CHAPTER 17 LIQUIDS Melting Freezing Most solids liquids







































- Slides: 45

CHAPTER 17 LIQUIDS

Melting & Freezing Most solids & liquids expand when heated l incr. kinetic energy • Particles forced farther apart – collide more & w/ greater force If temp. is incr. enough, particles will move far enough apart to slide over ea. other l ordered arrangement breaks down • MELTS

Melting & Freezing In liquids, @ a certain temp. particles travel so slowly they can’t slip past ea. other l FREEZES

Melting & Freezing All pure liquids have a definite freezing pt. All pure solids have a definite melting pt. For a pure subst. , the freezing pt. of the liquid is the same temp. as the melting pt. of the solid

Vapor Equilibrium Avg. kinetic energy of molecs. @ a given temp. is constant l not all moving same speed In liquids, surface molec. may gain enough K. E. to overcome attractive force & escape from surface of liquid. l l May also occur @ surface of solids These molecs. form a vapor

Vapor Equilibrium Gas - gaseous @ room temp. Vapor - gaseous state of substs. which are liquids or solids @ room temp. Vapor molec. may collide w/ surface of liquid. l If K. E. is low, may become part of liquid • little chance in open container • in closed container - greater chance

Vapor Equilibrium @ some pt. , there will be = # of molecs. leaving & returning to the suface l constant # of particles in liquid & vapor phase • - EQUILIBRIUM - no net change l l This is special type called DYNAMIC EQUILIBRIUM Rate of Evaporation = Rate of Condensation

Vapor Equilibrium When a subst. is in equilib. w/ its vapor, the gaseous phse is saturated w/ the vapor Physical change from liquid to vapor l l l X(l) X(g) Opposite process X(l) X(g)

Vapor Equilibrium 2 eqns. can be combined l X(l) X(g) • Reversible Change - Reach equilibrium when changes are occuring @ the same rate in both directions

Le. Chatelier’s Principle Vapor phase exerts a press. that’s dependent on temp. l The higher the temp. , the higher the vapor press. A liquid & its vapor will reach equilib. @ a specific press. for any temp.

Le. Chatelier’s Principle - A stress applied to a system @ equilibrium causes a readjustment to offset the stress (reduce stress) l This stress may be a chg. in temp. , press. , concentration, etc.

Le. Chatelier’s Principle Freezing & melting of H 2 O is a reversible syst. which can come to equilibrium H 2 O(l) H 2 O(s) When press. is applied to something, it gets smaller l If press. is applied to ice, it causes a stress - to relieve the stress, the ice will melt • liquid water takes up less space than ice

Measuring Vapor Pressure Vapor press. is a function of temp. l as temp. incr. , vapor press. incr. Substs. w/ low vapor press. have strong intermolec. forces Substs. w/ high vapor press. have weak intermolec. forces.

Melting Point In a mixture of solid & liquid, there is a dynamic equilib. l Solid Liquid • Ea. state is also in equilib. w/ its vapor • Only 1 vapor, so solid & liquid have the same vapor press.

Melting Point - The temp. @ which the vapor press. of the solid & vapor press. of the liquid are = l Melting pt. = Freezing pt.

Melting Point Melting Pt. depends on intermolec. forces in the subst. l l Substs. w/ weak intermolec. forces have lower melting pts. than substs. w/ strong forces Nonpolar substs. w/ low molar masses have lower melting pts. than polar substs. w/ low molar masses

Sublimation - The process of changing directly from a solid to a gas or vapor w/out passing thru the liquid state l l l Solid Vapor Solids have vapor press. large enough @ room temp. to vaporize readily Ex. dry ice & moth balls

Boiling Point Liquid & vapor can be in equilib. only in a closed container. l l in open container, molecs, escape into the air Evaporation - escape of molecs. from the surface of a liquid

Boiling Point At temp. incr. , K. E. incr, & vapor press. incr. l l K. E. eventually becomes large enough to overcome internal press. due to air pushing on the surface. Molecs. move fast enough, they are pushed far apart & form gas bubbles which rise to the surface.

Boiling Point Normal Boiling Point - The temp. @ which the vapor press. = std. atmospheric press. Boiling is a function of pressure l @ lower press. , boiling pt. is lower Evaporation occurs only @ the surface; boiling occurs throughout the liquid l Boiling pt. = Condensation pt.

