Question of the day QUESTION OF THE DAY
































- Slides: 50

Question of the day…. . QUESTION OF THE DAY: WHY DO YOU THINK THAT SCIENTISTS IN THE 20 TH CENTURY THOUGHT THAT IT WAS IMPORTANT TO “UNLOCK” THE STRUCTURE OF THE ATOM?

The History of the Atom……. Democritus was the first to theorize that all matter was composed of atoms! The name atom comes from his Greek word, “atomos”, which means not dividable or whole! Democritus’ ideas were crushed by a another Greek Philosopher, named……….

………………Aristotle!!!! Aristotle asked Democritus three questions: If we are made of atoms, what holds us together? Why can’t we see these atoms? Why don’t we fall down like a bag of marbles?

Democritus was unable to answer these questions……. And so, people continued to think that matter was continuous - your body, the world around us was one continuous piece…. This philosophy continued for centuries…….

During the middle ages, science was lost to the • The only science that was world…… done was performed by Arabs (Moors) out of Africa that conquered Spain! • They traveled from the Middle East to Western Europe, carrying the great library of Alexandria with them! • While the plague and the dark ages were killing millions, they had………. .

EVERYTHING…. . ! Running water…. . Dentistry…. . Reading, Writing, Geometry, Calculus… Breweries……. Complex gardens and Agriculture…. .

Science did not start up again until the Church was challenged…. The church was preventing people from coming up with new ideas and doing science! Who were some people that challenged the Church? Once the Church is challenged by thinkers, science experiments start to be done! With independence, came independent thought and ideas…. .

During the 1700’s and 1800’s…. Scientists were discovering concepts and relationships Scientists were doing large, observable, basic experiments They weren’t doing tests with microscopes or computers! They were doing experiments with stoves, pots, ovens, and basic glassware! With observable properties came explanations!

Joseph Priestley……. Prepared pure Oxygen Decomposed mercuric oxide into mercury and oxygen Invented soda pop Discovered carbon dioxide and nitrous oxide (laughing gas)

Priestley noticed that the gas produced by heating mercuric oxide burned a candle brightly…. He called the air, “perfect air”, or “dephlogisticated air” People thought that phlogiston was a substance that was in materials When substances burned, they released phlogiston, which eventually caused flames to go out. Is this true…. ? He had actually discovered oxygen, but didn’t realize it!

Antoine Lavoisier…. . Lived during the French Revolution Was a brilliant scientist, but a tax collector Had his head chopped off during the revolution with royalty

Lavoisier Proved…. . That substances did not give off phlogiston, but used oxygen! He burned metals in the air, and weighed them before and after The substances weighed more after, because they were combining with oxygen, not giving off phlogiston!

By weighing the materials before and after…. . Lavoisier came up with……. . The law of Conservation of Mass This states: - Matter cannot be created or destroyed in a chemical reaction, simply converted from one form into another The extra mass came from the oxygen in the air, which he later measured by reheating the compound formed

Joseph Proust…. . Examined copper carbonate No matter the amount, whenever decomposed it would yield 5. 3 parts copper, 4. 0 parts oxygen and 1. 0 parts carbon Came up with the law of definite composition All substances are composed of definite amounts of each element, no matter how much or how little of the matter there is! This compound is always 5. 3 parts Cu, 4. 0 parts O, and 1. 0 parts C!

John Dalton…… A schoolteacher! Devised the Law of Multiple Proportions Stated that when two elements form more than one compound, they come together in whole number ratios……

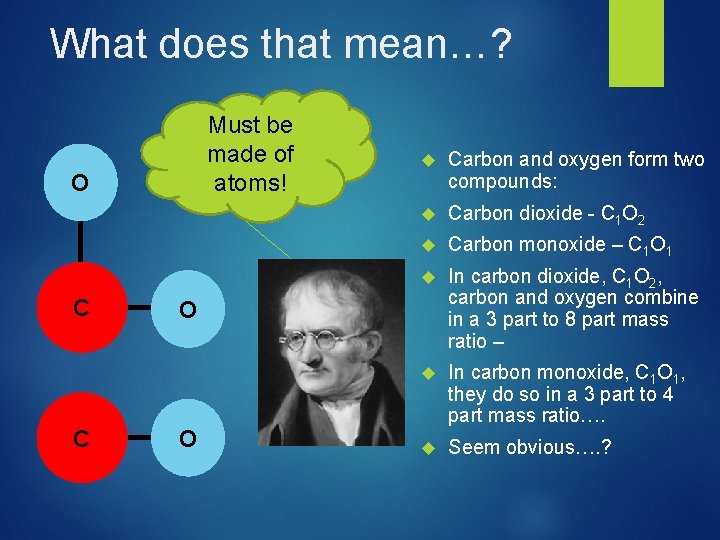
What does that mean…? Must be made of atoms! O C C Carbon and oxygen form two compounds: Carbon dioxide - C 1 O 2 Carbon monoxide – C 1 O 1 In carbon dioxide, C 1 O 2, carbon and oxygen combine in a 3 part to 8 part mass ratio – In carbon monoxide, C 1 O 1, they do so in a 3 part to 4 part mass ratio…. Seem obvious…. ? O O

IT WASN’T…… Scientists didn’t know what was making things come together to react! In fact, They didn’t even know that atoms existed! If compounds come together in ratios, they must be made of smaller pieces!

