UNIT9 OxidationReduction Electrochemistry Flint Water Crisis Corrosion of



























- Slides: 78

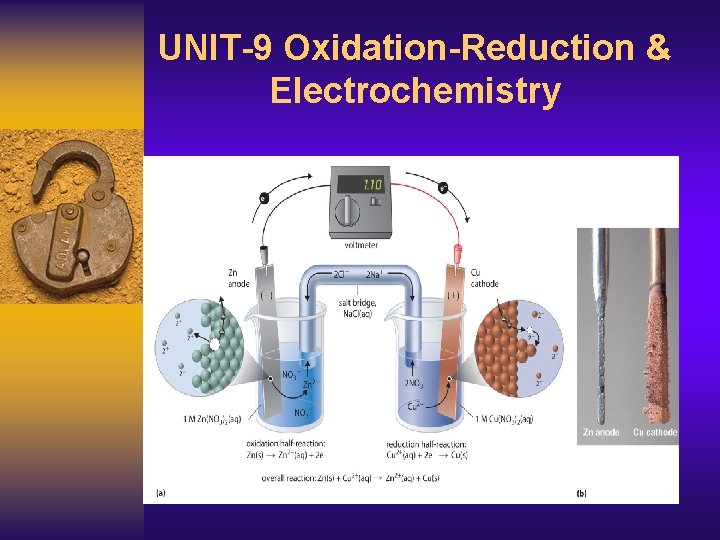
UNIT-9 Oxidation-Reduction & Electrochemistry

Flint Water Crisis • Corrosion of pipes lead to contaminated water • Lead poisoning in children VIDEO (Scientific American): Flint Water Crisis J Deutsch 2003 2

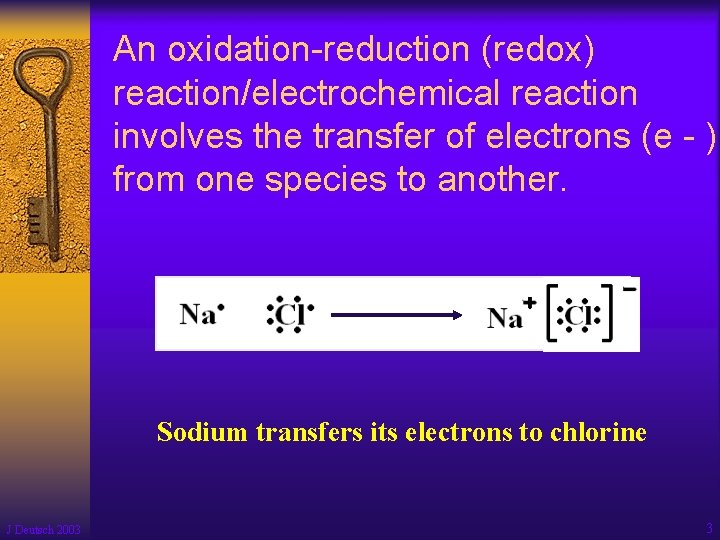
An oxidation-reduction (redox) reaction/electrochemical reaction involves the transfer of electrons (e - ) from one species to another. Sodium transfers its electrons to chlorine J Deutsch 2003 3

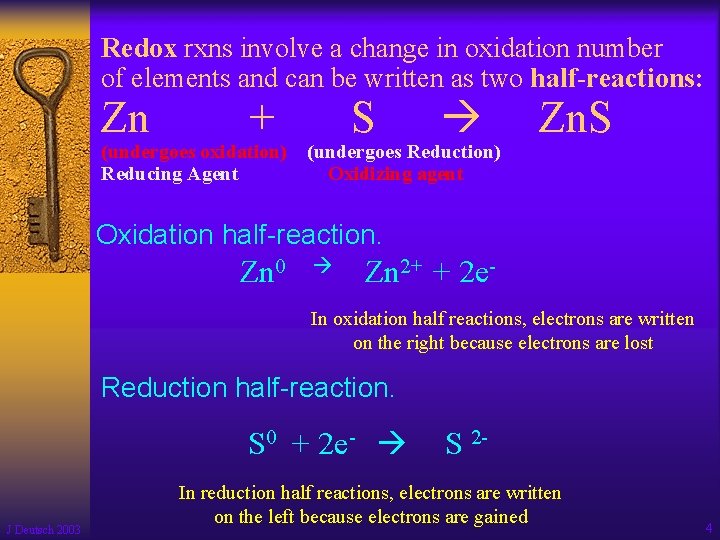
Redox rxns involve a change in oxidation number of elements and can be written as two half-reactions: Zn + (undergoes oxidation) Reducing Agent S (undergoes Reduction) Oxidizing agent Zn. S Oxidation half-reaction. Zn 0 Zn 2+ + 2 e- In oxidation half reactions, electrons are written on the right because electrons are lost Reduction half-reaction. S 0 + 2 e- J Deutsch 2003 S 2 - In reduction half reactions, electrons are written on the left because electrons are gained 4

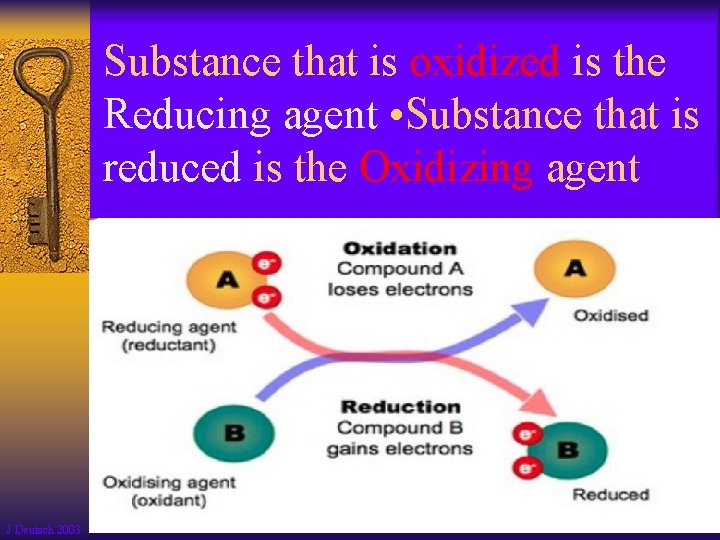
Substance that is oxidized is the Reducing agent • Substance that is reduced is the Oxidizing agent J Deutsch 2003 5

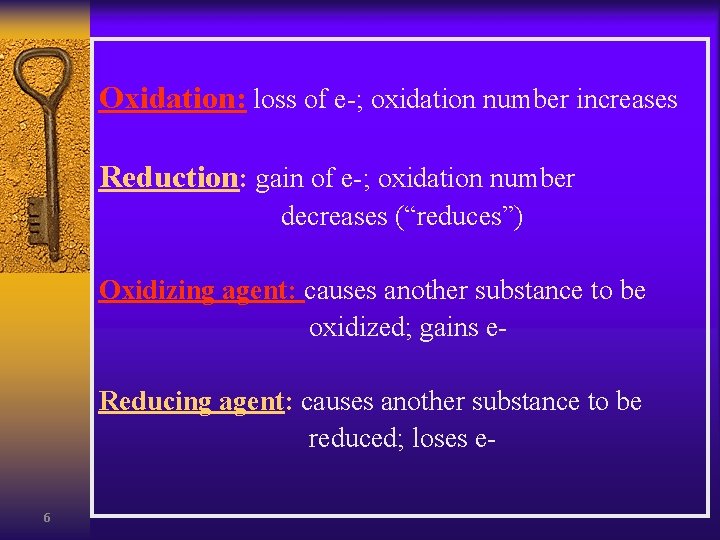
Oxidation: loss of e-; oxidation number increases Reduction: gain of e-; oxidation number decreases (“reduces”) Oxidizing agent: causes another substance to be oxidized; gains e. Reducing agent: causes another substance to be reduced; loses e 6

LEO growls GER Losing Electrons Oxidation J Deutsch 2003 Gaining Electrons Reduction 7

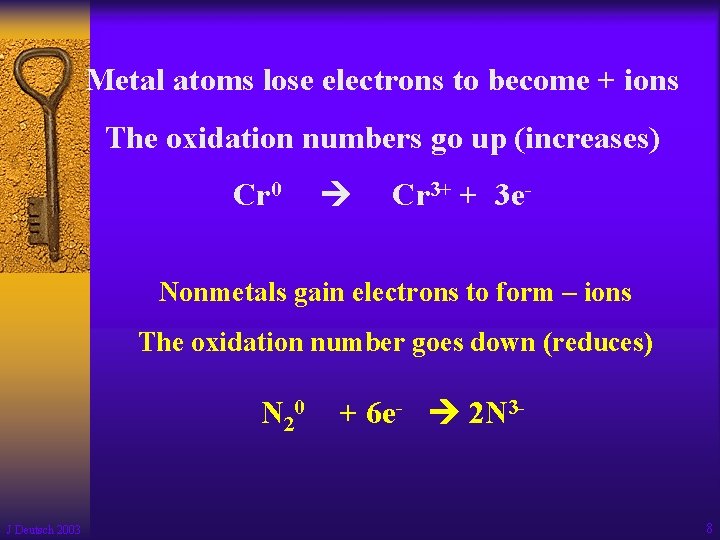
Metal atoms lose electrons to become + ions The oxidation numbers go up (increases) Cr 0 Cr 3+ + 3 e- Nonmetals gain electrons to form – ions The oxidation number goes down (reduces) N 20 J Deutsch 2003 + 6 e- 2 N 3 - 8

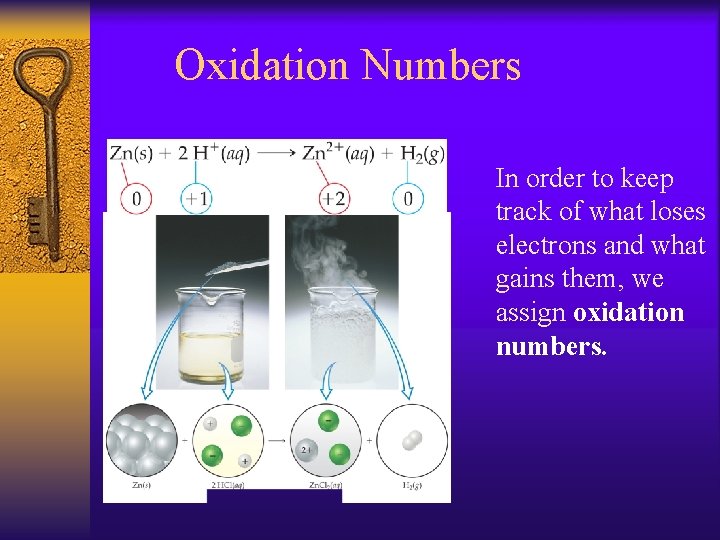
Oxidation Numbers In order to keep track of what loses electrons and what gains them, we assign oxidation numbers.

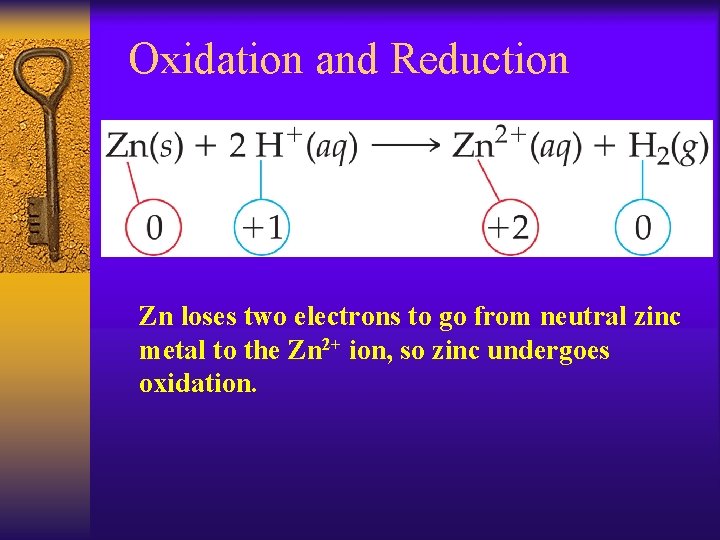
Oxidation and Reduction Zn loses two electrons to go from neutral zinc metal to the Zn 2+ ion, so zinc undergoes oxidation.

