CORROSION THEORY What Is Corrosion Why Does Corrosion







- Slides: 13

CORROSION THEORY What Is Corrosion? Why Does Corrosion Happen? How Does Corrosion Happen? What are some different types?

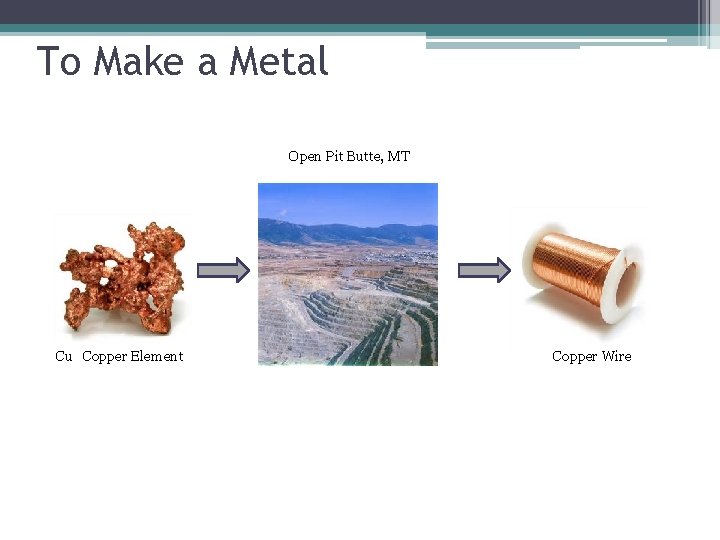
To Make a Metal Open Pit Butte, MT Cu Copper Element Copper Wire

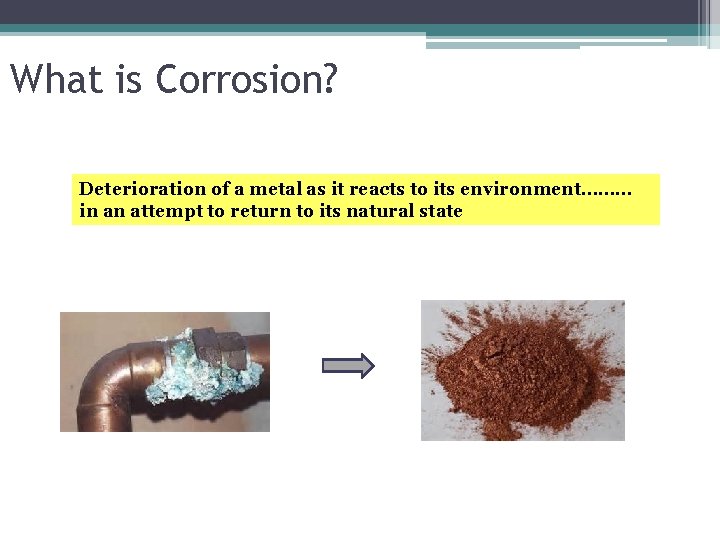
What is Corrosion? Deterioration of a metal as it reacts to its environment……… in an attempt to return to its natural state

Why Corrosion Happens Most Metals Not Stable in Normal Environments They Want to Revert to a More Stable Condition

Terms Chemical Properties: The way matter behaves when it is placed in contact with other kinds of matter Physical Properties: Color, Odor, Taste, Density, Freezing Corrosion Cell: Anode + Cathode + Metal Path + Electrolyte Oxidation & Reduction: § 1 Substance is being oxidized = Another substance is being reduced § Happens Simultaneously

How Do Metals Corrode? Direct Chemical Attack or Chemical Change + Production of Electrical Energy Electrochemical Reaction

Direct Chemical Attack Pitting Corrosion Uniform Surface Corrosion § § Salts Deposited From Coastal Waters Urine Spray Battery Acid Spillage Gases Absorbed From Environment § Localized Attacks

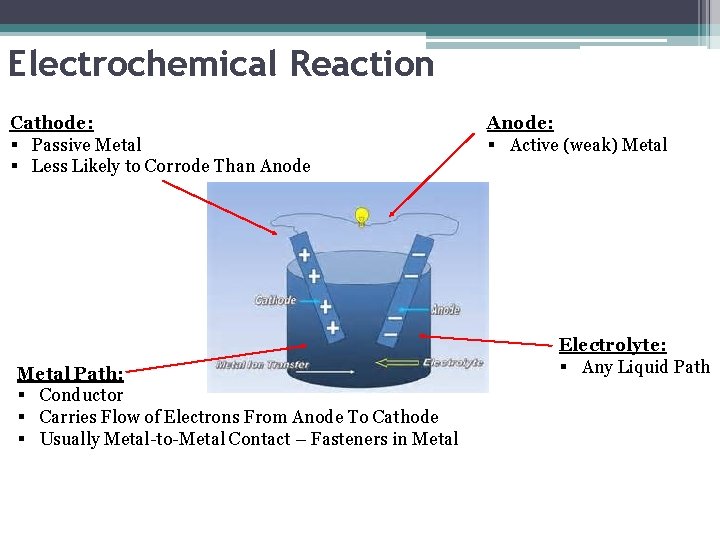
Electrochemical Reaction Cathode: § Passive Metal § Less Likely to Corrode Than Anode Metal Path: § Conductor § Carries Flow of Electrons From Anode To Cathode § Usually Metal-to-Metal Contact – Fasteners in Metal Anode: § Active (weak) Metal Electrolyte: § Any Liquid Path

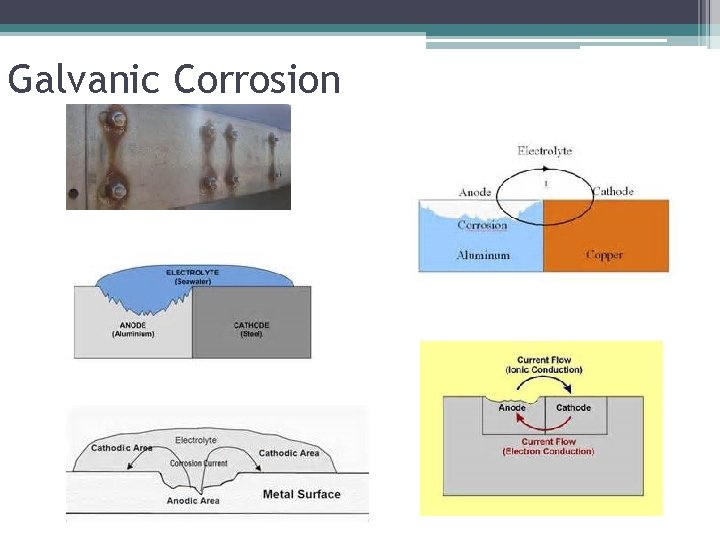
Galvanic Corrosion

Development Ø All Corrosive Attacks Start on Surface § Uniform Surface Corrosion § Pitting § Galvanic § Protect Surface or Remove 1 Condition = Stop Corrosion Ø Penetrates Into Granular Structure § Results in Extensive Surface & Structural Damage § Intergranular Corrosion § Exfoliation § Stress Cracking § Fatigue

Destructive Corrosion Exfoliation Stress Cracking Fatigue Failure

Corrosion Video Corrosion & Rust <iframe width="420" height="315" src="//www. youtube. com/embed/XMr 4 vse 7 Ybo" frameborder="0" allowfullscreen></iframe>

Corrosion Review Why Does Metal Corrode? What is Corrosion? How Does Metal Corrode? What are Some Different Kinds of Corrosion?