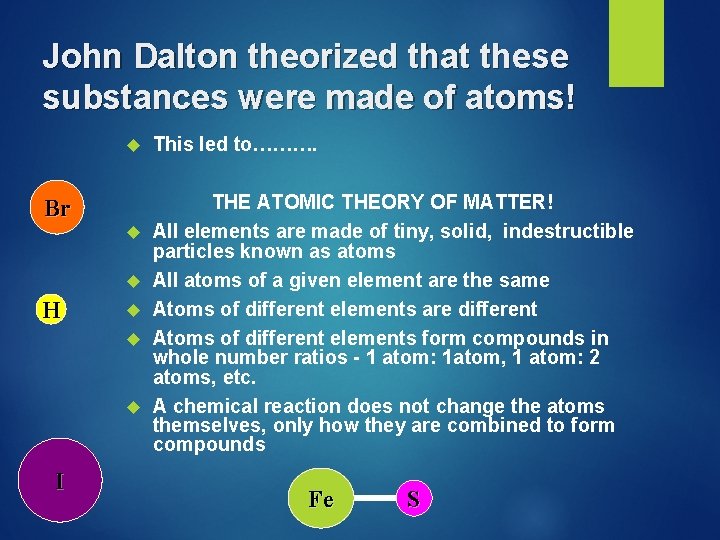
John Dalton theorized that these substances were made of atoms! Br H I This led to………. THE ATOMIC THEORY OF MATTER! All elements are made of tiny, solid, indestructible particles known as atoms All atoms of a given element are the same Atoms of different elements are different Atoms of different elements form compounds in whole number ratios - 1 atom: 1 atom, 1 atom: 2 atoms, etc. A chemical reaction does not change the atoms themselves, only how they are combined to form compounds Fe S

It took almost 2000 years before the idea of the atom came back! John Dalton brought Democritus’ idea back! It seems simple, but people did not believe that matter was made of little marblelike structures called atoms! John Dalton’s theory was wrong in one respect……. .

Atoms are not solid and indestructible like marbles…. . They contain different parts……. However, scientists did not know this until parts of the atom were discovered…. . What part of the atom do you think was discovered first…. . ?

THE ELECTRON…! Why do you think it was the first part of the atom to be discovered…. . ?

The electron is on the outside of the atom! The electron is the ONLY part of the atom that can be added or removed to an atom! The electron is tiny and light, and that is why it can be added or removed! This is what we call electricity…moving electrons! Technology wasn’t that good hundreds of years ago! e- e- e-

The Early 1800’s! Lots of basic work with electricity Matter has charge! There are two types of “charge” in the world: + charged objects - charged objects Ben Franklin is the first to come up with these names - + and -, and to discover that matter has charge! Franklin didn’t know that these charges were part of the atom he didn’t even know that atoms existed, yet!

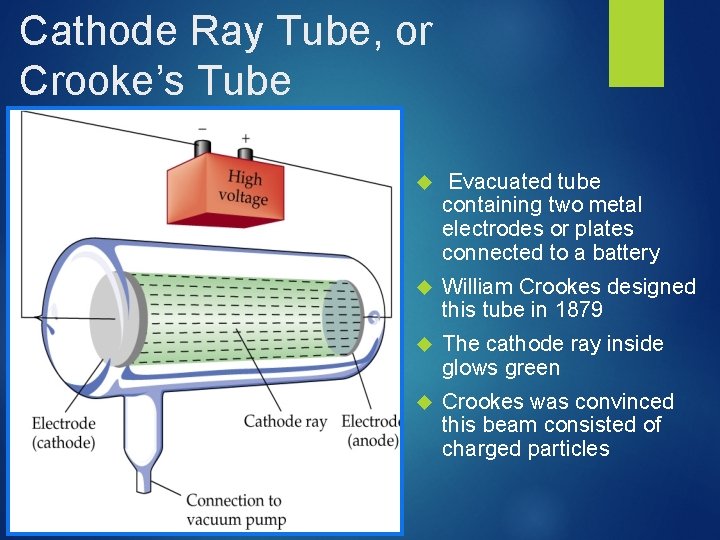
Cathode Ray Tube, or Crooke’s Tube Evacuated tube containing two metal electrodes or plates connected to a battery William Crookes designed this tube in 1879 The cathode ray inside glows green Crookes was convinced this beam consisted of charged particles