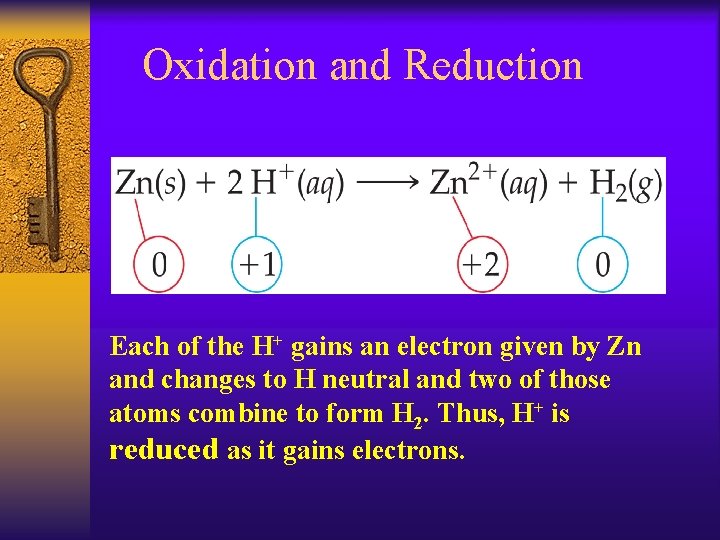
Oxidation and Reduction Each of the H+ gains an electron given by Zn and changes to H neutral and two of those atoms combine to form H 2. Thus, H+ is reduced as it gains electrons.

Assigning Oxidation Numbers 1. Elements in their elemental form have an oxidation number of 0. So, Na has an oxidation number of zero. 2. The oxidation number of a monatomic ion is the same as its charge. – Group 1 Metals are always 1+ – Group 2 Metals are always 2+

Assigning Oxidation Numbers 3. Nonmetals tend to have negative oxidation numbers, although some are positive in certain compounds or ions. ¨ Oxygen is always 2 - except when combined with F or in Peroxides (In OF 2 , O is 2+ and in H 2 O 2 , O is 1 - ) ¨ Hydrogen is − 1 when bonded to a metal, +1 when bonded to a nonmetal. ¨ Fluorine always has an oxidation number of − 1. ¨ The other halogens have an oxidation number of − 1 when they are negative; they can have positive oxidation numbers, however, most notably in oxyanions.

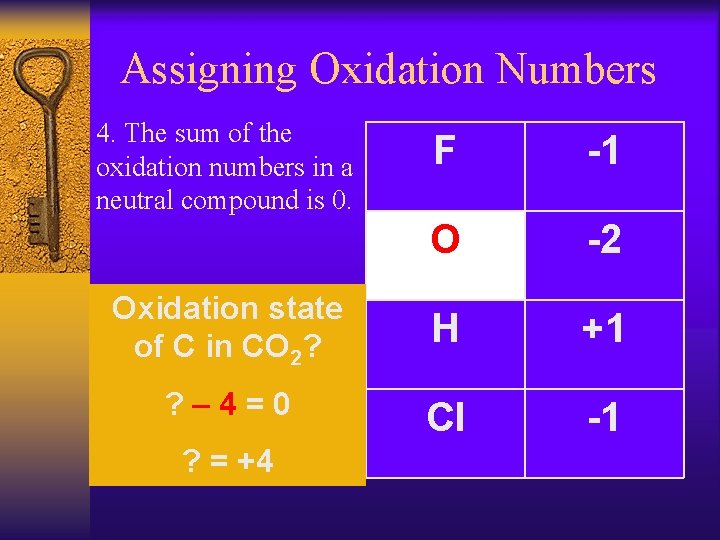
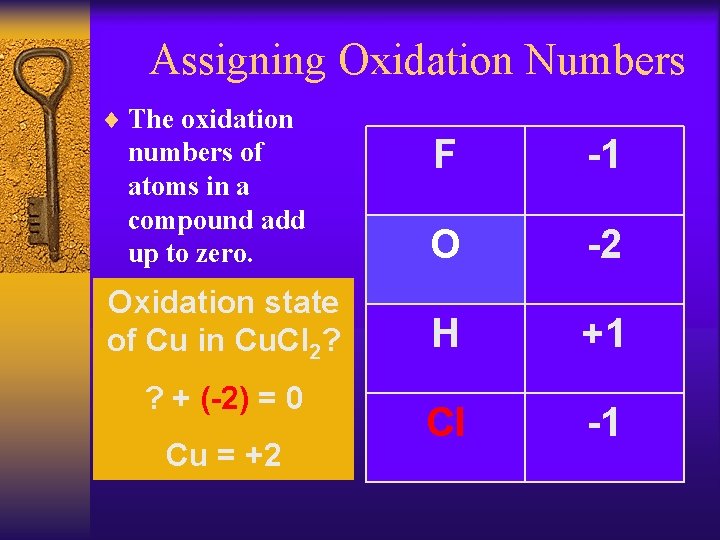
Assigning Oxidation Numbers 4. The sum of the oxidation numbers in a neutral compound is 0. F -1 O -2 Oxidation state of C in CO 2? H +1 ? – 4=0 Cl -1 ? = +4

Assigning Oxidation Numbers ¨ The oxidation numbers of atoms in a compound add up to zero. F -1 O -2 Oxidation state of Cu in Cu. Cl 2? H +1 Cl -1 ? + (-2) = 0 Cu = +2

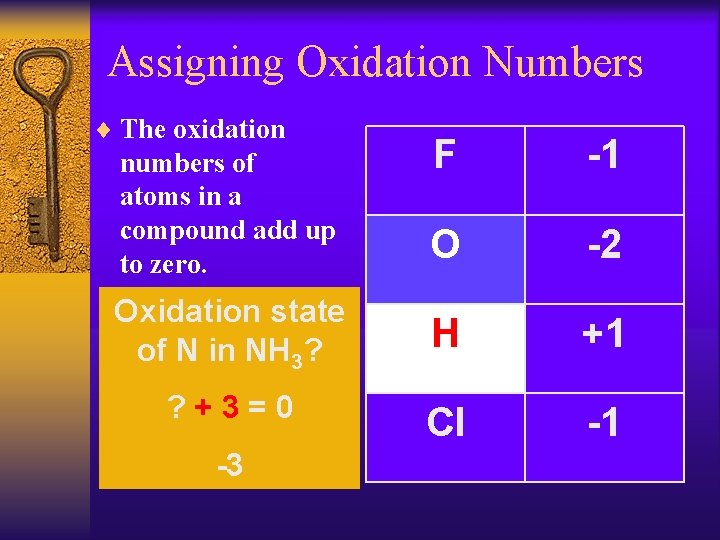
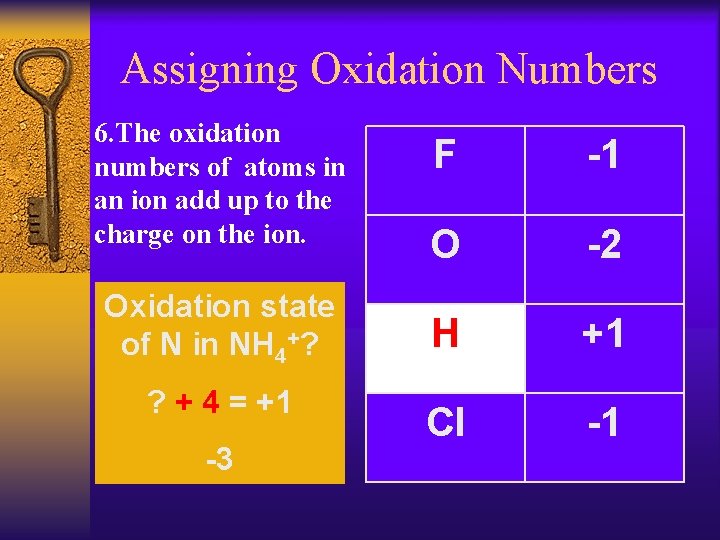
Assigning Oxidation Numbers ¨ The oxidation F -1 O -2 Oxidation state of N in NH 3? H +1 ? +3=0 Cl -1 numbers of atoms in a compound add up to zero. -3

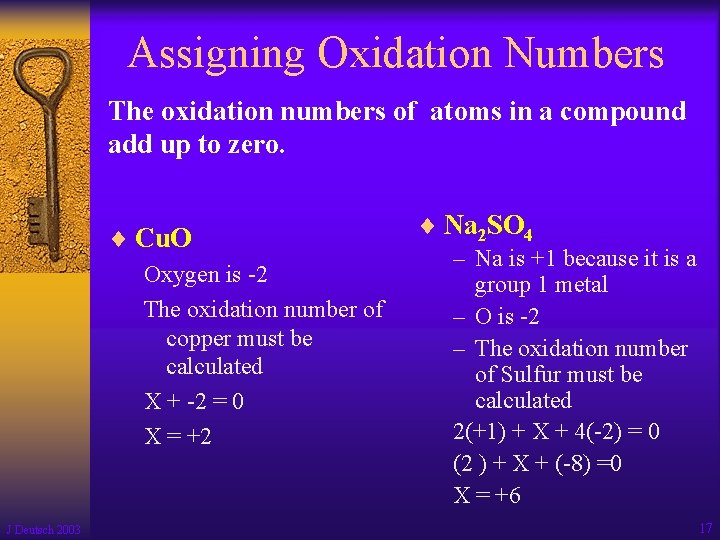
Assigning Oxidation Numbers The oxidation numbers of atoms in a compound add up to zero. ¨ Cu. O Oxygen is -2 The oxidation number of copper must be calculated X + -2 = 0 X = +2 J Deutsch 2003 ¨ Na 2 SO 4 – Na is +1 because it is a group 1 metal – O is -2 – The oxidation number of Sulfur must be calculated 2(+1) + X + 4(-2) = 0 (2 ) + X + (-8) =0 X = +6 17

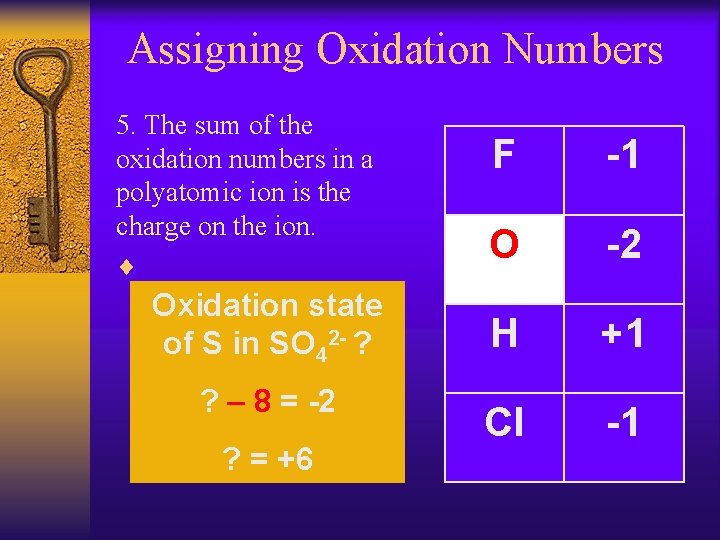
Assigning Oxidation Numbers 5. The sum of the oxidation numbers in a polyatomic ion is the charge on the ion. ¨ Oxidation state of S in SO 42 - ? ? – 8 = -2 ? = +6 F -1 O -2 H +1 Cl -1