Boiling Point Adding energy to a liquid @ its B. P. will chg it to a gas Removing energy from a gas @ its B. P will chg it to a liquid

Boiling Point Diff. liquids boil @ diff. temps. Volatile liquid - boils @ low temp. & evaporates readily l has high vapor press @ room temp. • Ex. - alcohol, acetone Nonvolatile Liquid - boils @ high temp & evaporates slowly @ room temp. l low vapor press. • Ex - oil, molasses, glycerin

Liquefaction of Gases - The condensation of substs. which are normally gases To liquefy: 1. Cool - slow dn. molecs so van der Waals forces can bind molecs. together 2. Compress - get molecs close enough for van der Waals forces to take effect

Liquefaction of Gases Tc - Critical Temperature - Temp. above which no amt. of press. will cause the gas to liquefy. Pc - Critical Pressure - Minimal press. that will cause a gas to liquefy @ its critical temp.

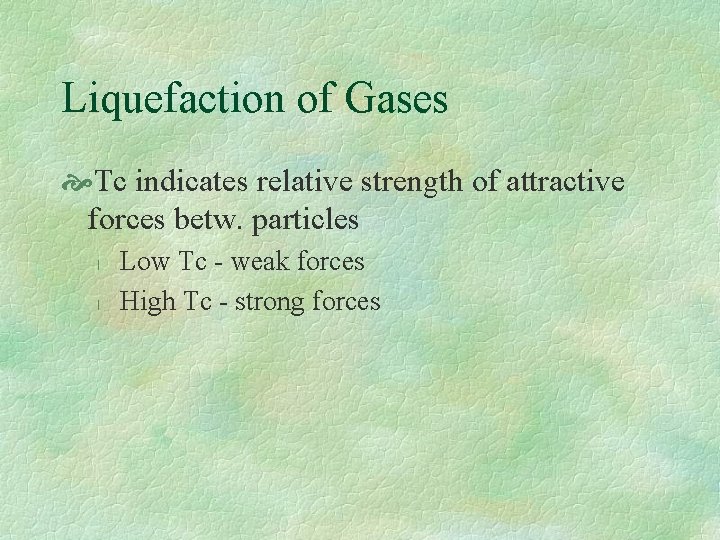
Liquefaction of Gases Tc indicates relative strength of attractive forces betw. particles l l Low Tc - weak forces High Tc - strong forces

Phase Diagrams - graphically represents changes of state @ varying temps. & press.

Phase Diagram for Water Line AB - solid-vapor line l represents vapor press. of ice from -100 o. C to pt. B Line BD - Liquid-vapor line l l l Vapor press. curve for liquid water Gives temp. & press. @ which liquid water & water vapor are in equilib. represents vapor press. of liquid water from pt. B to 374 o. C (Tc for water)

Phase Diagram for Water Pt. B - Triple Point - all 3 states are in equilibrium Pt. D - Critical Point - above this there is no vapor curve l Only gaseous state exists @ press. & temps. above this point

Phase Diagram for Water Tm - melting point - occurs where line BC is cut by std. atmos. press. l l l Vapor Press. of liquid & solid = atmos. press @ this pt. Line BC indicates press. -temp. conditions under which solid & liquid can be in equilib. Only line AD represents vapor press. info.

Phase Diagram for Water Tb - Boiling Pt. - Temp. @ which liquidvapor equilib. curve is cut by std. atmos. press. line

Phase Diagram for Water Line BC has (-) Slope - indicates that a rise in press. will lower the freezing pt. l - Water expands when it freezes Most substs. contract when they freeze l this line would have a (+) slope

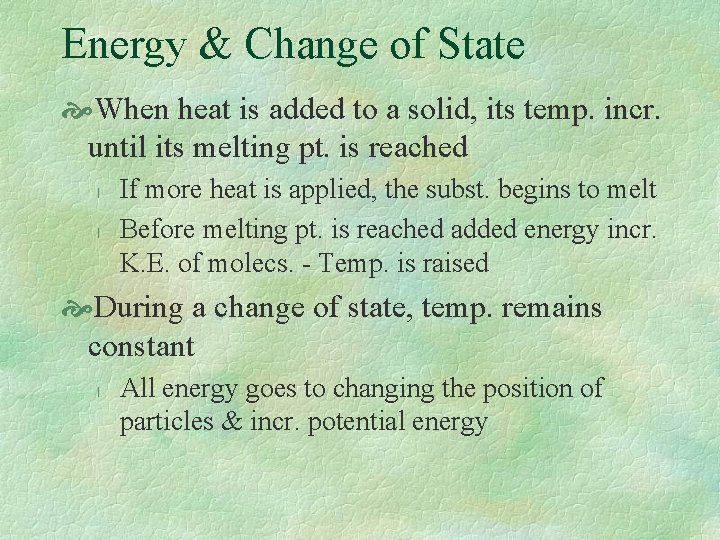
Energy & Change of State When heat is added to a solid, its temp. incr. until its melting pt. is reached l l If more heat is applied, the subst. begins to melt Before melting pt. is reached added energy incr. K. E. of molecs. - Temp. is raised During a change of state, temp. remains constant l All energy goes to changing the position of particles & incr. potential energy