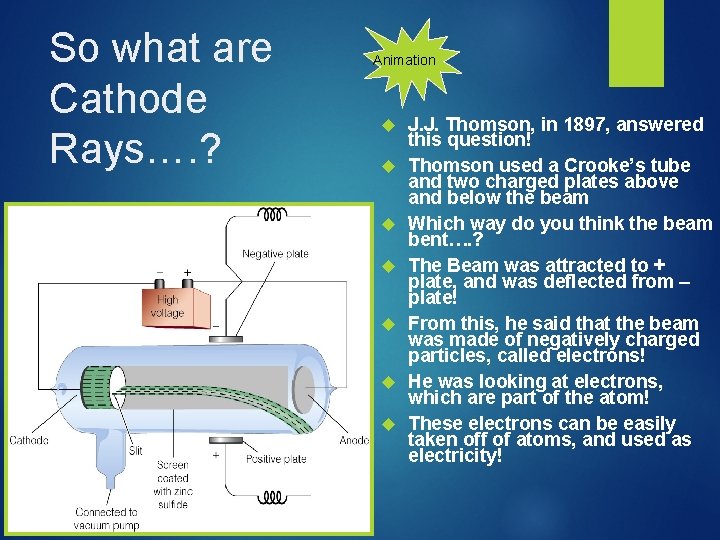
So what are Cathode Rays…. ? Animation J. J. Thomson, in 1897, answered this question! Thomson used a Crooke’s tube and two charged plates above and below the beam Which way do you think the beam bent…. ? The Beam was attracted to + plate, and was deflected from – plate! From this, he said that the beam was made of negatively charged particles, called electrons! He was looking at electrons, which are part of the atom! These electrons can be easily taken off of atoms, and used as electricity!


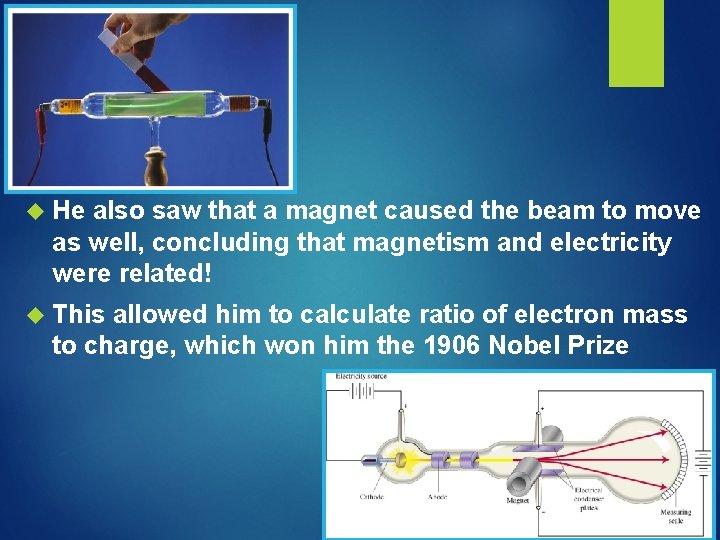
He also saw that a magnet caused the beam to move as well, concluding that magnetism and electricity were related! This allowed him to calculate ratio of electron mass to charge, which won him the 1906 Nobel Prize

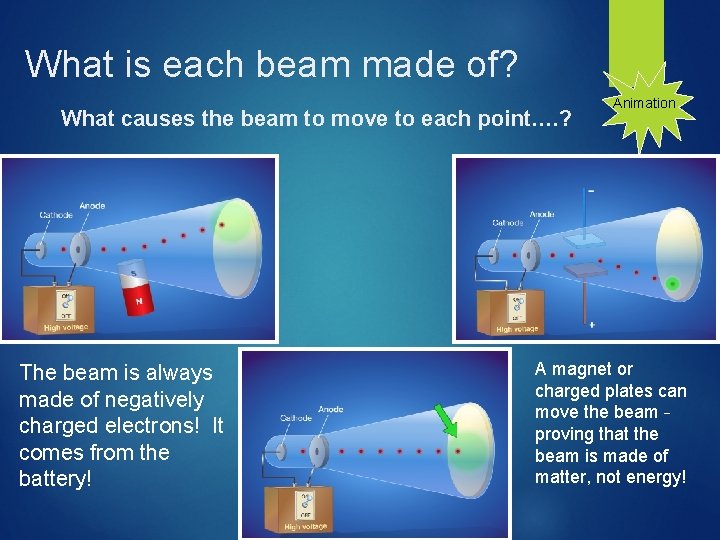
What is each beam made of? What causes the beam to move to each point…. ? The beam is always made of negatively charged electrons! It comes from the battery! Animation A magnet or charged plates can move the beam – proving that the beam is made of matter, not energy!

