Engineering Materials Corrosion Types of corrosion Corrosion can






- Slides: 23

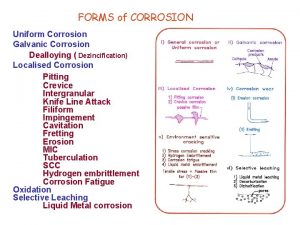
Engineering Materials Corrosion Types of corrosion Corrosion can be classified according to the appearance of the corroded metal; 1. 2. 3. 4. Uniform or general corrosion Pitting corrosion Galvanic corrosion Crevice corrosion 1. Uniform or General Corrosion The metal loss is uniform from the surface. Often combined with high-velocity fluid erosion, with or without abrasives.

Engineering Materials Corrosion Types of corrosion Pitting Corrosion The metal loss is randomly located on the metal surface. Often combined with stagnant fluid or in areas with low fluid velocity.

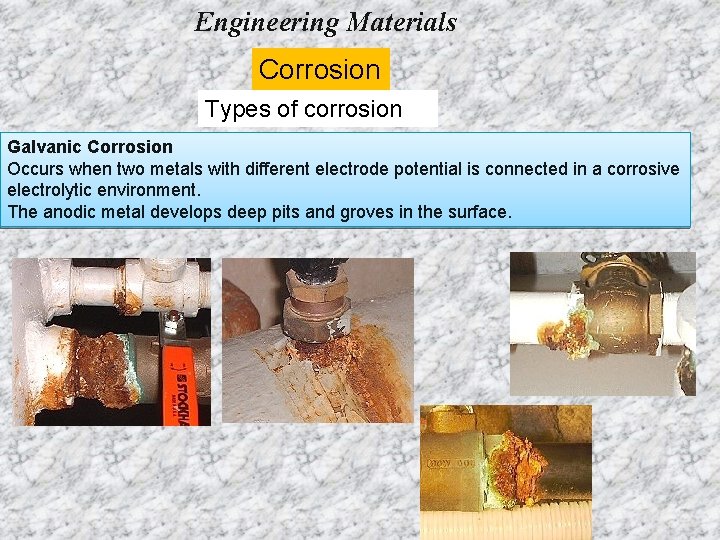
Engineering Materials Corrosion Types of corrosion Galvanic Corrosion Occurs when two metals with different electrode potential is connected in a corrosive electrolytic environment. The anodic metal develops deep pits and groves in the surface.

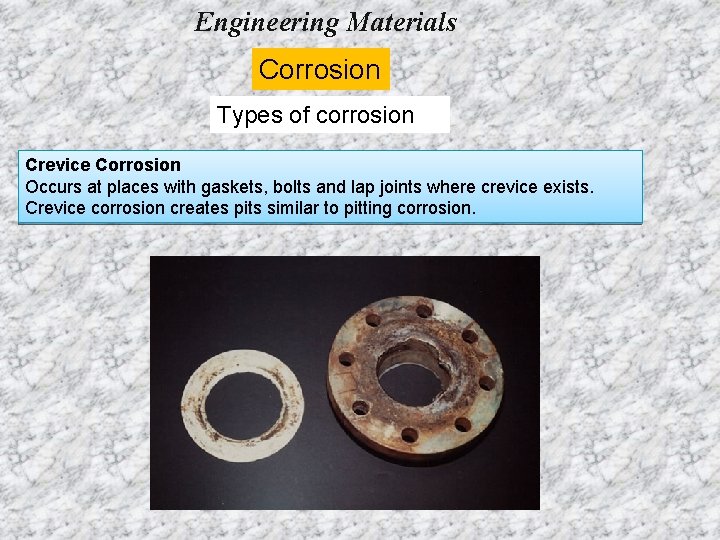
Engineering Materials Corrosion Types of corrosion Crevice Corrosion Occurs at places with gaskets, bolts and lap joints where crevice exists. Crevice corrosion creates pits similar to pitting corrosion.