Assigning Oxidation Numbers The sum of the oxidation numbers of all the atoms in a polyatomic ion is the charge of the ion. ¨ NO 3 Oxygen is 2 The oxidation number of nitrogen must be calculated X + 3(-2) = -1 X = 5+ J Deutsch 2003 ¨ PO 43 Oxygen is 2 The oxidation number of phosphorous must be calculated X + 4(-2) = -3 X + (-8) = -3 X = +5 19

Assigning Oxidation Numbers 6. The oxidation numbers of atoms in an ion add up to the charge on the ion. Oxidation state of N in NH 4+? ? + 4 = +1 -3 F -1 O -2 H +1 Cl -1

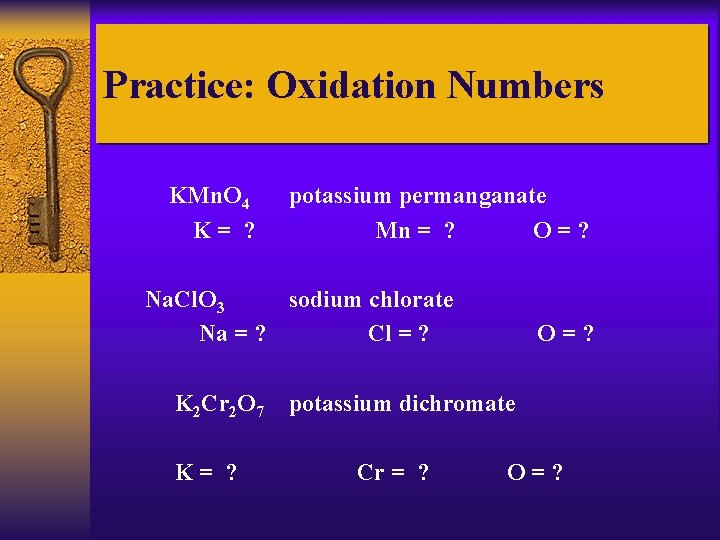
Practice: Oxidation Numbers KMn. O 4 K= ? potassium permanganate Mn = ? O=? Na. Cl. O 3 sodium chlorate Na = ? Cl = ? K 2 Cr 2 O 7 K= ? O=? potassium dichromate Cr = ? O=?

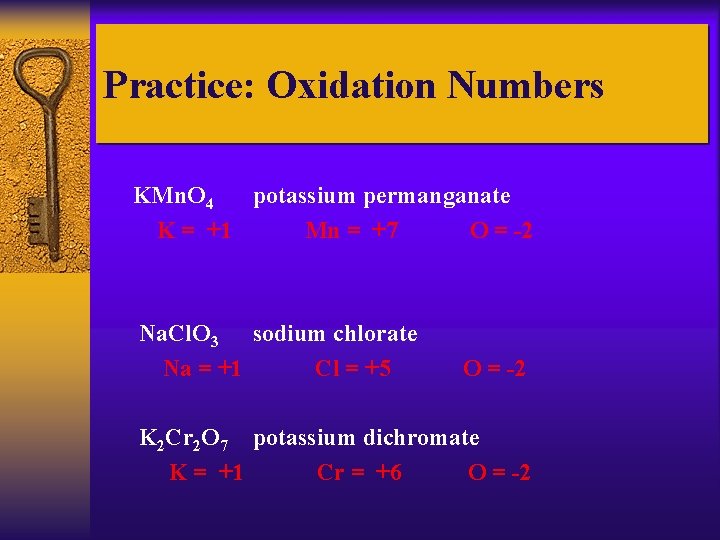
Practice: Oxidation Numbers KMn. O 4 potassium permanganate K = +1 Mn = +7 O = -2 Na. Cl. O 3 sodium chlorate Na = +1 Cl = +5 O = -2 K 2 Cr 2 O 7 potassium dichromate K = +1 Cr = +6 O = -2

Practice: Oxidation Numbers Find the oxidation numbers and see which ones change during the chemical reaction 0 2+ 5+ 2 - Zn + Cu(NO 3)2 2+ 5+ 2 - Zn(NO 3)2 + Cu 0 Nitrate NO 3 is -1 Since Nitrate ion is present before and after the reaction, it is called a spectator ion Zn 0 Zn 2+ + 2 e- Oxidation Cu 2+ + 2 e. Cu 0 Reduction J Deutsch 2003 23

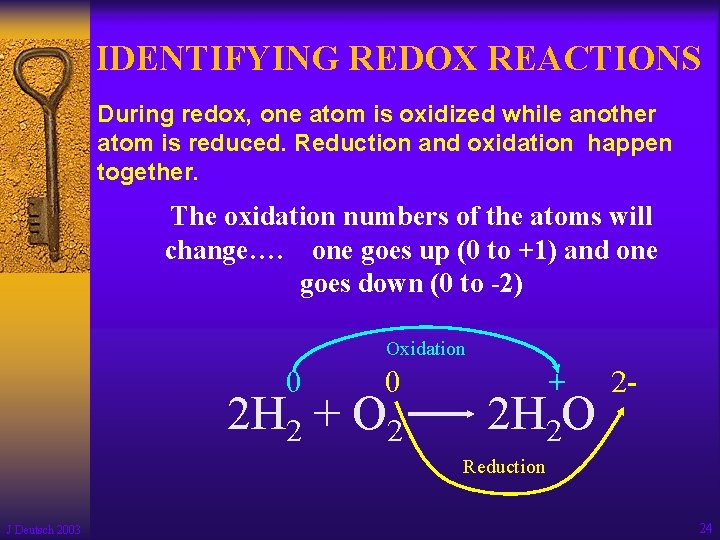
IDENTIFYING REDOX REACTIONS During redox, one atom is oxidized while another atom is reduced. Reduction and oxidation happen together. The oxidation numbers of the atoms will change…. one goes up (0 to +1) and one goes down (0 to -2) Oxidation 0 0 2 H 2 + O 2 + 2 H 2 O 2 - Reduction J Deutsch 2003 24

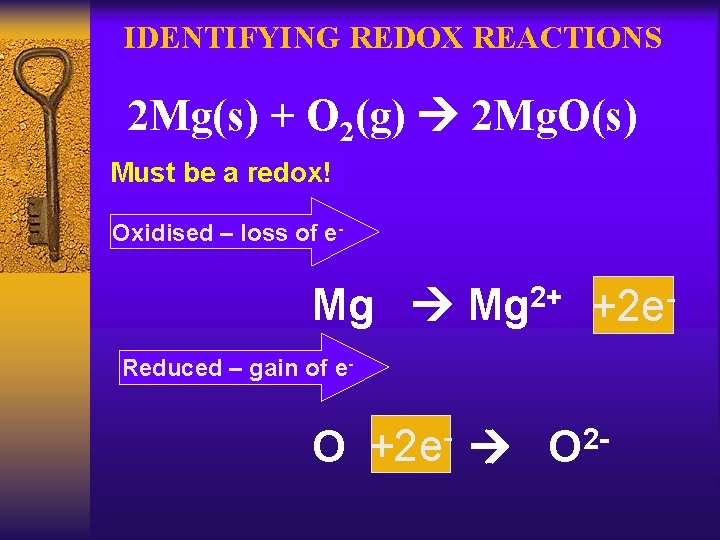
IDENTIFYING REDOX REACTIONS 2 Mg(s) + O 2(g) 2 Mg. O(s) Must be a redox! Oxidised – loss of e- Mg 2+ +2 e. Reduced – gain of e- O +2 e O 2 -

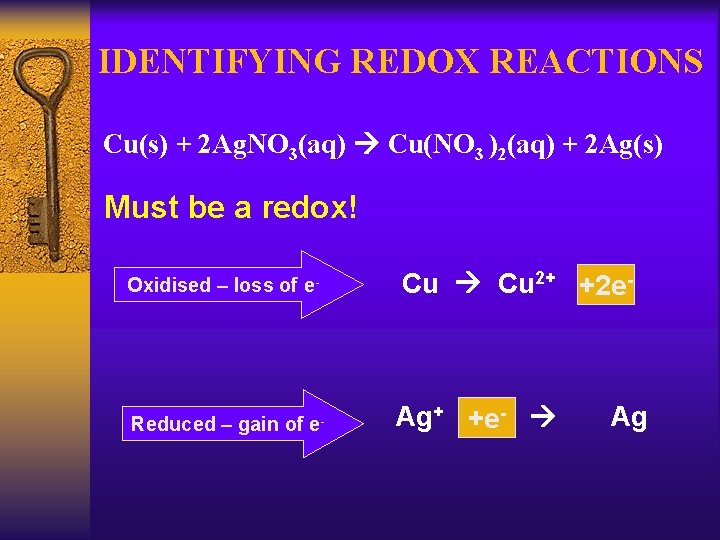
IDENTIFYING REDOX REACTIONS Cu(s) + 2 Ag. NO 3(aq) Cu(NO 3 )2(aq) + 2 Ag(s) Must be a redox! Oxidised – loss of e- Reduced – gain of e- Cu 2+ +2 e- Ag+ +e- Ag

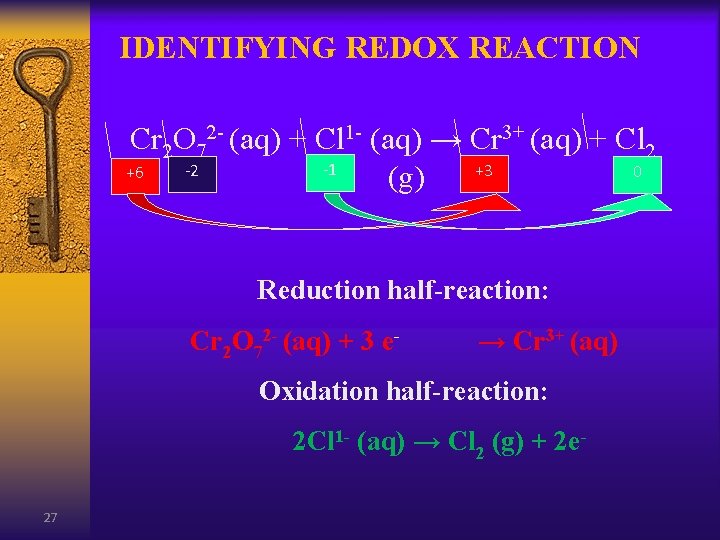
IDENTIFYING REDOX REACTION Cr 2 O 72 - (aq) + Cl 1 - (aq) → Cr 3+ (aq) + Cl 2 -1 +3 -2 0 +6 (g) Reduction half-reaction: Cr 2 O 72 - (aq) + 3 e- → Cr 3+ (aq) Oxidation half-reaction: 2 Cl 1 - (aq) → Cl 2 (g) + 2 e 27

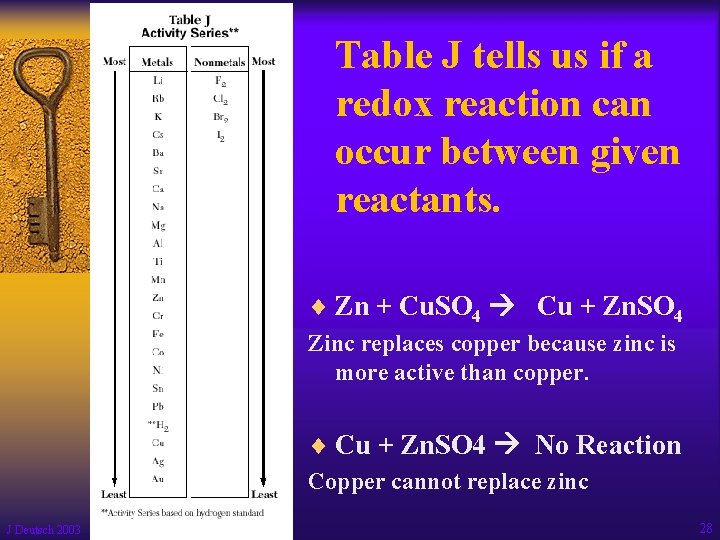
Table J tells us if a redox reaction can occur between given reactants. ¨ Zn + Cu. SO 4 Cu + Zn. SO 4 Zinc replaces copper because zinc is more active than copper. ¨ Cu + Zn. SO 4 No Reaction Copper cannot replace zinc J Deutsch 2003 28