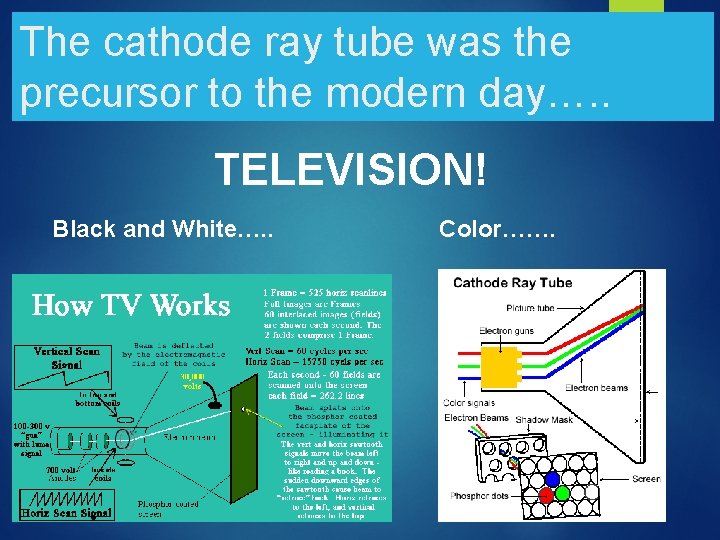
The cathode ray tube was the precursor to the modern day…. . TELEVISION! Black and White…. . Color…….

LCD Technology. . DLP 1 pixel!

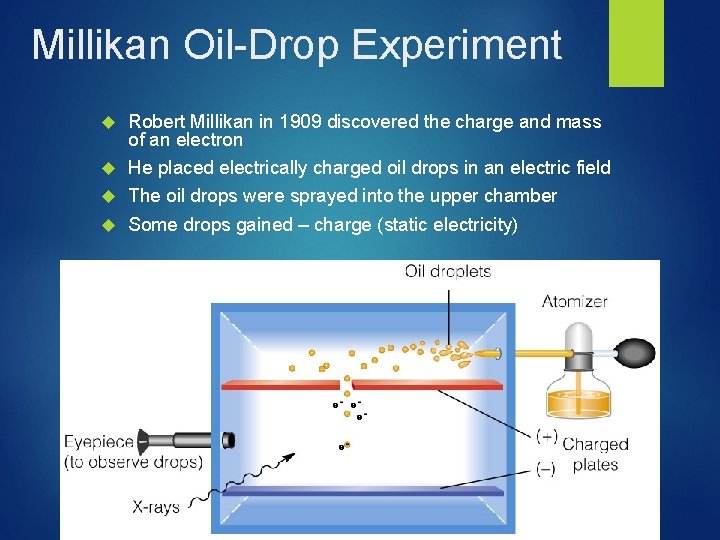
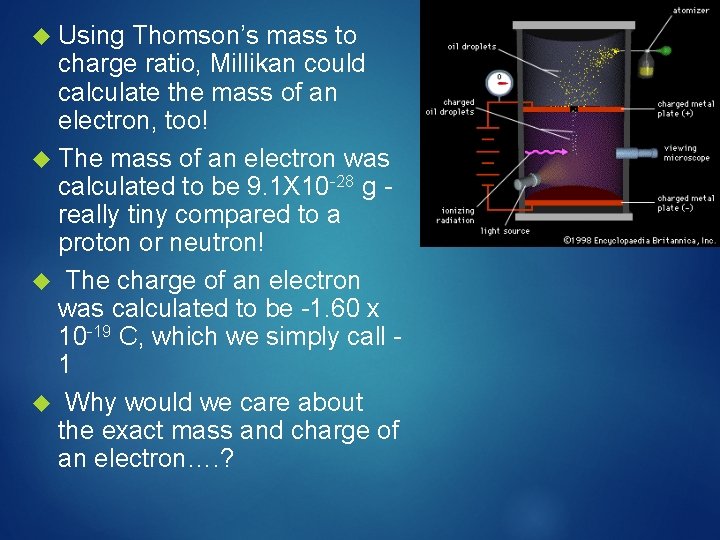
Millikan Oil-Drop Experiment Robert Millikan in 1909 discovered the charge and mass of an electron He placed electrically charged oil drops in an electric field The oil drops were sprayed into the upper chamber Some drops gained – charge (static electricity)