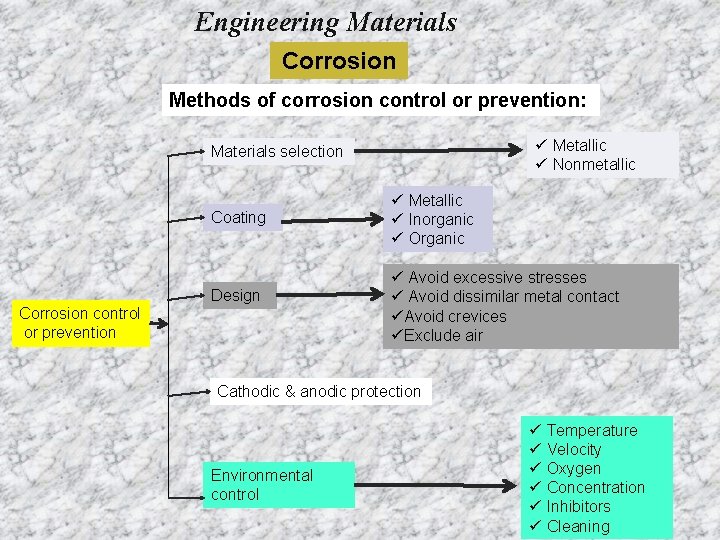
Engineering Materials Corrosion Methods of corrosion control or prevention: ü Metallic ü Nonmetallic Materials selection Coating Design Corrosion control or prevention ü Metallic ü Inorganic ü Organic ü Avoid excessive stresses ü Avoid dissimilar metal contact üAvoid crevices üExclude air Cathodic & anodic protection Environmental control ü Temperature ü Velocity ü Oxygen ü Concentration ü Inhibitors ü Cleaning

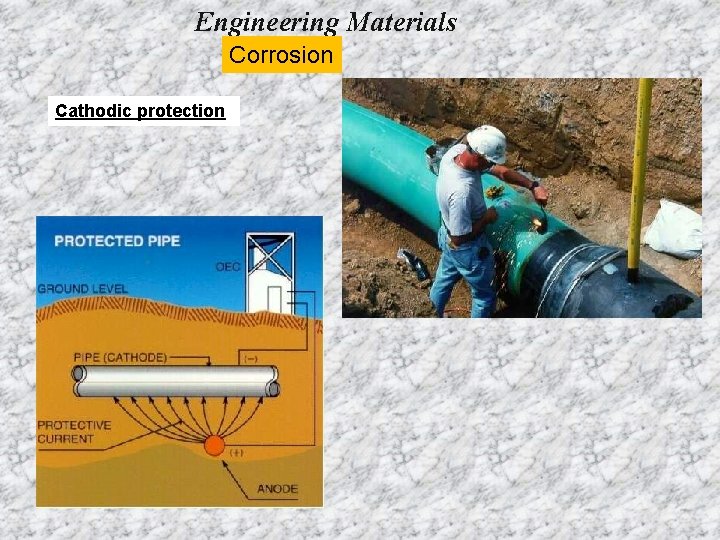
Engineering Materials Corrosion Cathodic protection

Engineering Materials Ceramic and Glasses materials Ceramic material: Inorganic, nonmetallic materials that consist of metallic and nonmetallic elements bonded together primarily by ionic and/or covalent bonds . Distinguishing Features – – – – Most have a regular arrangement of atoms (except glasses) Compounds of Metallic and Non-Metallic elements Density lower than Metals Hard and stronger than Metals Low resistance to Fracture Brittle (low ductility) High Melting Points (Refractory (ceramic) material) Poor Conductors of Electricity and Heat (except Graphite, Diamond) – Can be opaque, semi-transparent or transparent

Engineering Materials Ceramic and Glasses materials APPLICATIONS OF CERAMICS – Electrical Insulators – Thermal insulations and coatings – Abrasives – porcelain – Glasses (Windows, TV screens, Optical fibers) – Cement, Concrete – Ceramic tiles for space shuttles – Furnace Lining brick – Hi-tech ceramics => electronic, communication, computer hardware, aerospace industries Furnace Lining brick Ceramic tiles porcelain

