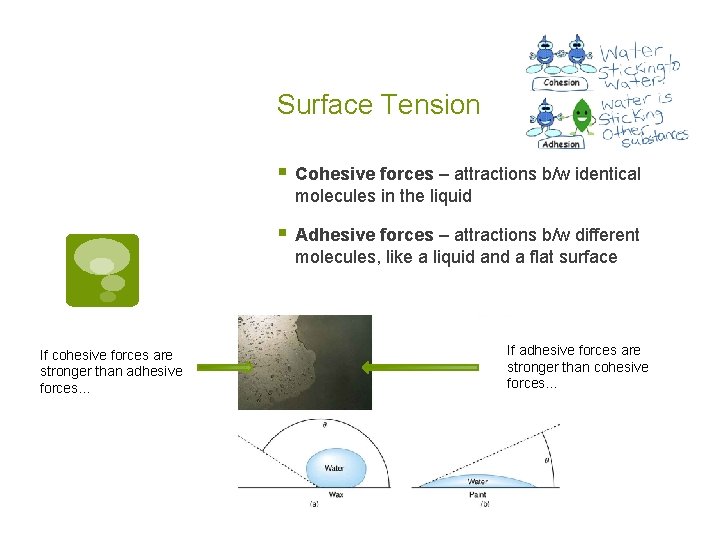
Surface Tension § Cohesive forces – attractions b/w identical molecules in the liquid § Adhesive forces – attractions b/w different molecules, like a liquid and a flat surface If cohesive forces are stronger than adhesive forces… If adhesive forces are stronger than cohesive forces…

Viscosity Low viscosity High viscosity § A liquid’s resistance to flow. § Attractive forces are responsible for viscosity. § Molecules move more freely in solutions with low attractive forces § Liquid alkanes have lower viscosities because they only have London forces § Water is more viscous because it has hydrogen bonding § Syrup is very viscous because all the bulky sugar molecules have lots of –OH groups, which hydrogen bond to the water in the mixture.

Viscosity § Decreases as the liquid’s T is increased. § Molecules have higher KE, weakens intermolecular forces (IMFs).

Evaporation § The process in which a liquid in an open container is slowly converted into a gas at the surface of the liquid. § Some liquids evaporate more rapidly than others. § Reverse of condensation, must have enough sufficient KE to escape the attractive forces

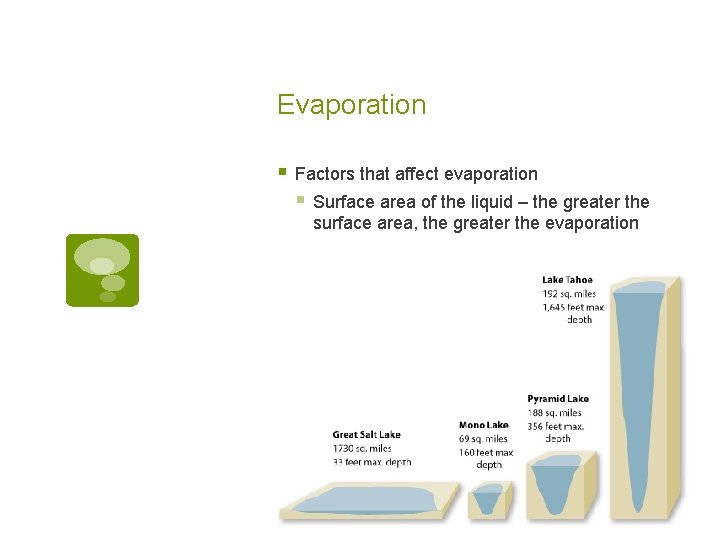
Evaporation § Factors that affect evaporation § Surface area of the liquid – the greater the surface area, the greater the evaporation

Evaporation § Factors that affect evaporation § Temperature – Increasing the T increases the # molecules with enough KE to escape as a gas