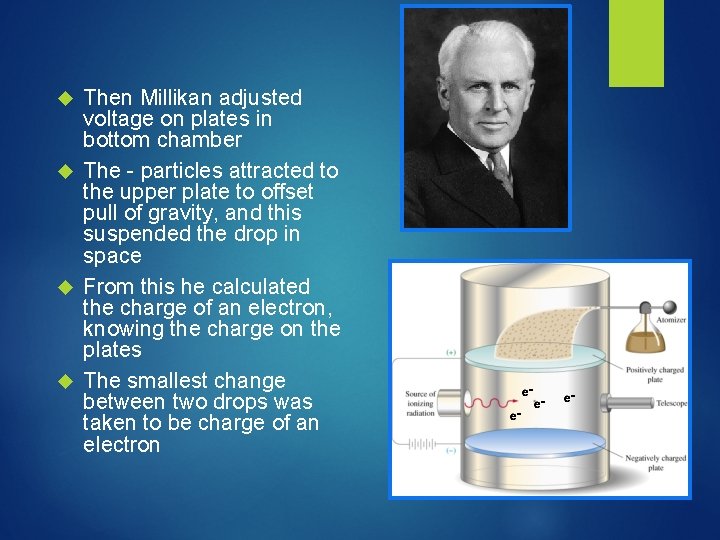
Then Millikan adjusted voltage on plates in bottom chamber The - particles attracted to the upper plate to offset pull of gravity, and this suspended the drop in space From this he calculated the charge of an electron, knowing the charge on the plates The smallest change between two drops was taken to be charge of an electron

Using Thomson’s mass to charge ratio, Millikan could calculate the mass of an electron, too! The mass of an electron was calculated to be 9. 1 X 10 -28 g really tiny compared to a proton or neutron! The charge of an electron was calculated to be -1. 60 x 10 -19 C, which we simply call 1 Why would we care about the exact mass and charge of an electron…. ?

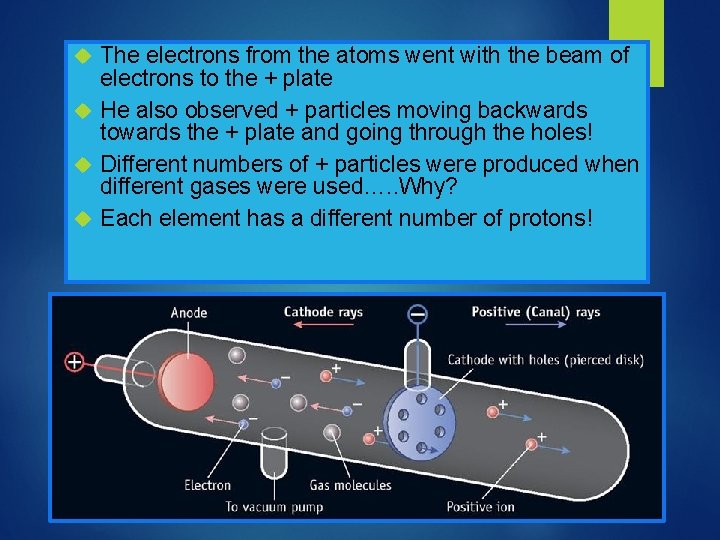
Now that we have found negative particles…. Eugen Goldstein in 1886 used a Crooke’s tube with holes in the cathode and discovered positive particles He shot a cathode beam (beam of electrons) at hydrogen atoms

The electrons from the atoms went with the beam of electrons to the + plate He also observed + particles moving backwards towards the + plate and going through the holes! Different numbers of + particles were produced when different gases were used…. . Why? Each element has a different number of protons!

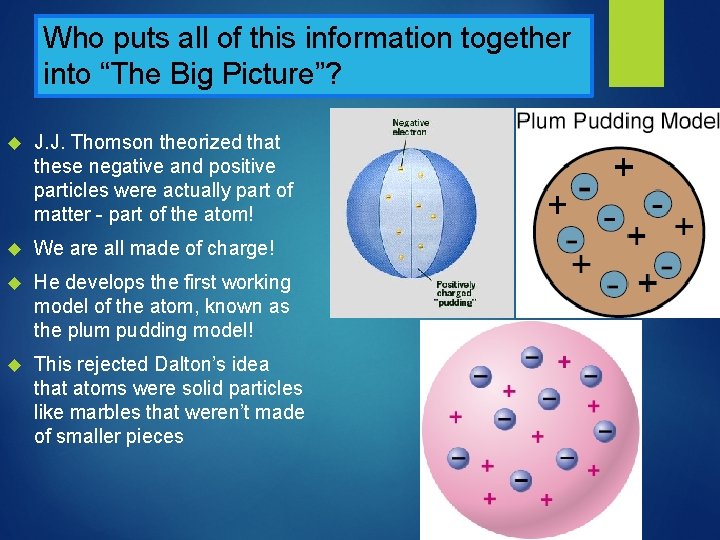
Who puts all of this information together into “The Big Picture”? J. J. Thomson theorized that these negative and positive particles were actually part of matter - part of the atom! We are all made of charge! He develops the first working model of the atom, known as the plum pudding model! This rejected Dalton’s idea that atoms were solid particles like marbles that weren’t made of smaller pieces

Why was any of this monumental…? People didn’t believe in atoms, and didn’t understand what things like electricity and light were! Thomson showed that electricity was nothing more than a flow of little particles called electrons! And electrons, along with protons, make up every atom, in every person, plant, building and object in the universe We are made of charged particles - the same particles that we use for electricity!

