Patient Lay Summaries PlainLanguage Summaries Understanding the differences
































- Slides: 55

Patient Lay Summaries? Plain-Language Summaries? Understanding the differences and how best to use these options Jan Seal-Roberts Publishing Director, Adis | Springer Healthcare Sarah Griffiths, Ph. D Communications Team Leader, Oxford Pharma. Genesis

Jan Seal-Roberts • Publishing Director, Adis (part of Springer Nature) – Adis currently publishes 33 journals – both hybrid and full open-access titles – All our journals focus on drugs and disease therapy – We work closely with pharma and agency publication planners to publish data • Background – – Adis has been accepting plain-language summaries (PLS) since 2017* We have seen a significant increase in the inclusion of PLS over the past 18 months Plain-language summaries are proving popular with our readership They are increasingly being regarded as useful to boost the impact of findings, and to facilitate information assimilation 1 *We were the first publisher to do so! 1. Pushparajah DS et al. Value of developing plain language summaries of scientific and clinical articles: a survey of patients and physicians. Available from: Http: //journals. sagepub. com/doi/abs/10. 1177/2168479017738723 (Accessed April 2020) 2

Sarah Griffiths • Communications Team Leader, Patient Engagement Team at Oxford Pharma. Genesis – Oxford Pharma. Genesis is an independently owned Health. Science consultancy • Medical writer with extensive publications and communications experience, across multiple disease therapy areas • Focus on patient engagement deliverables – – Plain-language summaries Trial results summaries Publications with patient authors Patient insights workshops • Work with CISCRP on trial results summaries and trained in Health Literacy 3

Part 1: setting the scene on PLS and TRS Jan Seal-Roberts, Adis | Springer Healthcare Sarah Griffiths, Oxford Pharma. Genesis

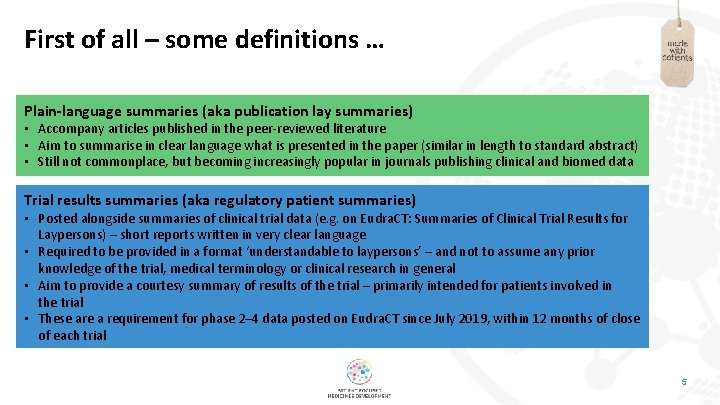
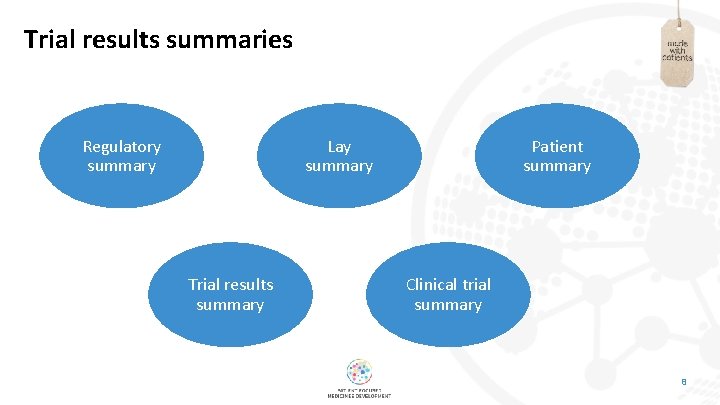
First of all – some definitions … Plain-language summaries (aka publication lay summaries) • Accompany articles published in the peer-reviewed literature • Aim to summarise in clear language what is presented in the paper (similar in length to standard abstract) • Still not commonplace, but becoming increasingly popular in journals publishing clinical and biomed data Trial results summaries (aka regulatory patient summaries) • Posted alongside summaries of clinical trial data (e. g. on Eudra. CT: Summaries of Clinical Trial Results for Laypersons) – short reports written in very clear language • Required to be provided in a format ‘understandable to laypersons’ – and not to assume any prior knowledge of the trial, medical terminology or clinical research in general • Aim to provide a courtesy summary of results of the trial – primarily intended for patients involved in the trial • These are a requirement for phase 2– 4 data posted on Eudra. CT since July 2019, within 12 months of close of each trial 5