Table J tells us if a redox reaction can occur between given reactants. ¨ A more active metal(high on table J) will replace a less active metal (below it on Table J) from a compound. Mg. Cl 2 + 2 Na. Cl + Mg ¨ A more active nonmetal (high on table J) will replace a less active non metal(below it on Table J) from a compound. Mg. Cl 2 + F 2 Mg F 2 + Cl 2 ¨ Any metal above H is more active than H and will react with an acid to produce H 2(g) – The higher up t table, the more readily the replacement will take place. J Deutsch 2003 Zn + 2 HCl Zn. Cl 2 + H 2 29

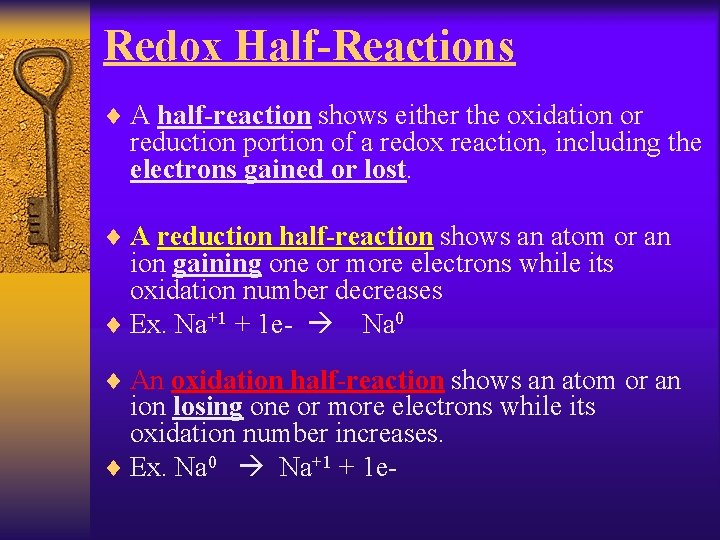
Redox Half-Reactions ¨ A half-reaction shows either the oxidation or reduction portion of a redox reaction, including the electrons gained or lost. ¨ A reduction half-reaction shows an atom or an ion gaining one or more electrons while its oxidation number decreases ¨ Ex. Na+1 + 1 e- Na 0 ¨ An oxidation half-reaction shows an atom or an ion losing one or more electrons while its oxidation number increases. ¨ Ex. Na 0 Na+1 + 1 e-

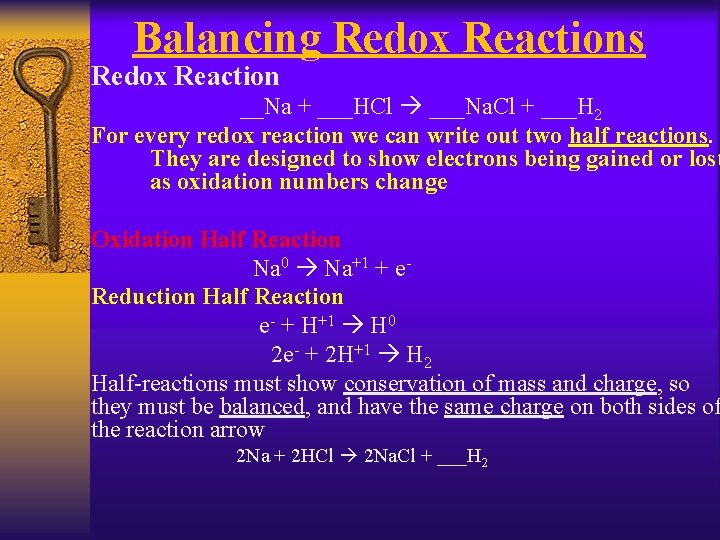
Balancing Redox Reactions Redox Reaction __Na + ___HCl ___Na. Cl + ___H 2 For every redox reaction we can write out two half reactions. They are designed to show electrons being gained or lost as oxidation numbers change Oxidation Half Reaction Na 0 Na+1 + e. Reduction Half Reaction e- + H+1 H 0 2 e- + 2 H+1 H 2 Half-reactions must show conservation of mass and charge, so they must be balanced, and have the same charge on both sides of the reaction arrow 2 Na + 2 HCl 2 Na. Cl + ___H 2

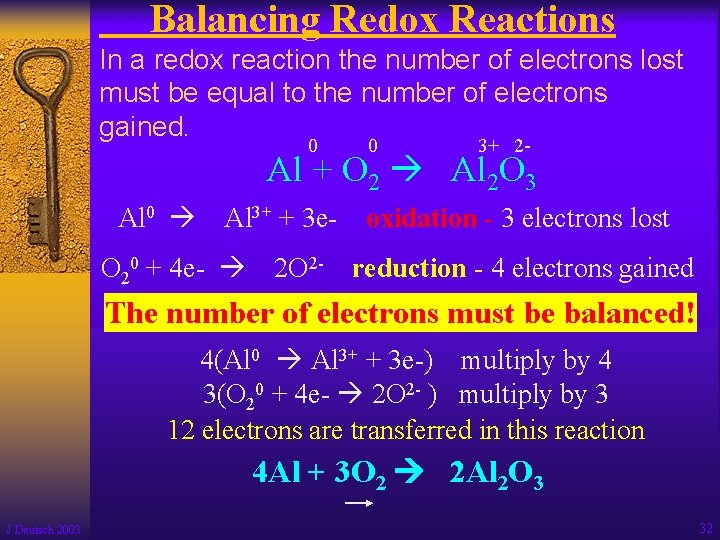
Balancing Redox Reactions In a redox reaction the number of electrons lost must be equal to the number of electrons gained. 0 0 3+ 2 - Al + O 2 Al 2 O 3 Al 0 Al 3+ + 3 e- O 20 + 4 e- 2 O 2 - oxidation - 3 electrons lost reduction - 4 electrons gained The number of electrons must be balanced! 4(Al 0 Al 3+ + 3 e-) multiply by 4 3(O 20 + 4 e- 2 O 2 - ) multiply by 3 12 electrons are transferred in this reaction 4 Al + 3 O 2 2 Al 2 O 3 J Deutsch 2003 32

Writing Half-Reactions ¨ To write the half-reactions for an equation such as ¨ ¨ Mg. Cl 2 + 2 Na. Cl + Mg Make sure the equation is balanced Next assign oxidation numbers to each atom, then write a partial half -reaction to show the change in oxidation number including the coefficients from the balanced equation. Oxidation: 2 Na 0 2 Na+1 Reduction Mg+2 Mg 0 Then place the correct number of electrons on one side of the equation to make the net charge equal on both sides. (remember electrons will always be a product in oxidation and a reactant in reduction) Oxidation: 2 Na 0 2 Na+1 + 2 e. Reduction: 2 e- + Mg+2 Mg 0 When you have written a correct oxidation and reduction half-reaction the electrons lost (oxidation) should be equal to the electrons gained (reduction)

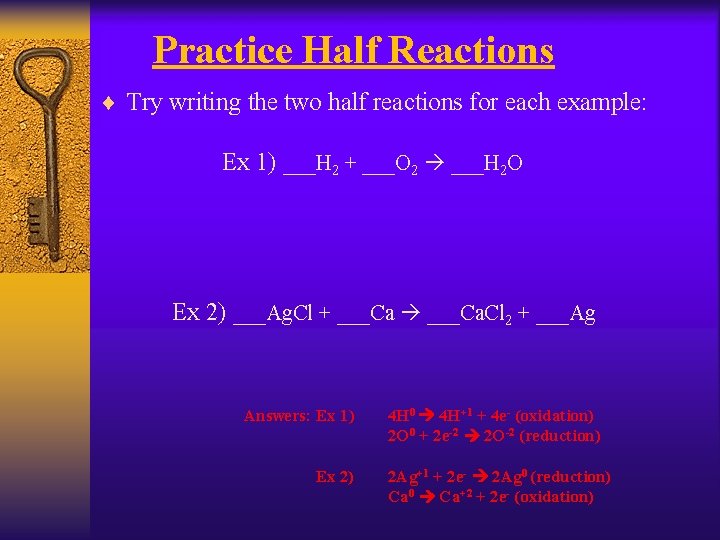
Practice Half Reactions ¨ Try writing the two half reactions for each example: Ex 1) ___H 2 + ___O 2 ___H 2 O Ex 2) ___Ag. Cl + ___Ca. Cl 2 + ___Ag Answers: Ex 1) Ex 2) 4 H 0 4 H+1 + 4 e- (oxidation) 2 O 0 + 2 e-2 2 O-2 (reduction) 2 Ag+1 + 2 e- 2 Ag 0 (reduction) Ca 0 Ca+2 + 2 e- (oxidation)

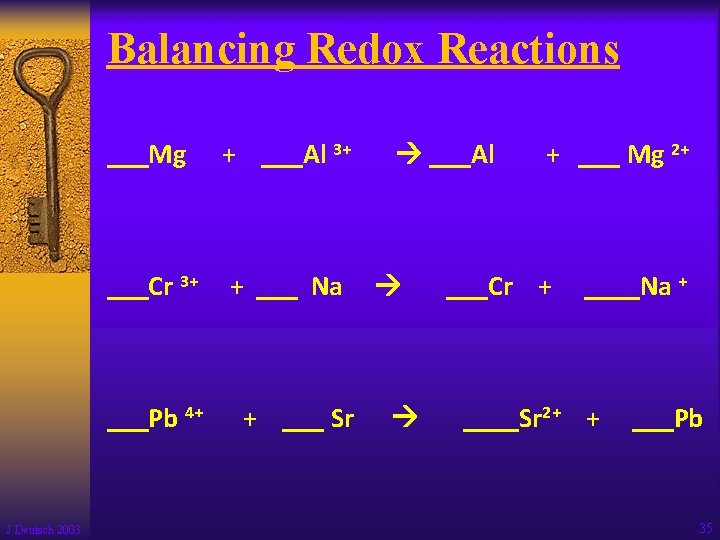
Balancing Redox Reactions ___Mg + ___Al 3+ ___Cr 3+ + ___ Na ___Pb 4+ J Deutsch 2003 + ___ Sr ___Al + ___ Mg 2+ ___Cr + ____Na + ____Sr 2+ + ___Pb 35

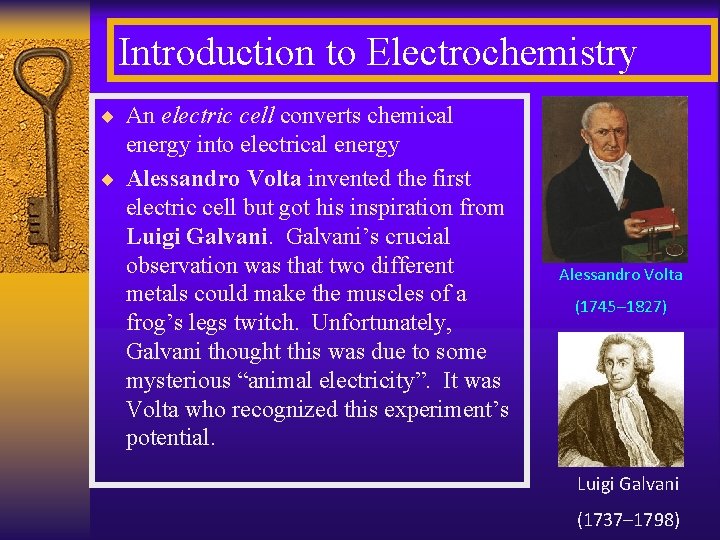
Introduction to Electrochemistry ¨ An electric cell converts chemical energy into electrical energy ¨ Alessandro Volta invented the first electric cell but got his inspiration from Luigi Galvani’s crucial observation was that two different metals could make the muscles of a frog’s legs twitch. Unfortunately, Galvani thought this was due to some mysterious “animal electricity”. It was Volta who recognized this experiment’s potential. Alessandro Volta (1745– 1827) Luigi Galvani (1737– 1798)