So atoms are made of + and - particles. How are some atoms “radioactive”? What is “radioactivity”? Henri Becquerel, in 1895, put a chunk of Uranium in a desk drawer with some photographic paper When he came back after the weekend, the paper had a picture of the Uranium chunk on it! Becquerel knew the Uranium atoms must be giving “something” off. He called this something “radioactivity” or “radiation”. The same year, William Roentgen had a similar experience - his Uranium actually caused photographic paper to develop in a nearby lab - through a wall! He called his rays, “X-Rays”. They were immediately used for medical purposes! What was this “radiation”, though? How did it relate to what Thomson had discovered?

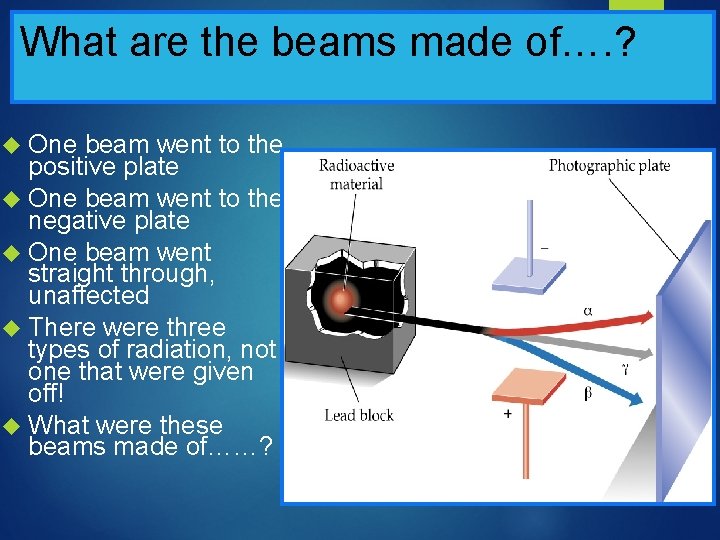
Ernest Rutherford was determined to figure out what this “radiation” was! Ernest Rutherford, a physicist from New Zealand, took a chunk of uranium, and set out to determine what it “gave off”! He placed the Uranium in a lead box He took two charged plates, and placed them above and below the beam of “radiation” that the Uranium was giving off It split into three beams!!! Animation

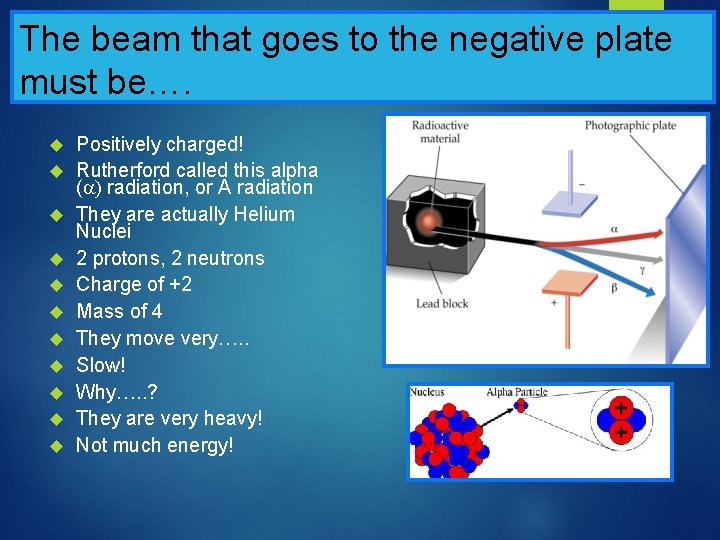
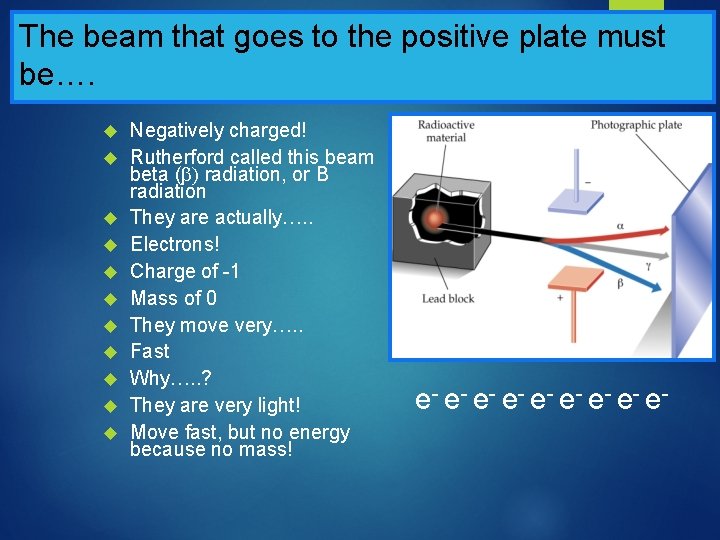
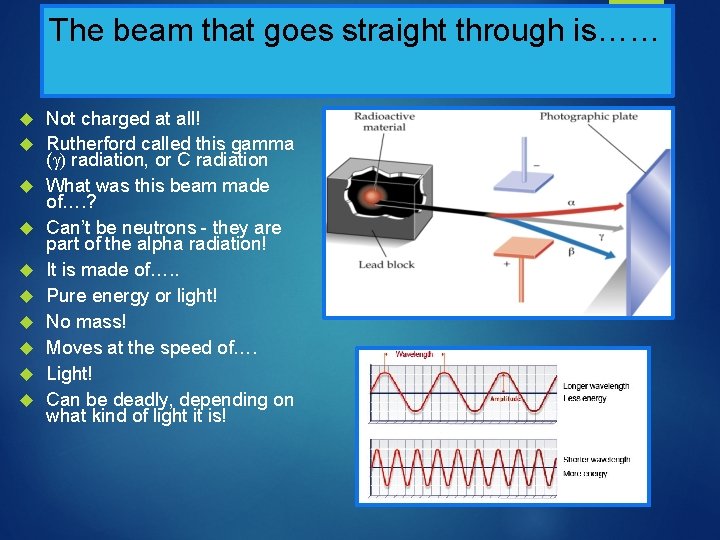
Rutherford’s Radiation Experiment