But beware! Both plain-language summaries and trial results summaries are still being called a number of different (but similar-sounding) names! 6

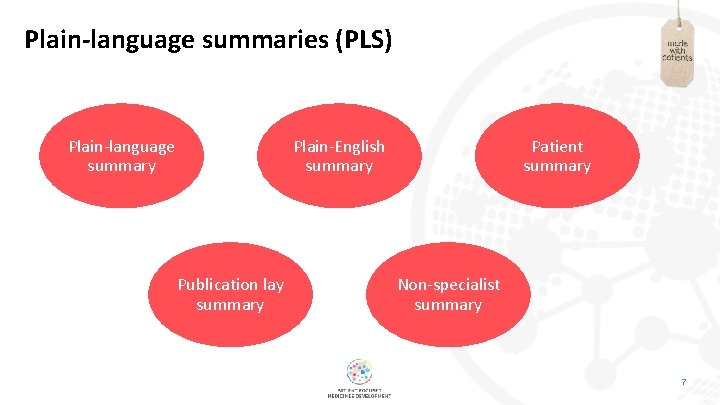
Plain-language summaries (PLS) Plain-language summary Plain-English summary Publication lay summary Patient summary Non-specialist summary 7

Trial results summaries Regulatory summary Lay summary Trial results summary Patient summary Clinical trial summary 8

Patient resources • And this is complicated by the increasing amount of patient-centric material now being made much more widely available for patients and carers, and also for/by patient advocate groups 9

Confused … ? • Yes – and you are not alone! • But don’t worry 10

Plain-language summaries What are they? Jan Seal-Roberts, Adis | Springer Healthcare

Plain-language summaries • These accompany a paper published in the peer-reviewed literature – usually submitted by the author for peer-review alongside the paper A plain-language summary describes only what is included in the paper • Aim is to provide an ‘expert-jargon-free’ tool for any reader requiring clarification of what has been presented in an article (like a secondary abstract) • Mainly intended to save time of readers and facilitate quick understanding • NB: these should not be ‘dumbed down’ abstracts, but instead written in a clear way that can assume some prior knowledge 1 • Recommended reading age: high-school educated = around 14– 18 years 1 1. Martínez Silvagnoli L et al. Evaluation of plain-language summaries (PLS): optimising readability and format. Available from: http: //www. completemc. com/ISMPP_EU 2019_poster_Martinez. Silvagnoli/ (Accessed April 2020) 12

Why include a plain-language summary? • Extremely useful in providing a quick entrée to an article – especially for non-specialist clinicians and other HCPs, including those reading in a non-native language • Clear language helps achieve quick assimilation of key points improved readability • But PLS are increasingly being used by time-poor specialists – especially those who don’t necessary want to read the whole paper • And are also useful for well-informed patients (and their carers) seeking clear and topical information in the peer-reviewed literature 1 Result: paper has increased clarity, transparency, readability and impact – and is likely to achieve greater reach 1 1. Pushparajah DS et al. Value of developing plain language summaries of scientific and clinical articles: a survey of patients and physicians. Available from: Http: //journals. sagepub. com/doi/abs/10. 1177/2168479017738723 (Accessed April 2020) 2. Martínez Silvagnoli L et al. Evaluation of plain-language summaries (PLS): optimising readability and format. Available from: http: //www. completemc. com/ISMPP_EU 2019_poster_Martinez. Silvagnoli/ (Accessed April 2020) 13