Engineering Materials Ceramic and Glasses materials CERAMIC MATERIAL EXAMPLES – Diamond, Graphite – Glasses – Building materials – Oxides (Si. O 2, Al 2 O 3) – Carbide tools v Compressive strength is typically ten times the tensile strength. This makes ceramics good structural materials under compression (e. g. , cement, bricks in building apartments, stone blocks in the pyramids). Diamond Graphite Carbide tools

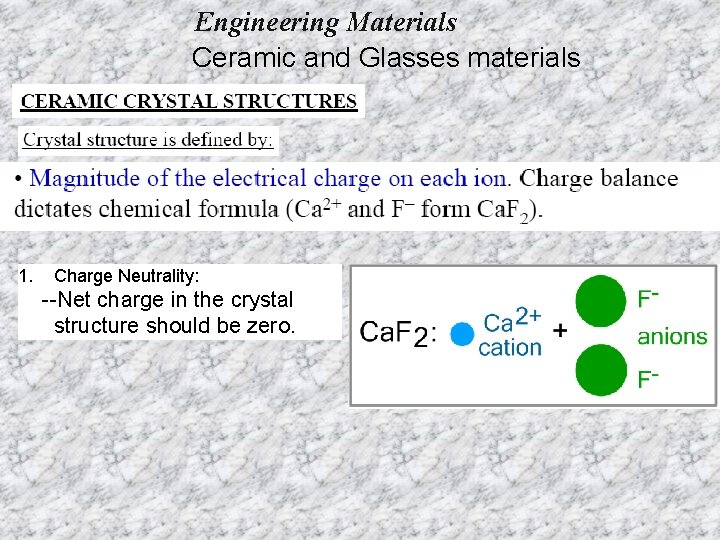
Engineering Materials Ceramic and Glasses materials 1. Charge Neutrality: --Net charge in the crystal structure should be zero.