Energy & Change of State Enthalpy of Fusion (DHfus) - Energy required to melt 1 g of a subst. @ its melting point Enthalpy of Vaporization (DHvap) - Energy required to vaporize 1 g of a subst. @ its boiling point Specific Heat Capacity (Cp) - Energy required to raise the temp. of 1 g of a subst. by 1 Co

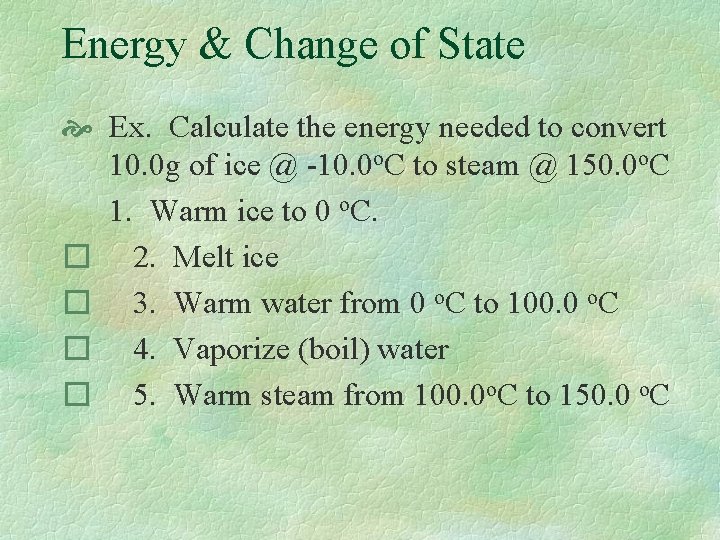
Energy & Change of State Ex. Calculate the energy needed to convert 10. 0 g of ice @ -10. 0 o. C to steam @ 150. 0 o. C 1. Warm ice to 0 o. C. � 2. Melt ice � 3. Warm water from 0 o. C to 100. 0 o. C � 4. Vaporize (boil) water � 5. Warm steam from 100. 0 o. C to 150. 0 o. C

Hydrogen Bonding In many substs. , the predicted m. p. ’s & b. p’s differ from actual ones These substs. have 2 things in common: 1. Contain H

Hydrogen Bonding 2. H is covalently bonded to a highly electroneg. atom l Electroneg. atom has almost complete possession of shared e- pr. • Molec. is highly polar • H has very strong partial (+) charge • almost like a bare p+

Hydrogen Bonding Only elems. electroneg. enough to cause this are N, O, &F H is always covalently bonded - H+ doesn’t exist l partial (+) charge on H end of molec. is much stronger than other dipoles • H is the only elem. that has this prop. • All others have inner e- levels

Hydrogen Bonding The attraction betw. the H end of one molec. & the (-) end of other molecs. is very strong. l l not nearly as strong as a chemical bond This attractive force in these substs. is called a Hydrogen Bond • H atom tends to hold the 2 molecs. firmly together • Considered apart from other dipole attractions bec. of its greater effect on the props. of substs.

Hydrogen Bonding in Water Effects of H-bonding can be seen in water l When frozen, a molec. of water is H-bonded to 4 other water molecs. • H atoms are attracted to the O atoms of neighboring molecs • Open crystalline structure

Hydrogen Bonding in Water When ice melts, many H bonds are broken not all l l The lattice collapses - molecs. move closer together water is more dense than ice

Hydrogen Bonding in Water As water is heated above 0 o. C, more H bonds are broken l @ 3. 98 o. C, most H bonds are broken • most dense l As water is heated more, water expands

Surface Tension & Capillary Rise Surface Tension - The apparent elasticity of a surface that’s due to unbalanced forces on surface particles l Particles in the middle of the liquid are subjected to attractive forces in all directions

Surface Tension & Capillary Rise Surface particles can’t be pulled in all directions l l creates a “skin” on the surface particles on surface have a net force inward this is why liquids form spheres when dropped surface molecs. are pulled inward by the rest of the molecs. in the drop

Surface Tension & Capillary Rise Unbalanced forces also account for Capillary Rise l The rise of a liquid in a tube of small diameter If there’s an attractive force betw. a liquid & the walls of the capillary tube, liquid will rise in tube l l attractive force relieves unbalanced forces Liquid will rise until forces are balanced

Surface Tension & Capillary Rise Mercury has a very strong surface tension, but won’t rise in a capillary tube l l does not “wet” the glass attractive force betw. Hg & glass (adhesive force) is not strong enough to overcome the attractive force betw. the Hg atoms (cohesive force)