Two examples of PLS …

First, the standard abstract Introduction The majority of elderly patients (≥ 65 years of age) with type 2 diabetes mellitus (T 2 DM) will eventually require insulin therapy, but they are particularly vulnerable to hypoglycemia and challenging to treat. Insulin degludec/insulin aspart (IDeg. Asp) is a novel co-formulation of 70% insulin degludec and 30% insulin aspart administered in a single injection, either once or twice daily with main meals. Methods A combined analysis of the phase 3 BOOST INTENSIFY PREMIX I (NCT 01009580) and BOOST INTENSIFY ALL (NCT 01059812) trials has previously reported lower rates of hypoglycemia during the maintenance period in patients with T 2 DM treated with IDeg. Asp twice daily (BID) versus biphasic insulin aspart 30 (BIAsp 30) BID. This post hoc analysis examined the safety and efficacy of IDeg. Asp versus BIAsp 30 in elderly patients from the global population of these two trials, and also from the Japanese cohort of BOOST INTENSIFY ALL. Results Change in Hb. A 1 c was similar for IDeg. Asp versus BIAsp 30 (p > 0. 5). Compared with BIAsp 30, IDeg. Asp resulted in significant reductions in fasting plasma glucose (p < 0. 0001), numerically lower rates of overall and nocturnal hypoglycemia (global estimated rate ratios: 0. 92 [0. 67; 1. 26]95% confidence interval [CI] , p = 0. 5980 and 0. 67 [0. 39; 1. 18] 95% CI, p = 0. 1676, respectively), and a significantly lower total daily insulin dose at end of trial (global estimated treatment difference 0. 79 [0. 73; 0. 87]95% CI, p < 0. 0001) in elderly patients. Conclusion The results described here are consistent with those of the overall trial populations, demonstrating that IDeg. Asp BID is efficacious in elderly patients and suggesting that there is no need for special safety precautions. Fulcher G et al. Diabetes Therapy 2019; 10: 107– 18. Available from: https: //link. springer. com/article/10. 1007%2 Fs 13300 -018 -0531 -0 (Accessed April 2020) 15

Plain-language summary IDeg. Asp is a new insulin therapy for people with diabetes. It is a combination of two insulins: insulin degludec (IDeg) and insulin aspart (IAsp). Previous studies have compared the IDeg. Asp combination with biphasic IAsp 30 (a premixed insulin therapy related to IAsp). These studies have shown that IDeg. Asp improves blood glucose levels with a low risk of harmful side effects. This study examined whether IDeg. Asp had the same positive effect on people who are aged 65 years or older. This age group is less well represented in clinical trials compared with younger adults, so this study pooled together elderly populations from two trials. Results showed that IDeg. Asp also improved blood glucose levels with a low risk of harmful side effects in elderly patients, and suggests that IDeg. Asp can be used in elderly people with diabetes just as it is used in younger adults. Fulcher G et al. Diabetes Ther 2019; 10: 107– 18. Available from: https: //link. springer. com/article/10. 1007%2 Fs 13300 -018 -0531 -0 (Accessed April 2020) 16