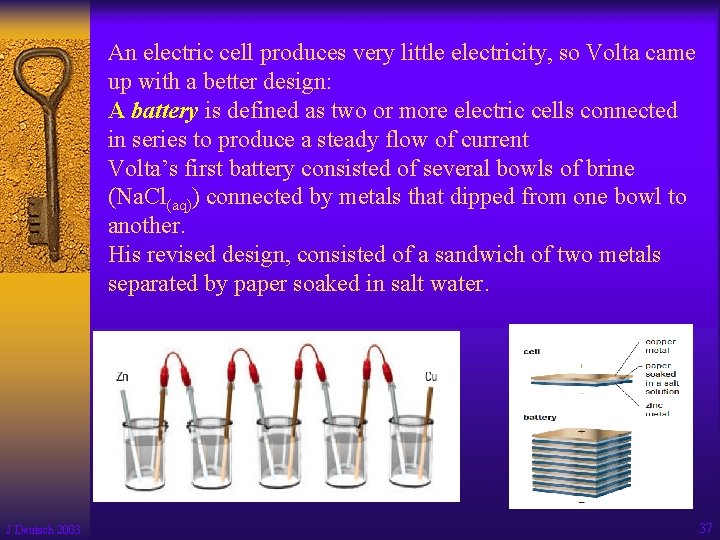
An electric cell produces very little electricity, so Volta came up with a better design: A battery is defined as two or more electric cells connected in series to produce a steady flow of current Volta’s first battery consisted of several bowls of brine (Na. Cl(aq)) connected by metals that dipped from one bowl to another. His revised design, consisted of a sandwich of two metals separated by paper soaked in salt water. J Deutsch 2003 37

An electrochemical cell can be either a voltaic or electrolytic cell Voltaic Cell/ Galvanic Cell: A Device that generates electric current from a spontaneous electrochemical reaction. Electrolytic Cell: A device that uses erxternal power source to make a non-spontaneous reaction happen. J Deutsch 2003 38

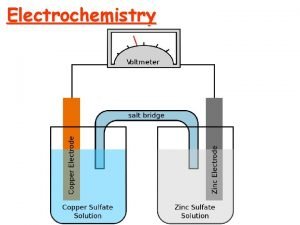
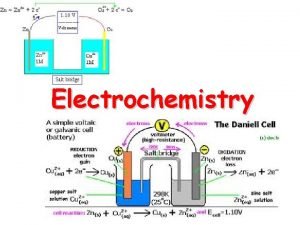
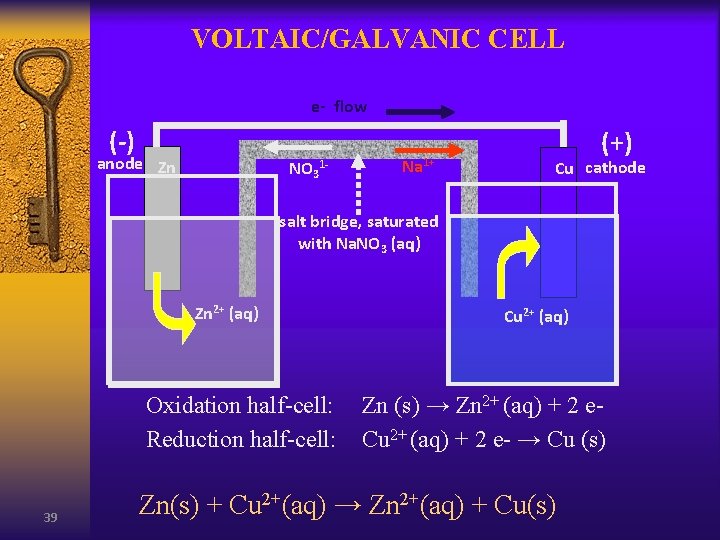
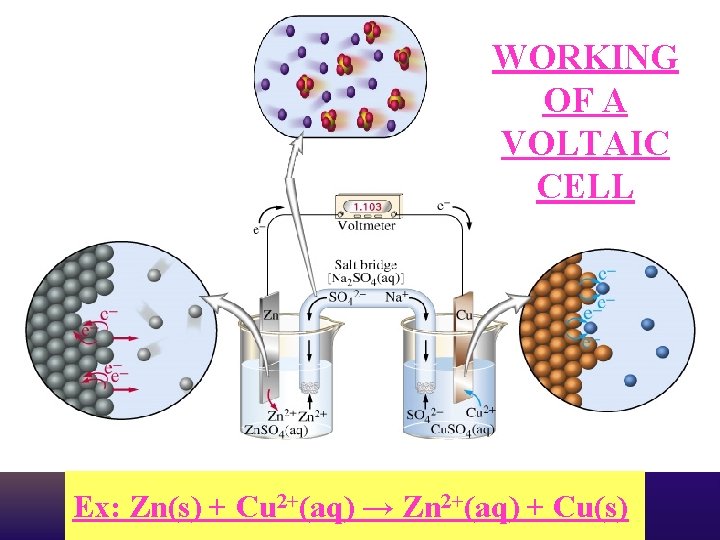
VOLTAIC/GALVANIC CELL e- flow (-) anode Zn NO 31 - Na 1+ (+) Cu cathode salt bridge, saturated with Na. NO 3 (aq) Zn 2+ (aq) Oxidation half-cell: Reduction half-cell: 39 Cu 2+ (aq) Zn (s) → Zn 2+ (aq) + 2 e. Cu 2+ (aq) + 2 e- → Cu (s) Zn(s) + Cu 2+(aq) → Zn 2+(aq) + Cu(s)

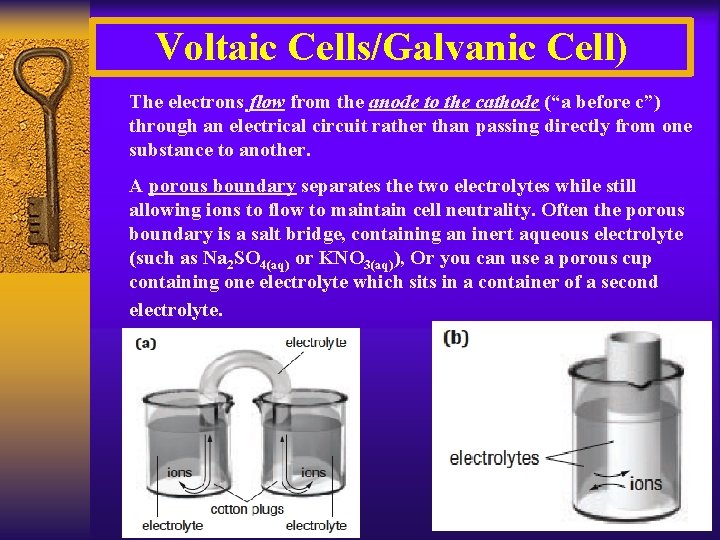
Voltaic Cells/Galvanic Cell) The electrons flow from the anode to the cathode (“a before c”) through an electrical circuit rather than passing directly from one substance to another. A porous boundary separates the two electrolytes while still allowing ions to flow to maintain cell neutrality. Often the porous boundary is a salt bridge, containing an inert aqueous electrolyte (such as Na 2 SO 4(aq) or KNO 3(aq)), Or you can use a porous cup containing one electrolyte which sits in a container of a second electrolyte.

VOLTAIC/GALVANIC CELL Electrodes: metals connected by an external circuit to allow the flow ofv electrons Anode: Electrode where oxidation occurs Cathode: Electrode where reduction occurs An Ox Red Cat Oxidation at the Anode Reduction at the Cathode 41

WORKING OF A VOLTAIC CELL Ex: Zn(s) + Cu 2+(aq) → Zn 2+(aq) + Cu(s)

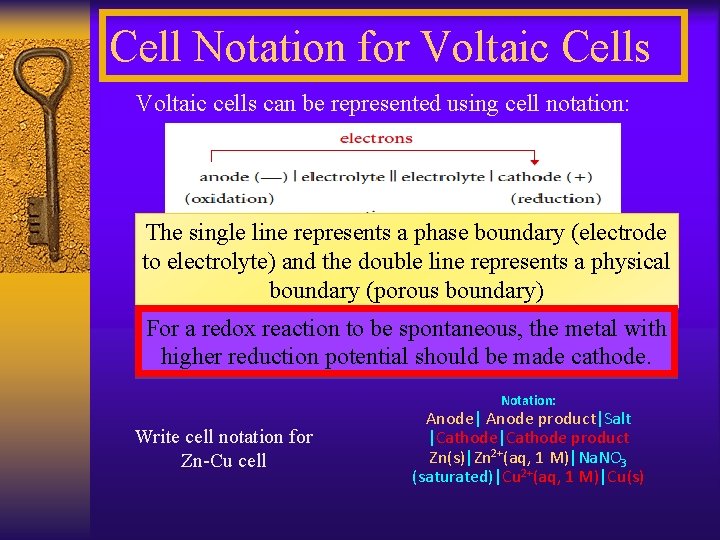
Cell Notation for Voltaic Cells Voltaic cells can be represented using cell notation: The single line represents a phase boundary (electrode to electrolyte) and the double line represents a physical boundary (porous boundary) For a redox reaction to be spontaneous, the metal with higher reduction potential should be made cathode. Notation: Write cell notation for Zn-Cu cell Anode| Anode product|Salt |Cathode product Zn(s)|Zn 2+(aq, 1 M)|Na. NO 3 (saturated)|Cu 2+(aq, 1 M)|Cu(s)

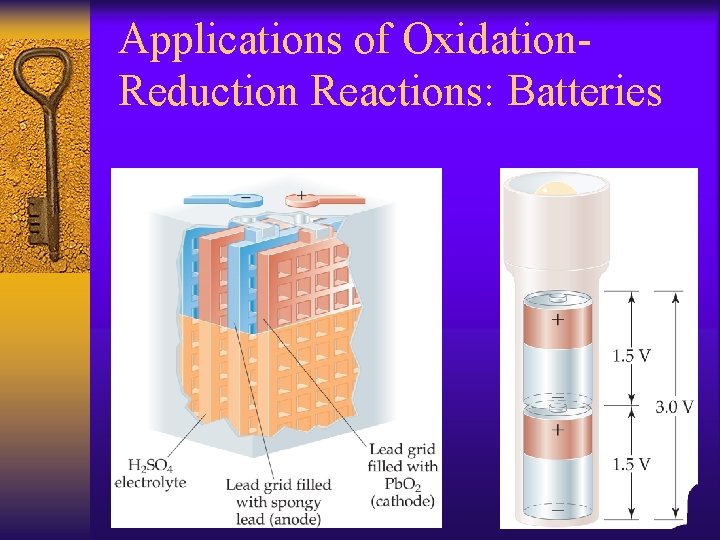
A voltaic cell spontaneously converts chemical energy to electrical energy. Batteries are voltaic cells J Deutsch 2003 44