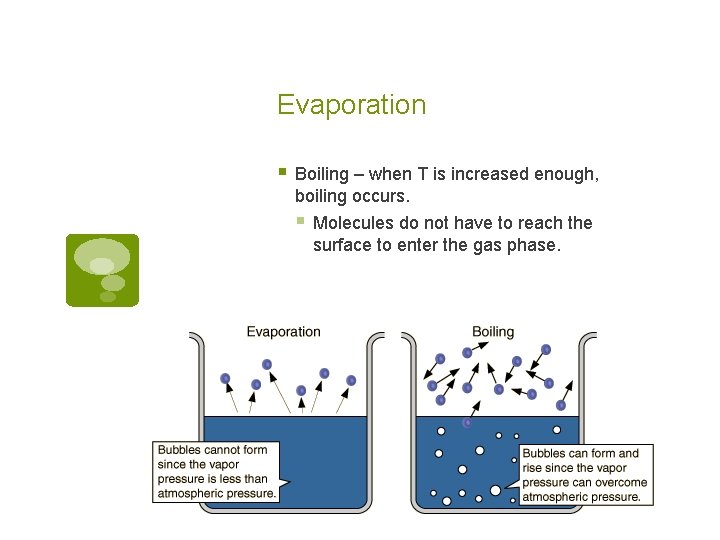
Evaporation § Boiling – when T is increased enough, boiling occurs. § Molecules do not have to reach the surface to enter the gas phase.

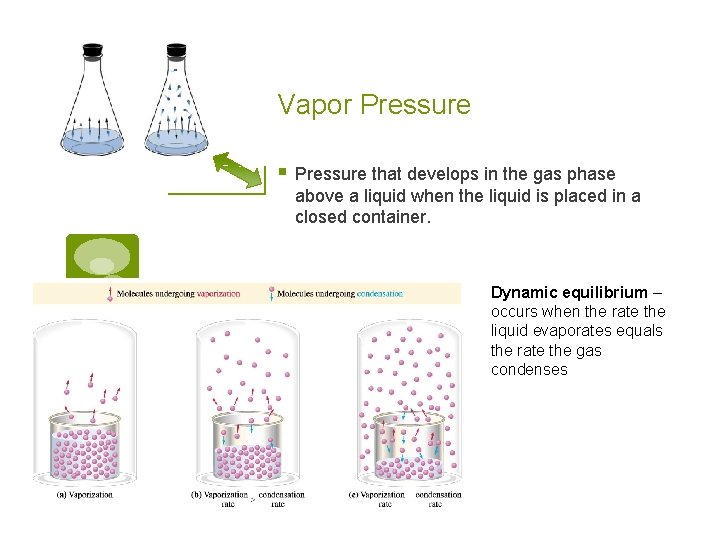
Vapor Pressure § Pressure that develops in the gas phase above a liquid when the liquid is placed in a closed container. Dynamic equilibrium – occurs when the rate the liquid evaporates equals the rate the gas condenses

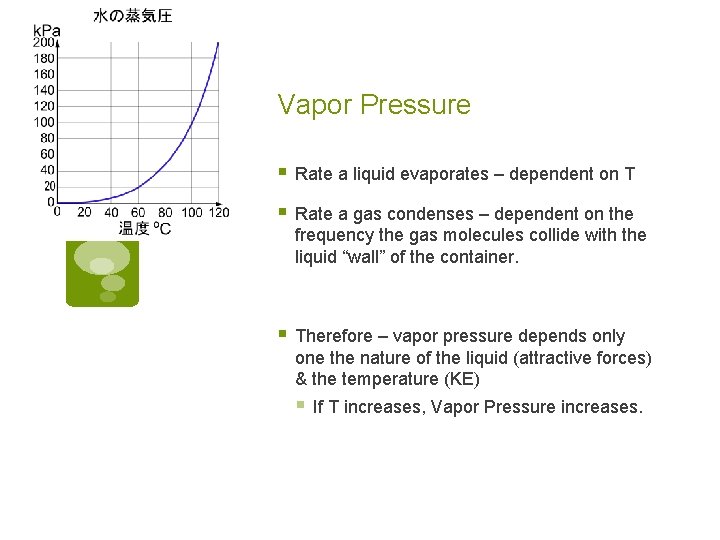
Vapor Pressure § Rate a liquid evaporates – dependent on T § Rate a gas condenses – dependent on the frequency the gas molecules collide with the liquid “wall” of the container. § Therefore – vapor pressure depends only one the nature of the liquid (attractive forces) & the temperature (KE) § If T increases, Vapor Pressure increases.