Engineering Materials Ceramic and Glasses materials

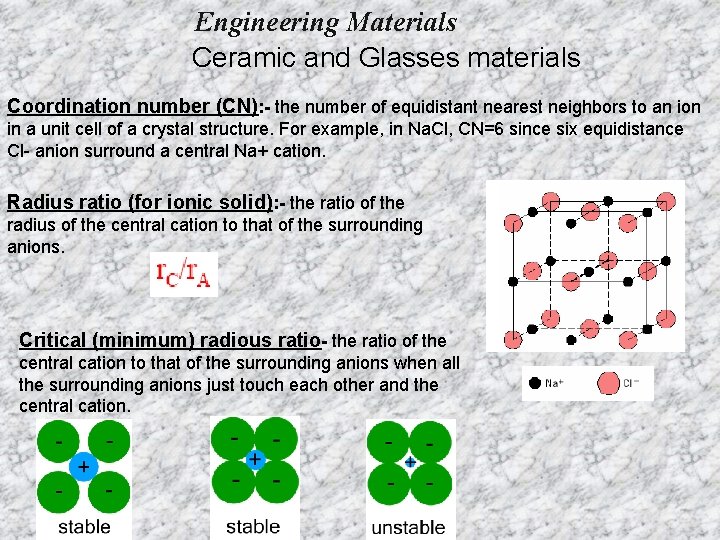
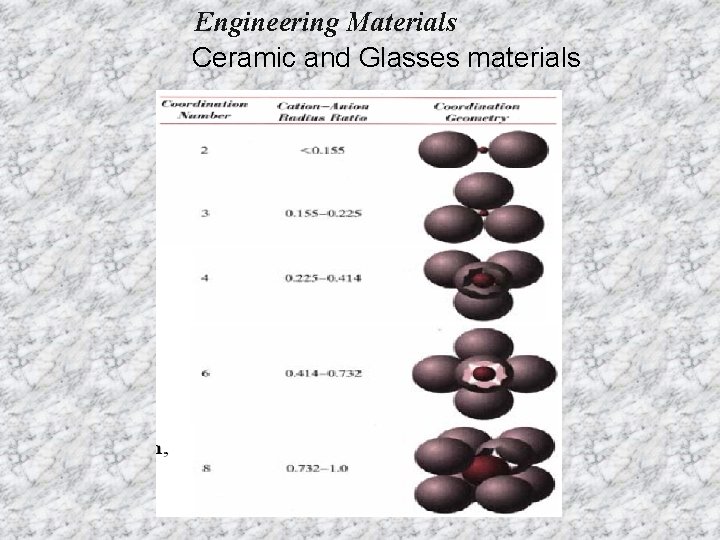
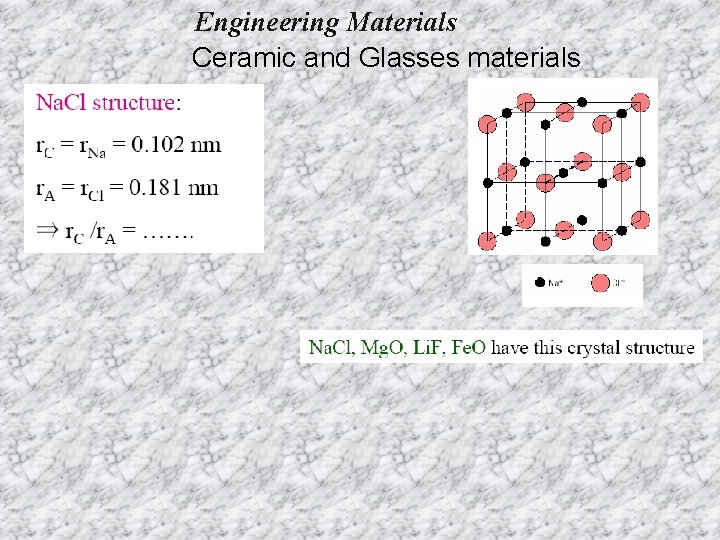
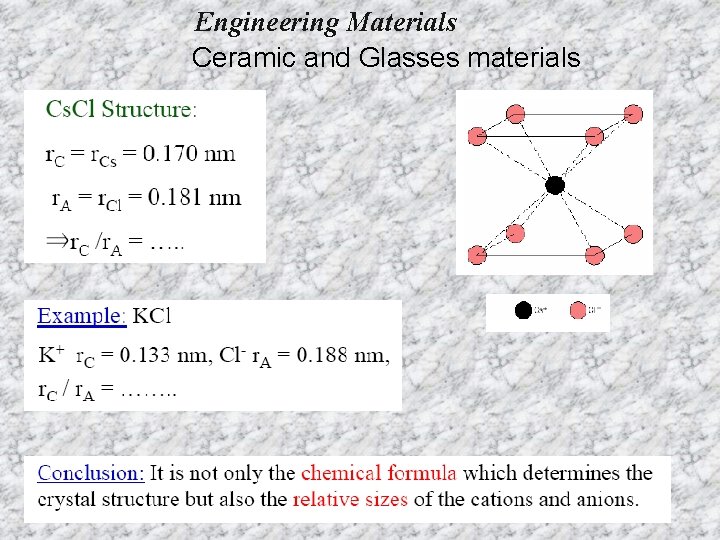
Engineering Materials Ceramic and Glasses materials Coordination number (CN): - the number of equidistant nearest neighbors to an ion in a unit cell of a crystal structure. For example, in Na. Cl, CN=6 since six equidistance Cl- anion surround a central Na+ cation. Radius ratio (for ionic solid): - the ratio of the radius of the central cation to that of the surrounding anions. Critical (minimum) radious ratio- the ratio of the central cation to that of the surrounding anions when all the surrounding anions just touch each other and the central cation.