What are the beams made of…. ? One beam went to the positive plate One beam went to the negative plate One beam went straight through, unaffected There were three types of radiation, not one that were given off! What were these beams made of……?

The beam that goes to the negative plate must be…. Positively charged! Rutherford called this alpha ( radiation, or A radiation They are actually Helium Nuclei 2 protons, 2 neutrons Charge of +2 Mass of 4 They move very…. . Slow! Why…. . ? They are very heavy! Not much energy!

The beam that goes to the positive plate must be…. Negatively charged! Rutherford called this beam beta ( radiation, or B radiation They are actually…. . Electrons! Charge of -1 Mass of 0 They move very…. . Fast Why…. . ? They are very light! Move fast, but no energy because no mass! e- e- e-

The beam that goes straight through is…… Not charged at all! Rutherford called this gamma ( radiation, or C radiation What was this beam made of…. ? Can’t be neutrons - they are part of the alpha radiation! It is made of…. . Pure energy or light! No mass! Moves at the speed of…. Light! Can be deadly, depending on what kind of light it is!

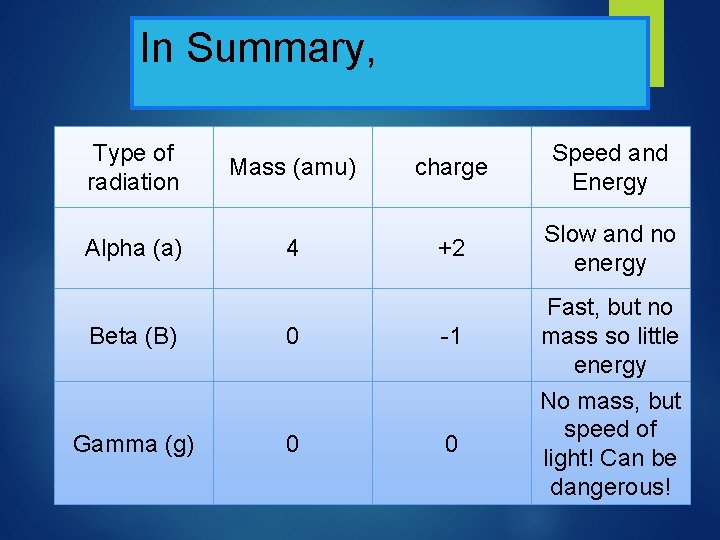
In Summary, Type of radiation Alpha (a) Mass (amu) 4 charge Speed and Energy +2 Slow and no energy Beta (B) 0 -1 Gamma (g) 0 0 Fast, but no mass so little energy No mass, but speed of light! Can be dangerous!

So why do some atoms give off radiation? ? Rutherford looked back at his model of the atom for answers! All of the protons are jammed into a tiny little nucleus! And they all have what charge…. ? The same charge! Positive! Which means…. . They are constantly trying to push each other out! Like charges REPEL! What keeps them in there…. . ?