Boiling Point § Boiling occurs when the vapor pressure of the liquid is equal to atmospheric pressure § Normal boiling point- refers to the boiling point when atmospheric pressure is 760 m. Hg

Heat of Vaporization § ΔHvap - the energy needed to convert 1 gram of liquid into 1 gram of gas at a temperature equal to the normal boiling point of the liquid. § Units are J/g or J/mol (if using molar heat of vaporization) § ΔHvap = -ΔHcond

Heat of Vaporization § There are differences in the heats of vaporization that can be related to the IMFs § For similar-size molecules, hydrogenbonded substances have largest ΔHvap. § Polar substances have higher ΔHvap than similar shape nonpolar substances § Increasing London forces increases ΔHvap

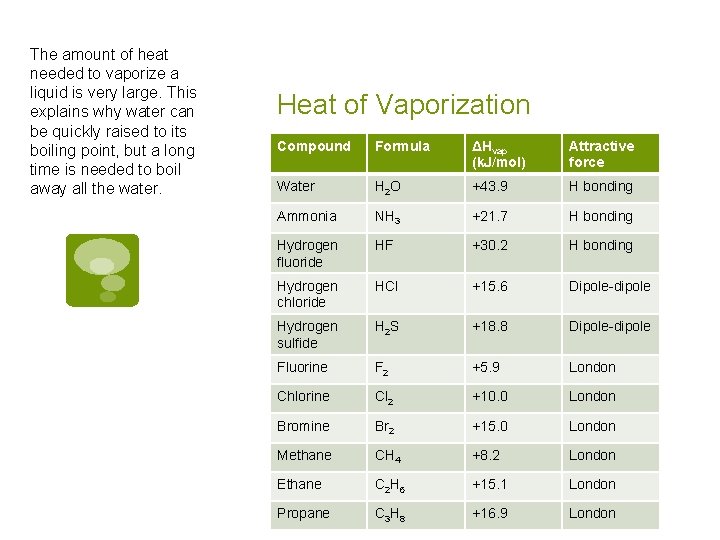
The amount of heat needed to vaporize a liquid is very large. This explains why water can be quickly raised to its boiling point, but a long time is needed to boil away all the water. Heat of Vaporization Compound Formula ΔHvap (k. J/mol) Attractive force Water H 2 O +43. 9 H bonding Ammonia NH 3 +21. 7 H bonding Hydrogen fluoride HF +30. 2 H bonding Hydrogen chloride HCl +15. 6 Dipole-dipole Hydrogen sulfide H 2 S +18. 8 Dipole-dipole Fluorine F 2 +5. 9 London Chlorine Cl 2 +10. 0 London Bromine Br 2 +15. 0 London Methane CH 4 +8. 2 London Ethane C 2 H 6 +15. 1 London Propane C 3 H 8 +16. 9 London
 Thermal expansion and contraction examples
Thermal expansion and contraction examples Solids liquids and gases section 2 properties of fluids
Solids liquids and gases section 2 properties of fluids Kinetic molecular theory of liquids and solids
Kinetic molecular theory of liquids and solids Solid to gas
Solid to gas Solid liquid venn diagram
Solid liquid venn diagram Properties of a solid
Properties of a solid Example of solid liquid and gas
Example of solid liquid and gas Adhesive force
Adhesive force Liquids and solids menu
Liquids and solids menu Lesson 1 thermal energy and the behavior of matter
Lesson 1 thermal energy and the behavior of matter Chapter 14 solids liquids and gases worksheet answers
Chapter 14 solids liquids and gases worksheet answers Kesler science.com
Kesler science.com Red liquid element
Red liquid element How does sound travel through solids liquids and gases
How does sound travel through solids liquids and gases Properties of solids liquids and gases
Properties of solids liquids and gases Motion of particles in solids, liquids and gases
Motion of particles in solids, liquids and gases Chapter 11 - states of matter: liquids and solids
Chapter 11 - states of matter: liquids and solids Why is gas easier to compress than liquid and solid
Why is gas easier to compress than liquid and solid Filtering solids from liquids
Filtering solids from liquids Limit comparison theorem
Limit comparison theorem 5 parts of nourishing element
5 parts of nourishing element A yolk and cream mixture that is used to thicken liquids.
A yolk and cream mixture that is used to thicken liquids. Molecular theory of gases and liquids
Molecular theory of gases and liquids Classification of liquid dielectrics
Classification of liquid dielectrics Separating mixtures worksheet grade 5
Separating mixtures worksheet grade 5 Regional metamorphism
Regional metamorphism Intersection in technical drawing
Intersection in technical drawing Similar figures
Similar figures Similar congruent or neither
Similar congruent or neither Regular and irregular solids
Regular and irregular solids