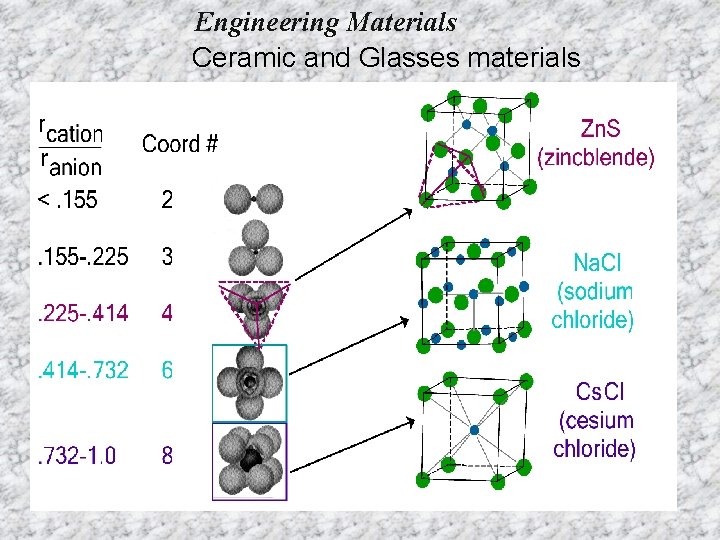
Engineering Materials Ceramic and Glasses materials For a specific coordination number there is a critical or minimum cation/anion radius ratio r. C/r. A for which this contact can be maintained.

Engineering Materials Ceramic and Glasses materials

Engineering Materials Ceramic and Glasses materials

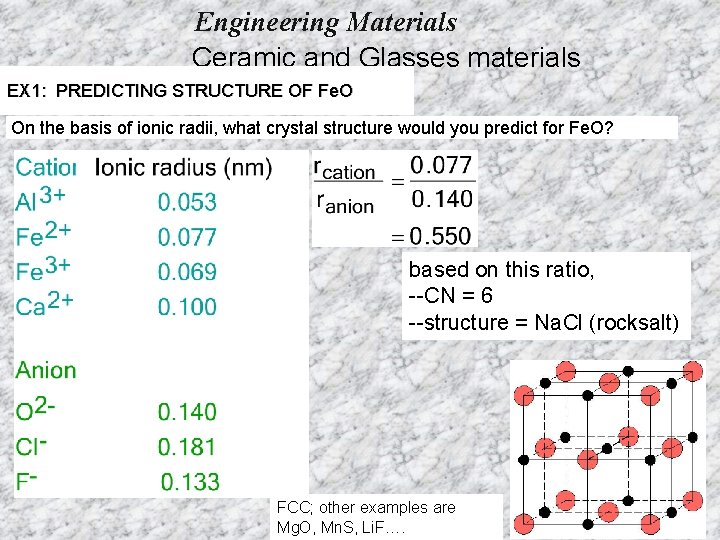
Engineering Materials Ceramic and Glasses materials EX 1: PREDICTING STRUCTURE OF Fe. O On the basis of ionic radii, what crystal structure would you predict for Fe. O? based on this ratio, --CN = 6 --structure = Na. Cl (rocksalt) FCC; other examples are Mg. O, Mn. S, Li. F….

Engineering Materials Ceramic and Glasses materials

Engineering Materials Ceramic and Glasses materials

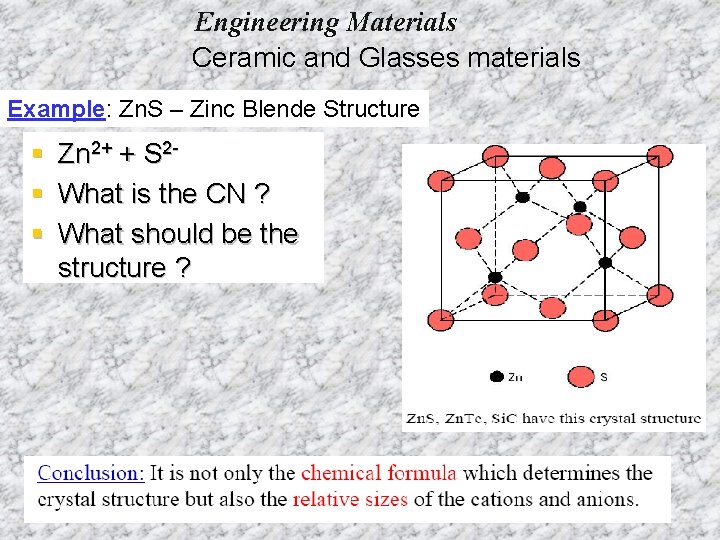
Engineering Materials Ceramic and Glasses materials Example: Zn. S – Zinc Blende Structure § § § Zn 2+ + S 2 What is the CN ? What should be the structure ?