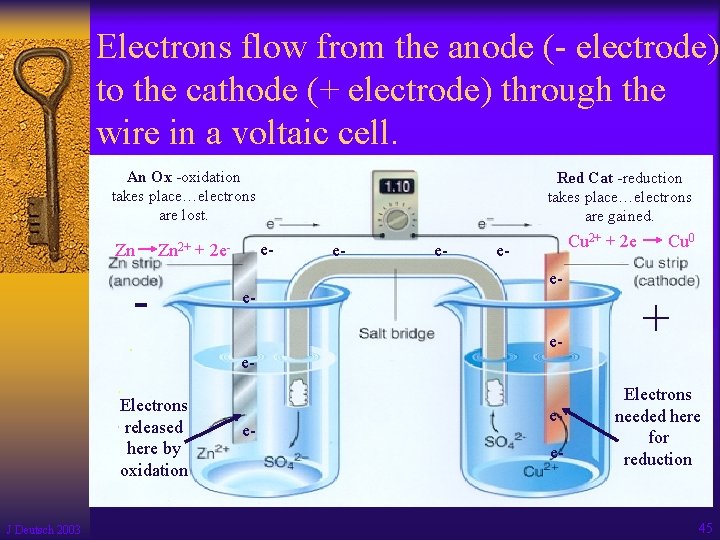
Electrons flow from the anode (- electrode) to the cathode (+ electrode) through the wire in a voltaic cell. An Ox -oxidation takes place…electrons are lost. Zn e- Zn 2+ + 2 e- - Red Cat -reduction takes place…electrons are gained. e- e- e- Cu 2+ + 2 e e- - Cu 0 ee- + e. Electrons released here by oxidation J Deutsch 2003 e- ee- Electrons needed here for reduction 45

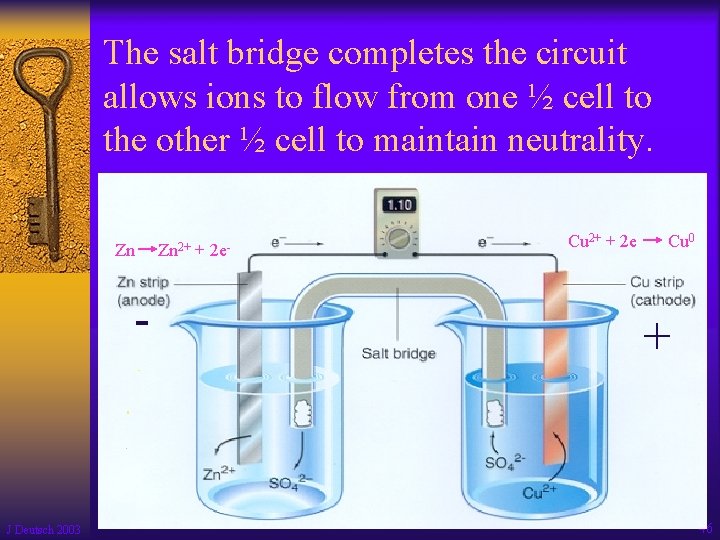
The salt bridge completes the circuit allows ions to flow from one ½ cell to the other ½ cell to maintain neutrality. Zn - J Deutsch 2003 Zn 2+ + 2 e- Cu 2+ + 2 e - Cu 0 + 46

Voltaic Cells & Table J ¨ Oxidation and reduction occur at the electrodes. ¨ To identify which electrode is your anode or the site of oxidation and which is your cathode or the sight of reduction you must compare the reactivity of the metals on Table J in your reference table. ¨ The more reactive metal will always lose electrons (oxidation) which makes it your Anode. ¨ The less reactive metal will always gain electrons (reduction) it is always your cathode. ¨ Remember “Red Cat An Ox”

Table J ¨ So in summary the metal higher up on Table J is your Anode (oxidation) the metal lower on Table J is your cathode (reduction). ¨ Once you identify your anode and cathode you can identify the flow of electrons they flow from the Anode to the Cathode. ¨ Try the practice questions on the next page !!!

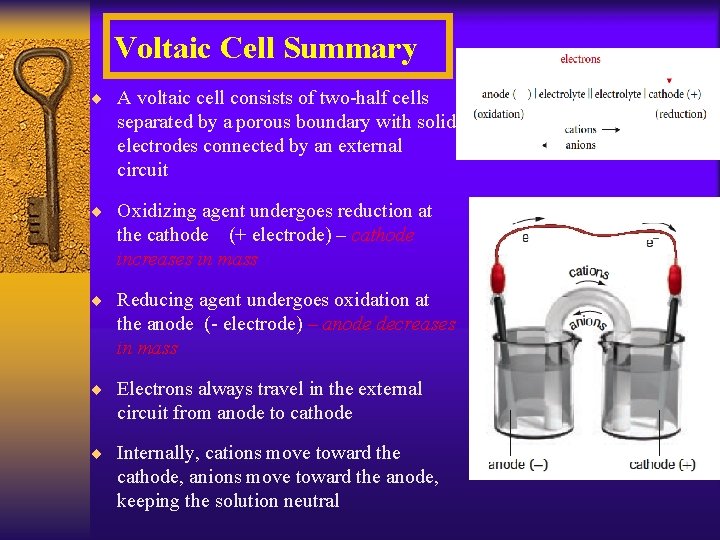
Voltaic Cell Summary ¨ A voltaic cell consists of two-half cells separated by a porous boundary with solid electrodes connected by an external circuit ¨ Oxidizing agent undergoes reduction at the cathode (+ electrode) – cathode increases in mass ¨ Reducing agent undergoes oxidation at the anode (- electrode) – anode decreases in mass ¨ Electrons always travel in the external circuit from anode to cathode ¨ Internally, cations move toward the cathode, anions move toward the anode, keeping the solution neutral

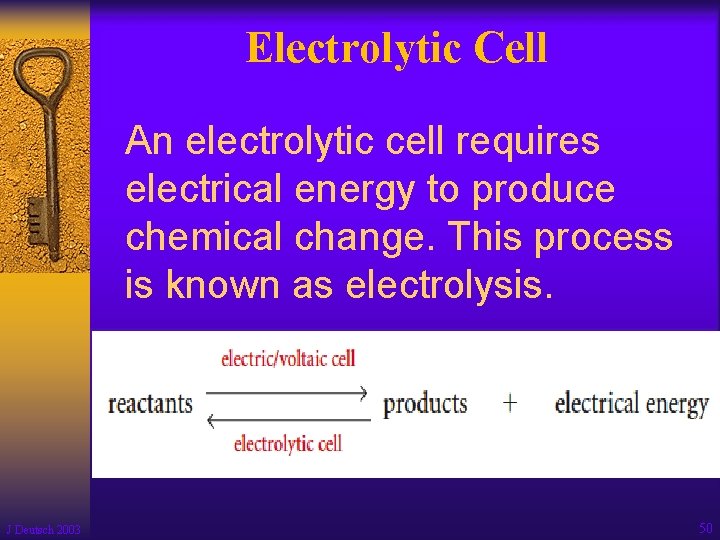
Electrolytic Cell An electrolytic cell requires electrical energy to produce chemical change. This process is known as electrolysis. J Deutsch 2003 50

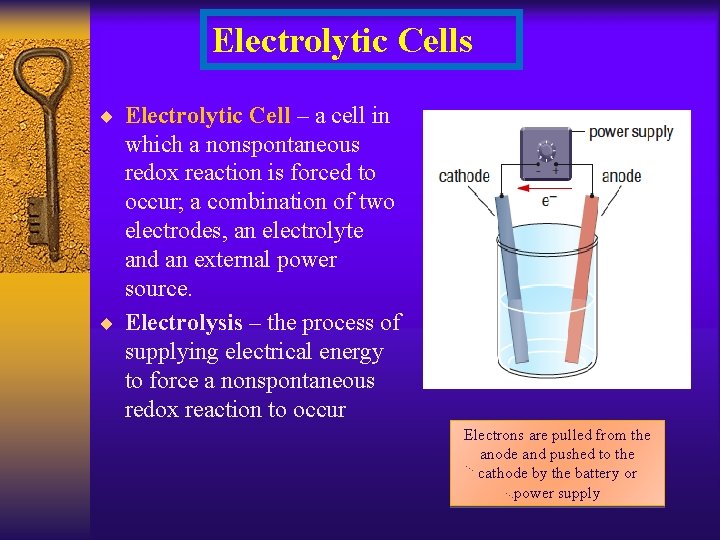
Electrolytic Cells ¨ Electrolytic Cell – a cell in which a nonspontaneous redox reaction is forced to occur; a combination of two electrodes, an electrolyte and an external power source. ¨ Electrolysis – the process of supplying electrical energy to force a nonspontaneous redox reaction to occur Electrons are pulled from the anode and pushed to the cathode by the battery or power supply

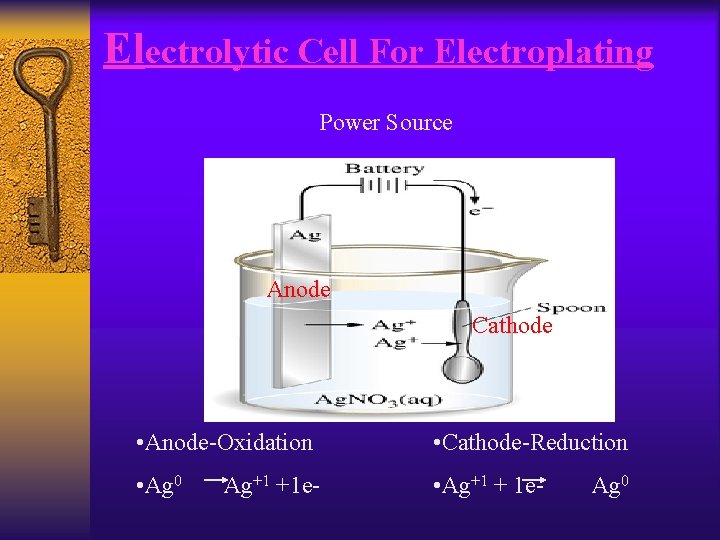
Electroplating ¨ The most common use of electrolytic cells is an electroplating cell. ¨ Electroplating involves plating a small layer of usually a precious metal on another metal. ¨ The material to be plated for example a spoon or a piece of jewelry, would be the cathode were reduction occurs. ¨ The metal being used to plate for example gold or silver would be the anode were oxidation occurs.

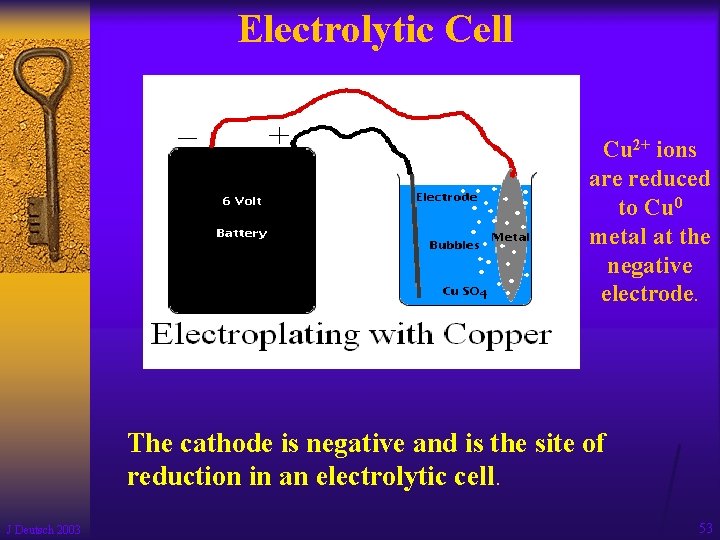
Electrolytic Cell Cu 2+ ions are reduced to Cu 0 metal at the negative electrode. The cathode is negative and is the site of reduction in an electrolytic cell. J Deutsch 2003 53

Electrolytic Cell For Electroplating Power Source Anode Cathode • Anode-Oxidation • Cathode-Reduction • Ag 0 • Ag+1 + 1 e- Ag+1 +1 e- Ag 0