Standard abstract Introduction Synthesis of evidence on the long-term use of first-line biologic therapy in patients with early rheumatoid arthritis (RA) is required. We compared the efficacy of up to 5 years’ treatment with first-line tumor necrosis factor inhibitors (TNFis) versus other treatment strategies in this population. Methods Previous systematic reviews, Pub. Med and the Cochrane Central Register of Controlled Trials were searched for randomized controlled trials (RCTs) involving treatment of methotrexate-naïve RA patients with first-line TNFis. Literature was synthesized qualitatively, and a meta-analysis conducted to evaluate American College of Rheumatology (ACR) responses, clinical remission defined by any standard measure, and Health Assessment Questionnaire Disability Index (HAQ) at Years 2 and/or 5. Results Ten RCTs involving 4306 patients [first-line TNFi, n = 2234; other treatment strategies (control), n = 2072] were included in the meta-analysis. Three studies were double-blind for the first 2 years, while seven were partly/completely open label during this period. Five studies reported data at Year 5; all were open label at this time point. At Year 2, ACR 50 response, ACR 70 response and remission rates were significantly improved with first-line TNFi versus control in double-blind RCTs [log-odds ratio (OR) 0. 32 [95% confidence interval (CI) 0. 02, 0. 62; p = 0. 035], log-OR 0. 48 (95% CI 0. 20, 0. 77; p = 0. 001), and log-OR 0. 44 (95% CI 0. 13, 0. 74; p = 0. 005), respectively], but not in open-label studies. No significant between-group differences were observed in mean HAQ at Year 2 in double-blind or open-label RCTs or in ACR response or remission outcomes at Year 5. Conclusion In double-blind studies, 2 -year efficacy outcomes were significantly improved with first-line TNFi versus other treatment strategies in patients with MTXnaïve RA. No significant differences in these outcomes were observed when data from open-label RCTs were considered on their own. Further data on the efficacy of TNFi therapy over ≥ 2 years in patients with methotrexate-naïve RA are required. Gulácsi L et al. Adv Ther 2019; 36: 721– 45. Available from: https: //link. springer. com/article/10. 1007%2 Fs 12325 -018 -0869 -8 (Accessed April 2020) 17

Plain-language summary Rheumatoid arthritis (RA) is a disease of the joints and surrounding tissue, which often becomes worse over time. It causes inflammation, pain, stiffness and swelling that can destroy the joints and lead to disability. Biologics are a type of diseasemodifying antirheumatic drug (DMARD) that suppress the immune system and reduce inflammation in the joints, thereby preventing joint damage. Messenger proteins such as tumor necrosis factor (TNF) play an important role in inflammation. Tumor necrosis factor inhibitors (TNFis) are biologic drugs that block TNF and can reduce or stop inflammation in patients with RA. Currently, TNFis are not often used as the first treatment for patients with RA. Patients usually receive other drugs (conventional synthetic DMARDs) first. Doctors face the challenge of identifying which type of DMARD to use first in patients with RA. Although previous studies have demonstrated the short-term benefits of using biologics as the first treatment, the longer-term effects of doing this have yet to be proven. We conducted this analysis to find out if patients with RA who were first treated with TNFis in clinical trials had long-term improvements in their disease compared with patients receiving other treatments. We conducted a thorough review of the literature and used a meta-analysis approach to combine data from relevant randomized controlled trials. We found that, in certain types of tightly controlled trials, using TNFis as the first treatment can improve disease in the long term (up to 2 years at least). However, further studies are required to confirm this finding. Gulácsi L et al. Adv Ther 2019; 36: 721– 45. Available from: https: //link. springer. com/article/10. 1007%2 Fs 12325 -018 -0869 -8 (Accessed April 2020) 18