……. . The neutron does! The neutron was the last particle to be discovered! Why was it the last particle to be discovered…. ? IT HAS NO CHARGE! It was discovered when James Chadwick noticed that Beryllium atoms were giving off some “unknown particle” when hit with alpha radiation! These particles were neutrons! Why are the neutrons even in the nucleus? They have a special force, known as “STRONG NUCLEAR FORCE”, that holds the protons together, because the protons are always pushing each other out! I call it “NEUTRON GLUE”!


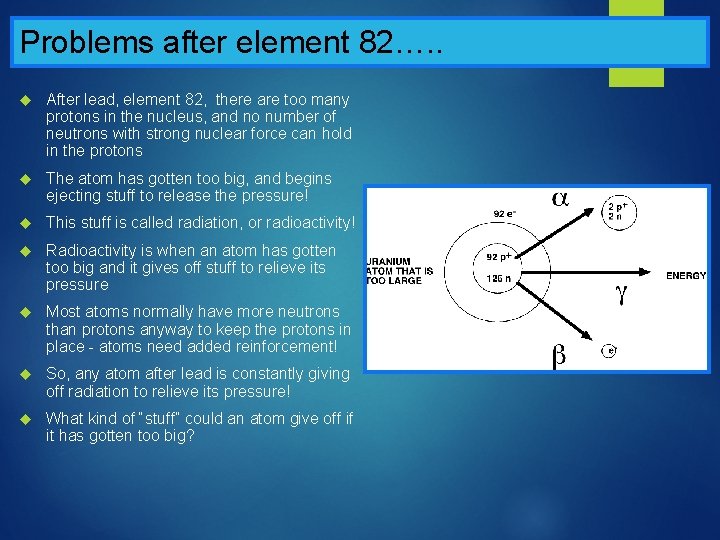
Problems after element 82…. . After lead, element 82, there are too many protons in the nucleus, and no number of neutrons with strong nuclear force can hold in the protons The atom has gotten too big, and begins ejecting stuff to release the pressure! This stuff is called radiation, or radioactivity! Radioactivity is when an atom has gotten too big and it gives off stuff to relieve its pressure Most atoms normally have more neutrons than protons anyway to keep the protons in place - atoms need added reinforcement! So, any atom after lead is constantly giving off radiation to relieve its pressure! What kind of “stuff” could an atom give off if it has gotten too big?

In summary…. . We know that the nucleus contains… Protons And and neutrons! the electrons are…. . Orbiting around the outside! Atoms that have too many protons in the nucleus (more than 82) will give off… Radiation! To relieve the pressure!
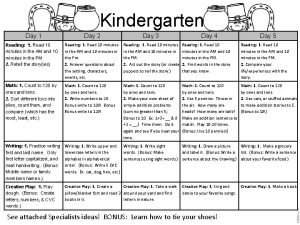
 Day 1 day 2 day 3 day 4
Day 1 day 2 day 3 day 4 Costa's level of questions
Costa's level of questions Day 1 day 2 day 817
Day 1 day 2 day 817 Hổ đẻ mỗi lứa mấy con
Hổ đẻ mỗi lứa mấy con Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Diễn thế sinh thái là
Diễn thế sinh thái là Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Lp html
Lp html Làm thế nào để 102-1=99
Làm thế nào để 102-1=99 Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Lời thề hippocrates
Lời thề hippocrates Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Tư thế worms-breton
Tư thế worms-breton đại từ thay thế
đại từ thay thế Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Dot
Dot Biện pháp chống mỏi cơ
Biện pháp chống mỏi cơ Bổ thể
Bổ thể độ dài liên kết
độ dài liên kết Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan Tia chieu sa te
Tia chieu sa te Bài hát chúa yêu trần thế alleluia
Bài hát chúa yêu trần thế alleluia điện thế nghỉ
điện thế nghỉ Một số thể thơ truyền thống
Một số thể thơ truyền thống Hệ hô hấp
Hệ hô hấp Công thức tiính động năng
Công thức tiính động năng Các số nguyên tố
Các số nguyên tố Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới ưu thế lai là gì
ưu thế lai là gì Thẻ vin
Thẻ vin Môn thể thao bắt đầu bằng từ đua
Môn thể thao bắt đầu bằng từ đua Tư thế ngồi viết
Tư thế ngồi viết Cái miệng nó xinh thế chỉ nói điều hay thôi
Cái miệng nó xinh thế chỉ nói điều hay thôi Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Cách giải mật thư tọa độ
Cách giải mật thư tọa độ Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Tư thế ngồi viết
Tư thế ngồi viết Gấu đi như thế nào
Gấu đi như thế nào Thế nào là giọng cùng tên
Thế nào là giọng cùng tên Thể thơ truyền thống
Thể thơ truyền thống Contoh open question
Contoh open question Example of compelling question
Example of compelling question Factor relating questions
Factor relating questions Question without question words
Question without question words Direct and indirect questions with answers
Direct and indirect questions with answers Nudging probe questions
Nudging probe questions