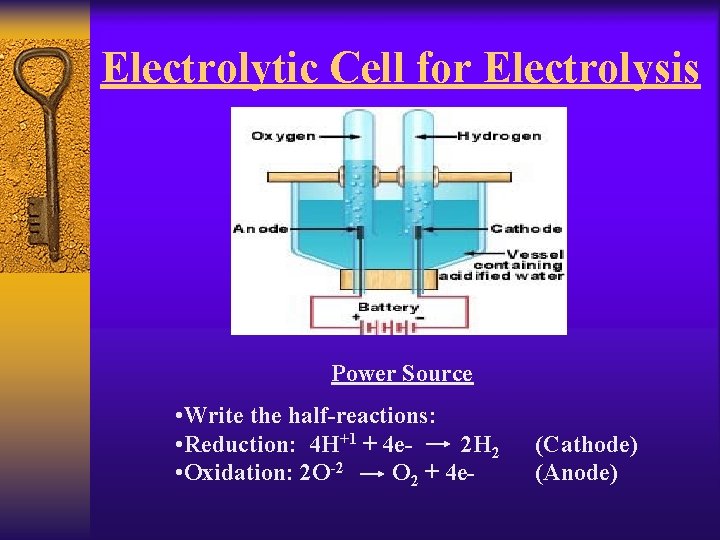
Electrolysis ¨ Electrolysis can be used to break up compounds to reform the elements it is made up of. ¨ For instance if you wanted to break up water molecules into hydrogen and oxygen gas you could use an Electrolytic Cell. ¨ Since the reaction will be non-spontaneous a power source must be used to force the reaction to occur. ¨ The power source is usually a battery. 2 H 2 O + electrical energy 2 H 2 + O 2

Electrolytic Cell for Electrolysis Power Source • Write the half-reactions: • Reduction: 4 H+1 + 4 e 2 H 2 • Oxidation: 2 O-2 O 2 + 4 e- (Cathode) (Anode)

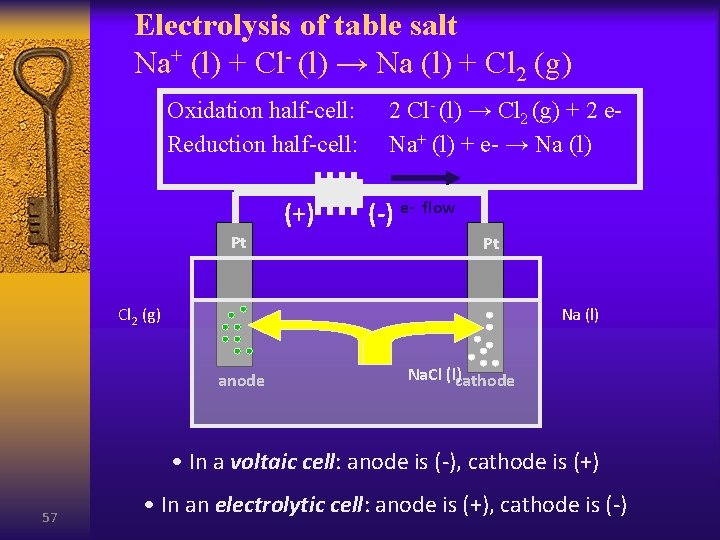
Electrolysis of table salt Na+ (l) + Cl- (l) → Na (l) + Cl 2 (g) Oxidation half-cell: Reduction half-cell: (+) Pt 2 Cl- (l) → Cl 2 (g) + 2 e. Na+ (l) + e- → Na (l) (-) e- flow Pt Cl 2 (g) Na (l) anode Na. Cl (l)cathode • In a voltaic cell: anode is (-), cathode is (+) 57 • In an electrolytic cell: anode is (+), cathode is (-)

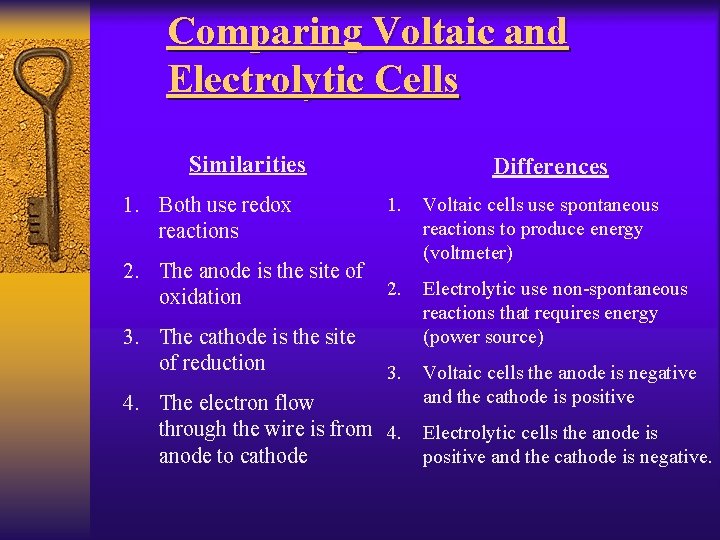
Comparing Voltaic and Electrolytic Cells Similarities 1. Both use redox reactions Differences 1. 2. The anode is the site of oxidation Voltaic cells use spontaneous reactions to produce energy (voltmeter) 2. 3. The cathode is the site of reduction Electrolytic use non-spontaneous reactions that requires energy (power source) 3. Voltaic cells the anode is negative and the cathode is positive 4. The electron flow through the wire is from 4. Electrolytic cells the anode is anode to cathode positive and the cathode is negative.

Applications of Oxidation. Reduction Reactions: Batteries

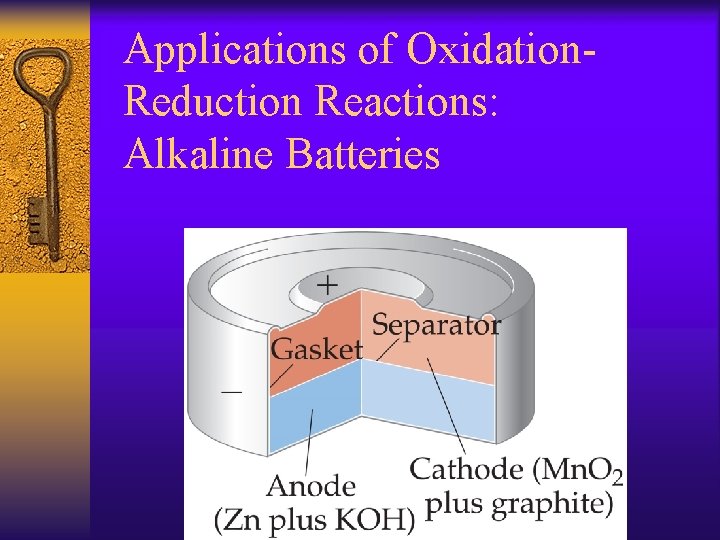
Applications of Oxidation. Reduction Reactions: Alkaline Batteries

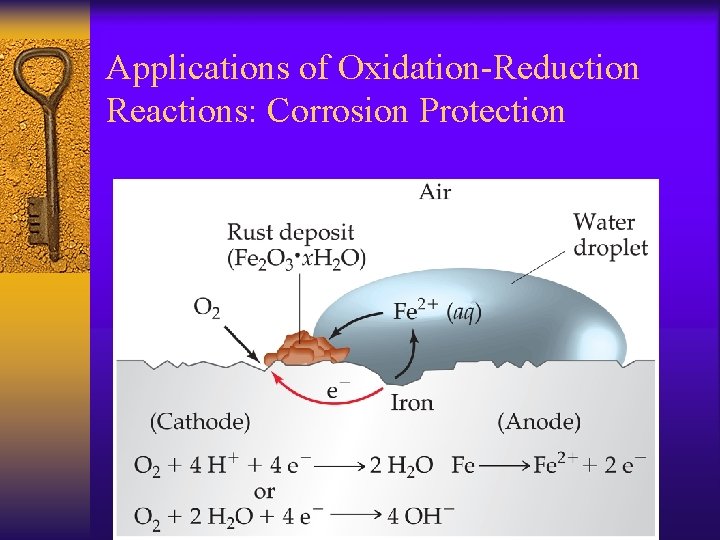
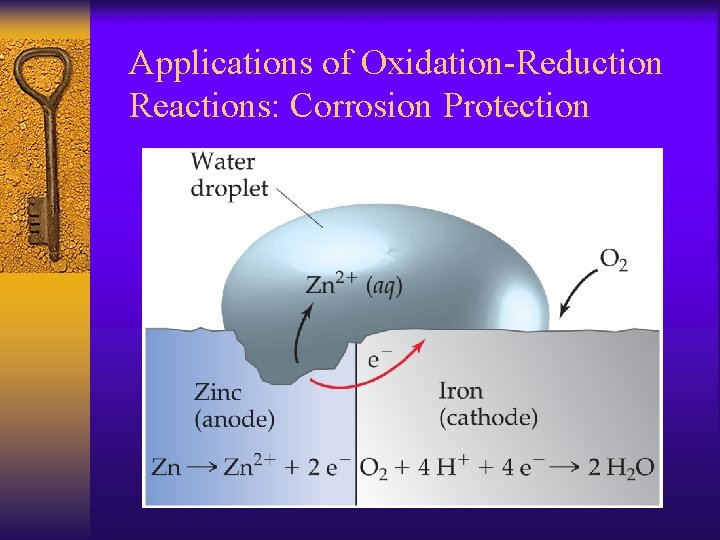
Applications of Oxidation-Reduction Reactions: Corrosion Protection

Applications of Oxidation-Reduction Reactions: Corrosion Protection

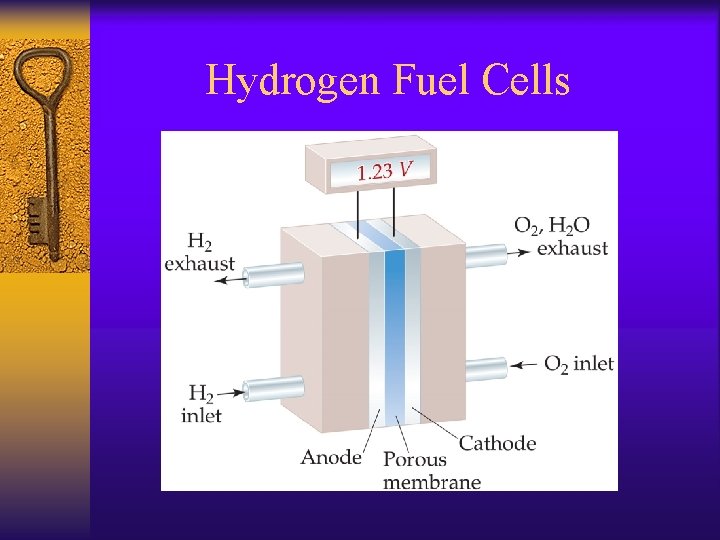
Hydrogen Fuel Cells

Regents Question: 06/02 #22 Which process requires an external power source? (1) neutralization (2) synthesis (3) fermentation (4) electrolysis J Deutsch 2003 64

Regents Question: 08/02 #29 As a Ca atom undergoes oxidation to Ca 2+ , the number of neutrons in its nucleus (1) decreases (2) increases (3) remains the same J Deutsch 2003 65

Regents Question: 06/02 #20 Which particles are gained and lost during a redox reaction? (1) electrons (2) Protons (3) Neutrons (4) positrons J Deutsch 2003 66

Regents Question: 06/03 #27 Which statement is true for any electrochemical cell? (1) Oxidation occurs at the anode, only. (2) Reduction occurs at the anode, only. (3) Oxidation occurs at both the anode and the cathode. (4) Reduction occurs at both the anode and the cathode. J Deutsch 2003 67

Regents Question: 06/03 #26 In which substance does chlorine have an oxidation number of +1? (1) Cl 2 (2) HCl (3) HCl. O (4) HCl. O 2 J Deutsch 2003 68

Regents Question: 01/03 #46 According to Reference Table J, which of these metals will react most readily with 1. 0 M HCl to produce H 2(g)? (1) Ca (2) K (3) Mg (4) Zn J Deutsch 2003 69