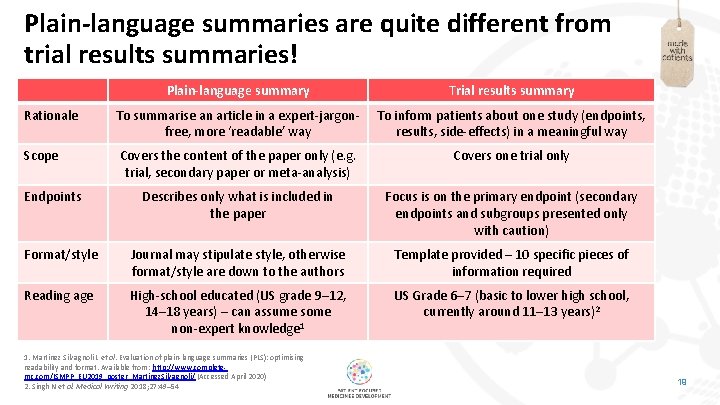
Plain-language summaries are quite different from trial results summaries! Plain-language summary Trial results summary Rationale To summarise an article in a expert-jargonfree, more ‘readable’ way To inform patients about one study (endpoints, results, side-effects) in a meaningful way Scope Covers the content of the paper only (e. g. trial, secondary paper or meta-analysis) Covers one trial only Describes only what is included in the paper Focus is on the primary endpoint (secondary endpoints and subgroups presented only with caution) Format/style Journal may stipulate style, otherwise format/style are down to the authors Template provided – 10 specific pieces of information required Reading age High-school educated (US grade 9– 12, 14– 18 years) – can assume some non-expert knowledge 1 US Grade 6– 7 (basic to lower high school, currently around 11– 13 years)2 Endpoints 1. Martínez Silvagnoli L et al. Evaluation of plain-language summaries (PLS): optimising readability and format. Available from: http: //www. completemc. com/ISMPP_EU 2019_poster_Martinez. Silvagnoli/ (Accessed April 2020) 2. Singh N et al. Medical Writing 2018; 27: 49– 54 19

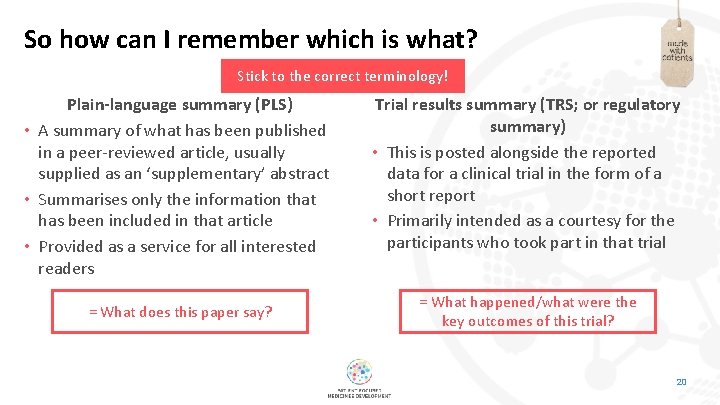
So how can I remember which is what? Stick to the correct terminology! Plain-language summary (PLS) • A summary of what has been published in a peer-reviewed article, usually supplied as an ‘supplementary’ abstract • Summarises only the information that has been included in that article • Provided as a service for all interested readers = What does this paper say? Trial results summary (TRS; or regulatory summary) • This is posted alongside the reported data for a clinical trial in the form of a short report • Primarily intended as a courtesy for the participants who took part in that trial = What happened/what were the key outcomes of this trial? 20

Part 2: thinking about readability Jan Seal-Roberts, Adis | Springer Healthcare Sarah Griffiths, Oxford Pharma. Genesis

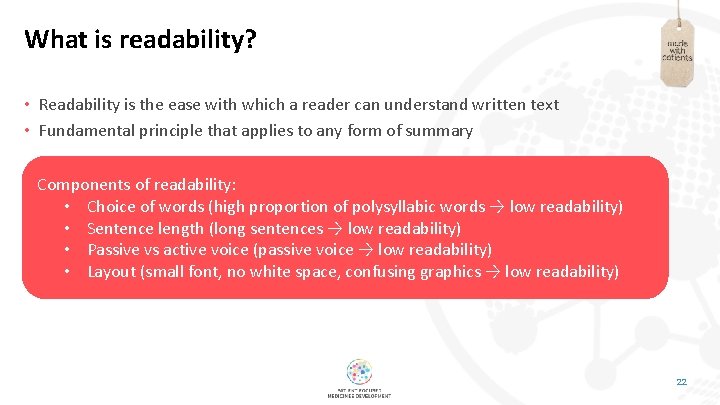
What is readability? • Readability is the ease with which a reader can understand written text • Fundamental principle that applies to any form of summary Components of readability: • Choice of words (high proportion of polysyllabic words → low readability) • Sentence length (long sentences → low readability) • Passive vs active voice (passive voice → low readability) • Layout (small font, no white space, confusing graphics → low readability) 22