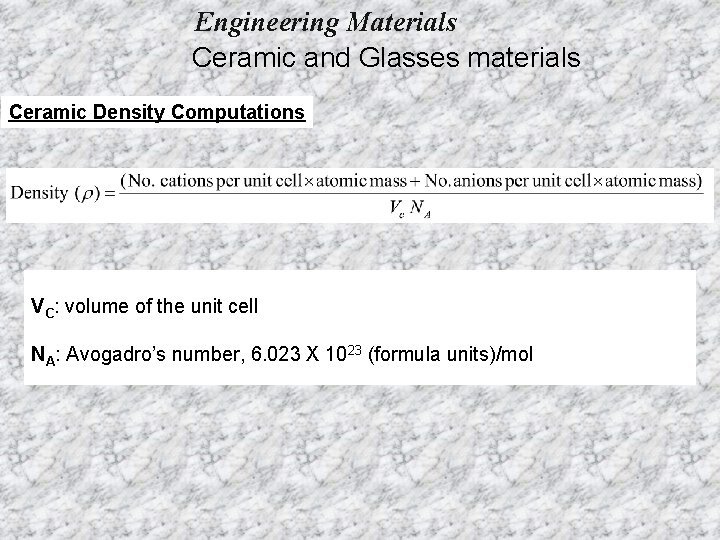
Engineering Materials Ceramic and Glasses materials Ceramic Density Computations VC: volume of the unit cell NA: Avogadro’s number, 6. 023 X 1023 (formula units)/mol

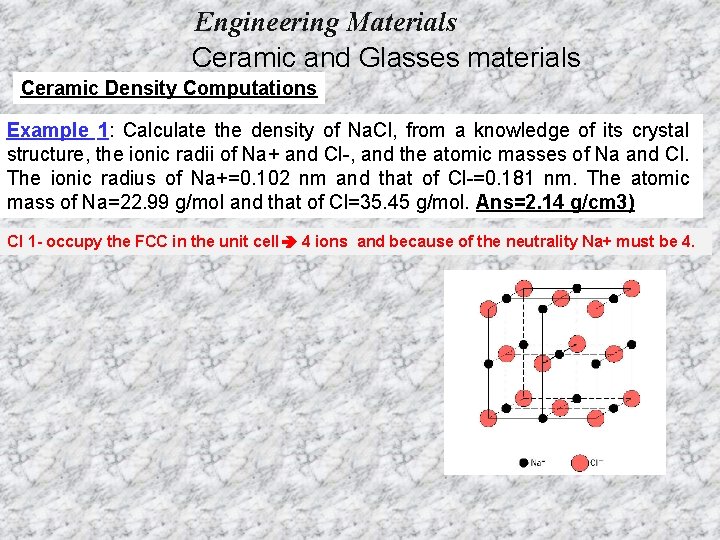
Engineering Materials Ceramic and Glasses materials Ceramic Density Computations Example 1: Calculate the density of Na. Cl, from a knowledge of its crystal structure, the ionic radii of Na+ and Cl-, and the atomic masses of Na and Cl. The ionic radius of Na+=0. 102 nm and that of Cl-=0. 181 nm. The atomic mass of Na=22. 99 g/mol and that of Cl=35. 45 g/mol. Ans=2. 14 g/cm 3) Cl 1 - occupy the FCC in the unit cell 4 ions and because of the neutrality Na+ must be 4.