Regents Question: 08/02 #22 In any redox reaction, the substance that undergoes reduction will (1) lose electrons and have a decrease in oxidation number (2) lose electrons and have an increase in oxidation number (3) gain electrons and have a decrease in oxidation number (4) gain electrons and have an increase in oxidation number J Deutsch 2003 70

Regents Question: 06/02 #21 What is the oxidation number of chromium in K 2 Cr 2 O 7 ? (1) +12 (2) +2 (3) +3 (4) +6 J Deutsch 2003 71

Regents Question: 06/02 #18 Given the equation: C(s) + H 2 O(g) CO(g) + H 2 (g) Which species undergoes reduction? (1) C(s) (2) H + LEO growls GER (3) C 2+ (4) H 2 (g) J Deutsch 2003 72

Regents Question: 06/02 #18 Given the reaction: Mg(s) + 2 H+(aq) + 2 Cl–(aq) Mg 2+(aq) + 2 Cl–(aq) + H 2(g) Which species undergoes oxidation? (1) Mg(s) (2) H+(aq) (3) Cl– (aq) LEO growls GER (4) H 2 (g) J Deutsch 2003 73

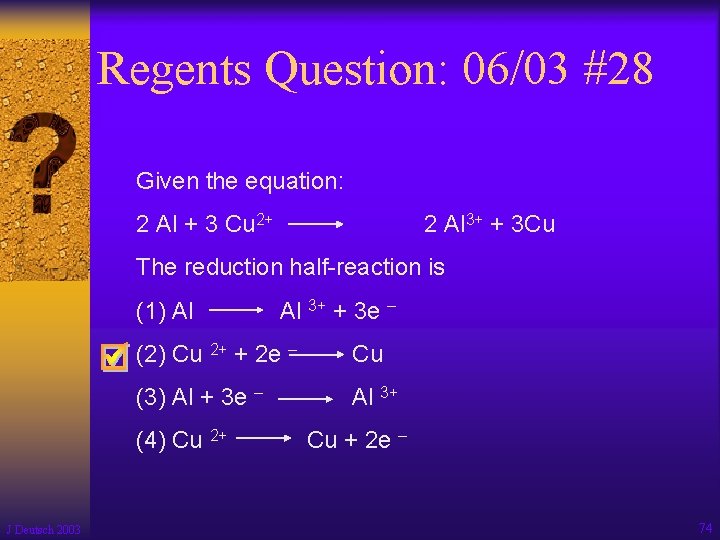
Regents Question: 06/03 #28 Given the equation: 2 Al + 3 Cu 2+ 2 Al 3+ + 3 Cu The reduction half-reaction is (1) Al (2) Cu 2+ + 2 e – Cu (3) Al + 3 e – Al 3+ (4) Cu 2+ J Deutsch 2003 Al 3+ + 3 e – Cu + 2 e – 74

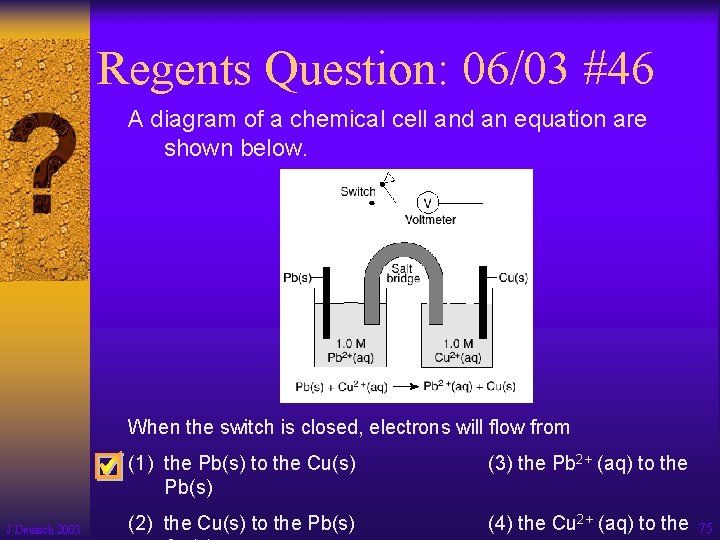
Regents Question: 06/03 #46 A diagram of a chemical cell and an equation are shown below. When the switch is closed, electrons will flow from J Deutsch 2003 (1) the Pb(s) to the Cu(s) Pb(s) (3) the Pb 2+ (aq) to the (2) the Cu(s) to the Pb(s) (4) the Cu 2+ (aq) to the 75

Regents Question: 06/02 #70 -75 Base your answers to the next six questions on the following redox reaction, which occurs spontaneously in an electrochemical cell. Zn + Cr 3+ § Write the half-reaction for the reduction that occurs. Cr 3+ + 3 e- § Cr 0 Write the half-reaction for the oxidation that occurs. Zn 0 § Zn 2+ + Cr Zn 2+ + 2 e- Balance the equation using the smallest wholenumber coefficients. The number of e-s gained = the number of e-s lost 3 Zn + 2 Cr 3+ 3 Zn 2+ + 2 Cr J Deutsch 2003 76

Regents Question: 06/02 #70 -75 Base your answers to the next five questions on the following redox reaction, which occurs spontaneously in an electrochemical cell. Zn + Cr 3+ Zn 2+ + Cr § Which species loses electrons and which species gains electrons? Zn 0 loses electrons, Cr 3+ gains electrons § Which half-reaction occurs at the cathode? Cr 3+ § + 3 e- Cr 0 Hint: Red Cat State what happens to the number of protons in a Zn atom when it changes to Zn 2+ as the redox reaction occurs. The number of protons remains the same. J Deutsch 2003 77

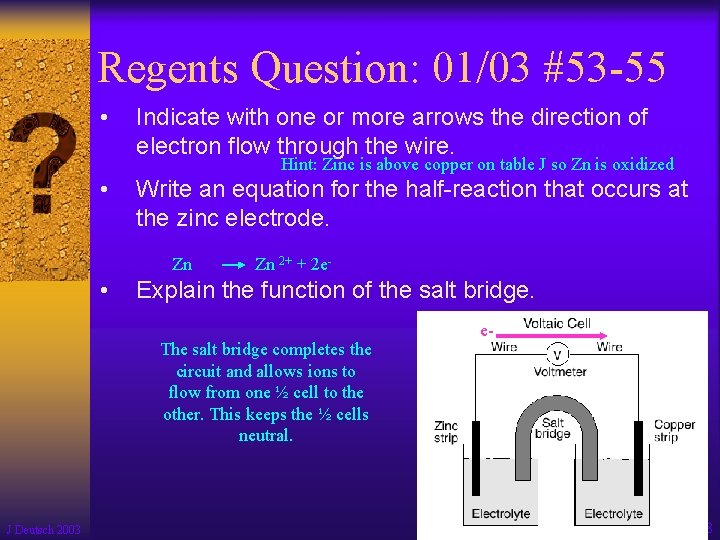
Regents Question: 01/03 #53 -55 • Indicate with one or more arrows the direction of electron flow through the wire. Hint: Zinc is above copper on table J so Zn is oxidized • Write an equation for the half-reaction that occurs at the zinc electrode. Zn • Zn 2+ + 2 e- Explain the function of the salt bridge. The salt bridge completes the circuit and allows ions to flow from one ½ cell to the other. This keeps the ½ cells neutral. J Deutsch 2003 e- 78
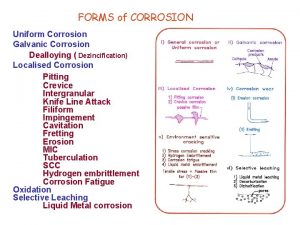
 Differentiate between dry corrosion and wet corrosion
Differentiate between dry corrosion and wet corrosion Dry corrosion and wet corrosion
Dry corrosion and wet corrosion Water and water and water water
Water and water and water water Myasthenic vs cholinergic crisis
Myasthenic vs cholinergic crisis Austin flint murmur
Austin flint murmur Ast levels and exercise
Ast levels and exercise Murmurs made easy
Murmurs made easy Austin flint murmur
Austin flint murmur Chapter 13 flint
Chapter 13 flint Flint health centre
Flint health centre Hardened piece of steel
Hardened piece of steel It consulting flint
It consulting flint Printable rfid tags cost
Printable rfid tags cost The basic building block of the silicate minerals
The basic building block of the silicate minerals Richard flint england golf
Richard flint england golf Flint hills regional council
Flint hills regional council White flint sector plan
White flint sector plan Um flint rec center
Um flint rec center Tlc pediatrics flint
Tlc pediatrics flint Rotary club assembly agenda
Rotary club assembly agenda Who is your favourite heroine
Who is your favourite heroine Pmi flint
Pmi flint Austin flint üfürümü
Austin flint üfürümü Basic chemistry tutorial
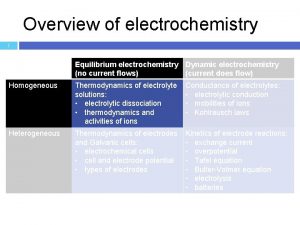
Basic chemistry tutorial Hittorf law
Hittorf law Junction potential
Junction potential Ap chemistry chapter 18 electrochemistry test
Ap chemistry chapter 18 electrochemistry test Electrochemistry equations
Electrochemistry equations What is electrochemical
What is electrochemical Electrochemistry balancing equations
Electrochemistry balancing equations Electrochemistry stoichiometry
Electrochemistry stoichiometry Intro to electrochemistry
Intro to electrochemistry Nernst equation khan academy
Nernst equation khan academy Aee cd20f
Aee cd20f Electrochemistry balancing equations
Electrochemistry balancing equations Chapter 20 review electrochemistry
Chapter 20 review electrochemistry Electrochemistry ap chem
Electrochemistry ap chem Redox reaction
Redox reaction Chapter 21 electrochemistry
Chapter 21 electrochemistry E cell formula
E cell formula Electroanalytical techniques
Electroanalytical techniques Electrochemistry eds
Electrochemistry eds Fundamentals of electrochemistry
Fundamentals of electrochemistry What is electrochemistry
What is electrochemistry What is electrochemistry in chemistry
What is electrochemistry in chemistry Cell notation
Cell notation Diagonal rule electrochemistry
Diagonal rule electrochemistry Polarization in electrochemistry
Polarization in electrochemistry Electrochemistry lesson plan
Electrochemistry lesson plan Slidesdgo
Slidesdgo Oxidation reduction memory tricks
Oxidation reduction memory tricks Mass transport electrochemistry
Mass transport electrochemistry Electroanalytical chemistry
Electroanalytical chemistry Fundamentals of electrochemistry
Fundamentals of electrochemistry What is electrochemistry
What is electrochemistry Diagonal rule electrochemistry
Diagonal rule electrochemistry Coupon technology
Coupon technology Electrochemistry balancing equations
Electrochemistry balancing equations Analytical electrochemistry
Analytical electrochemistry Red cat electrochemistry
Red cat electrochemistry The cell reaction for the zn-h2 cell is
The cell reaction for the zn-h2 cell is Koh alkaline
Koh alkaline Electrochemistry
Electrochemistry Electrolysis vs voltaic cell
Electrolysis vs voltaic cell Chapter 20 electrochemistry
Chapter 20 electrochemistry Prevention of corrosion
Prevention of corrosion Uniform corrosion mechanism
Uniform corrosion mechanism Trenton corrosion protection
Trenton corrosion protection Corrosion under insulation primer
Corrosion under insulation primer Aluminium corrosion equation
Aluminium corrosion equation Skin corrosion symbol
Skin corrosion symbol Stress corrosion
Stress corrosion Filiform corrosion
Filiform corrosion Waterline corrosion
Waterline corrosion Galvanic corrosion
Galvanic corrosion Sulfidation corrosion
Sulfidation corrosion Oxidative corrosion
Oxidative corrosion Corrosion intercept
Corrosion intercept Corrosion coupon rack installation
Corrosion coupon rack installation