Corrosion pp 412 415 What is Corrosion Corrosion









- Slides: 13

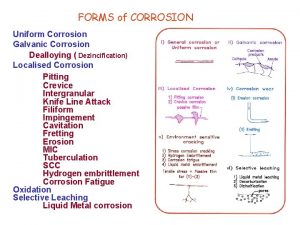
Corrosion pp. 412 -415

What is Corrosion? Corrosion is the break-down of metals as a result of OXIDATION.

Disadvantages of Corrosion Advantages of Corrosion

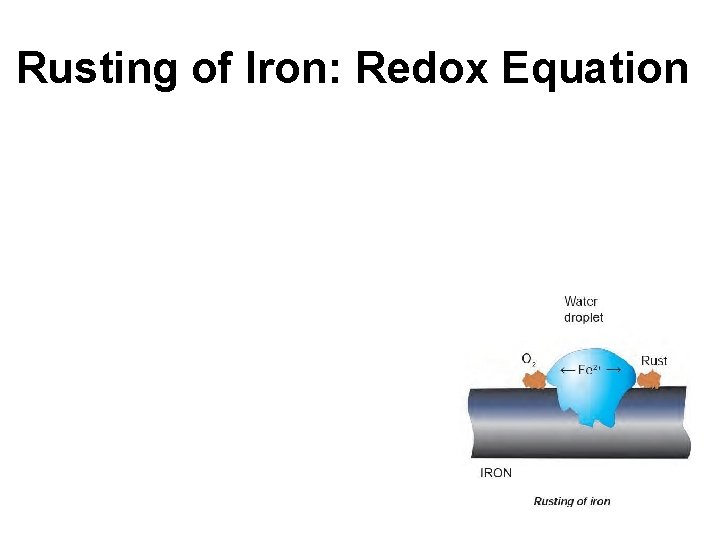
Rusting of Iron • Redox reaction • Involves Fe (s), O 2 (g) and H 2 O (l). The water comes from the moisture in the air. • Corrosion is in the form of rust, which is a mix of oxides and hydroxides of iron. It’s red and flakes off easily allows for further corrosion.

Rusting of Iron: Redox Equation

Factors that Affect the Rate of Corrosion 1. Moisture (presence of water) – The presence of water speeds up the corrosion rate – Corrosion is nearly impossible without water – Metals in desert climates last longer than those in temperate climates (where humidity is higher)

2. Electrolytes – Salts (eg. Na. Cl) are electrolytes that do NOT cause corrosion, BUT help speed up the process – When mixed with water (eg. snow, rain), the negative Cl- ion pulls away the positive Fe 2+ ions from the surface of the metal. This removes iron. – Road salt is bad for cars – Cars within reach of the ocean also suffer from the salt-water spray in the air

3. Contact with Less Reactive Metals – Corrosion can happen when 2 different metals come into contact (eg. Copper and steel, copper and iron) – Metals differ in their reactivity (pg. 386), some are more reactive than others – To prevent this, use the same metal (copper pipes and copper nails)

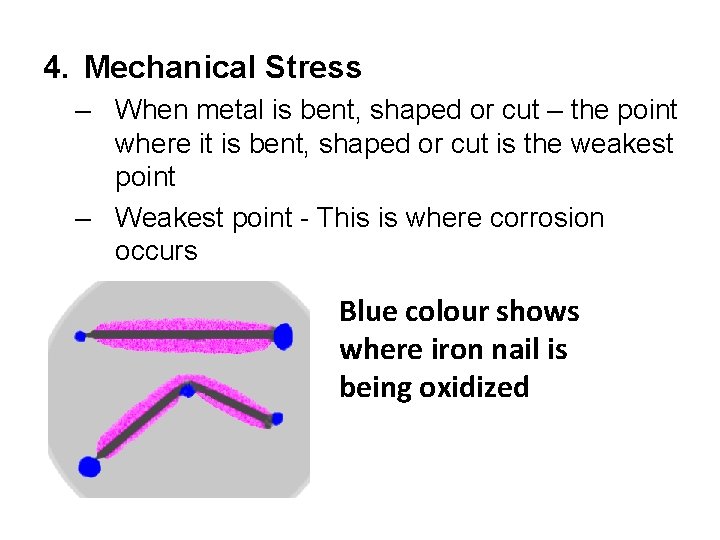
4. Mechanical Stress – When metal is bent, shaped or cut – the point where it is bent, shaped or cut is the weakest point – Weakest point - This is where corrosion occurs Blue colour shows where iron nail is being oxidized

Preventing Corrosion • Corrosion can be costly, especially within the trade industries (eg. metals, automobiles, etc). • There are three methods to prevent the onset of corrosion, some more effective than others

1. Protective Coatings • Simplest method • Cover the metal with: – Rust-preventative paint – Tape – Plastic • Problem: If chips or scratches occur, then corrosion may start to set in

2. Corrosion-Resistant Metals • Use metals that are NOT susceptible to corrosion – Stainless steel (mix of iron, chromium, tin) – Aluminum • Recent models of cars feature these metals, as well as essential items (eg. Sinks, artificial hips, etc) • Problem: Can be costly.

3. Cathodic Protection • Using a layer of metal to protect the layer of a similar or different metal underneath – To cover iron or steel, you must use a metal more reactive than iron (such as zinc) becomes galvanized. • Problem: May not last as long. Once the metal layer is corroded, you must replace it.