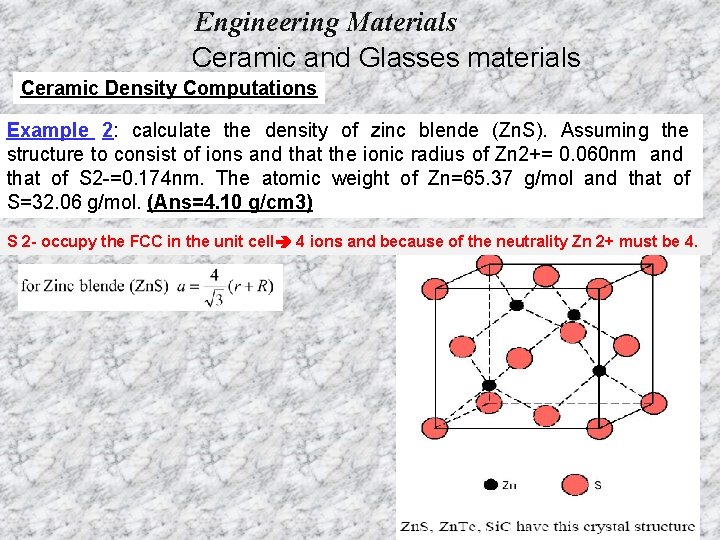
Engineering Materials Ceramic and Glasses materials Ceramic Density Computations Example 2: calculate the density of zinc blende (Zn. S). Assuming the structure to consist of ions and that the ionic radius of Zn 2+= 0. 060 nm and that of S 2 -=0. 174 nm. The atomic weight of Zn=65. 37 g/mol and that of S=32. 06 g/mol. (Ans=4. 10 g/cm 3) S 2 - occupy the FCC in the unit cell 4 ions and because of the neutrality Zn 2+ must be 4.

Engineering Materials Ceramic and Glasses materials Ceramic Density Computations Example 3: calculate the density of Uranium oxide (UO 2). Which has the calcium fluoride (Ca. F 2) structure. (Ionic radii: U 4+=0. 105 nm and O 2 -=0. 132 nm). The atomic mass of U=238 g/mol and that of O=16 g/mol. (Ans=10. 9 g/cm 3) U 4+ occupy the FCC in the unit cell 4 ions and because of the neutrality O 2 - must be 8.
 Differentiate between dry corrosion and wet corrosion
Differentiate between dry corrosion and wet corrosion Classification of corrosion
Classification of corrosion Types of engineering materials
Types of engineering materials Erosion corrosion can be controlled by
Erosion corrosion can be controlled by Types of electrochemical corrosion
Types of electrochemical corrosion Go noodle cant stop the feeling
Go noodle cant stop the feeling Harmful useful materials
Harmful useful materials Man made materials
Man made materials Adapting and adopting materials
Adapting and adopting materials Direct materials budget with multiple materials
Direct materials budget with multiple materials Manufacturing processes for engineering materials 5th
Manufacturing processes for engineering materials 5th What is the engineering materials
What is the engineering materials Optical properties of engineering materials
Optical properties of engineering materials Hot materials
Hot materials Properties of engineering materials
Properties of engineering materials Necking
Necking Materials for engineering
Materials for engineering Integrated computational materials engineering
Integrated computational materials engineering Eacademics iitd student login
Eacademics iitd student login Integrated computational materials engineering
Integrated computational materials engineering Civil engineering source
Civil engineering source Things that block light
Things that block light Computer based system engineering in software engineering
Computer based system engineering in software engineering Forward engineering in software engineering
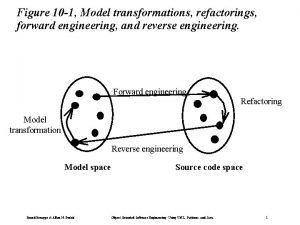
Forward engineering in software engineering