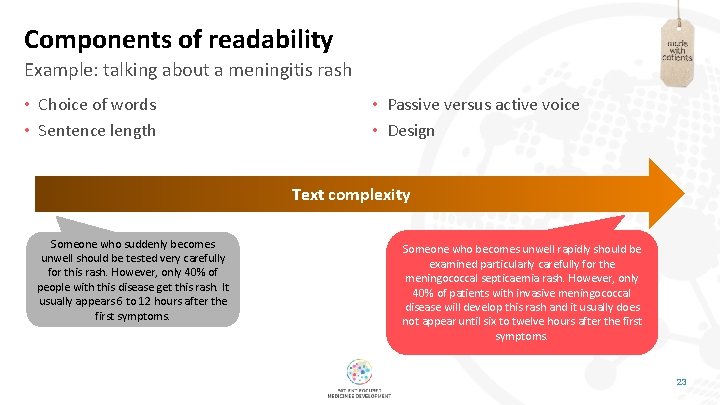
Components of readability Example: talking about a meningitis rash • Choice of words • Sentence length • Passive versus active voice • Design Text complexity Someone who suddenly becomes unwell should be tested very carefully for this rash. However, only 40% of people with this disease get this rash. It usually appears 6 to 12 hours after the first symptoms. 12 Someone who becomes unwell rapidly should be examined particularly carefully for the meningococcal septicaemia rash. However, only 40% of patients with invasive meningococcal disease will develop this rash and it usually does not appear until six to twelve hours after the first symptoms. 23

Health literacy in the USA • Approximately 36% of adults have limited health literacy 1 • Only 12% of the population has proficient health literacy 1 “Nearly 9 out of 10 adults have difficulty using the everyday health information that is routinely available in our health care facilities, retail outlets, media and communities” 2 1. Kutner M et al. The health literacy of America’s adults: results from the 2003 National Assessment of Adult Literacy (NCES 2006– 486). 2006. Available from: http: //nces. ed. gov/pubs 2006/2006483. pdf (Accessed 8 September 2016) 2. US Department of Health and Human Services, Office of Disease Prevention and Health Promotion. National action plan to improve health literacy. 2010. Available from: https: //health. gov/communication/HLAction. Plan/pdf/Health_Lit_Action_Plan_Summary. pdf (Accessed 8 September 2016) 24

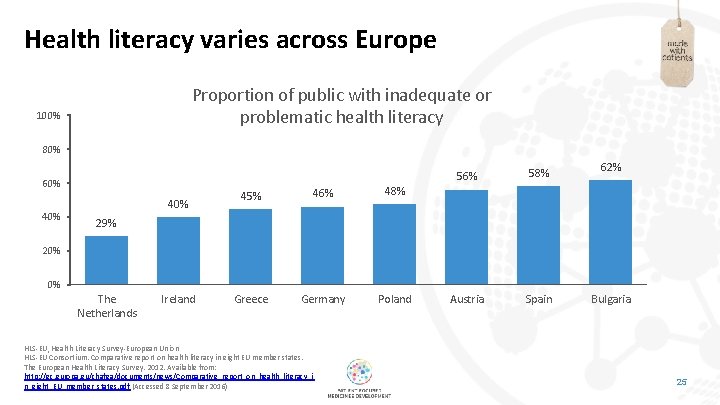
Health literacy varies across Europe Proportion of public with inadequate or problematic health literacy 100% 80% 60% 40% 45% 46% 48% Greece Germany Poland 56% 58% Austria Spain 62% 29% 20% 0% The Netherlands Ireland HLS-EU, Health Literacy Survey-European Union HLS-EU Consortium. Comparative report on health literacy in eight EU member states. The European Health Literacy Survey. 2012. Available from: http: //ec. europa. eu/chafea/documents/news/Comparative_report_on_health_literacy_i n_eight_EU_member_states. pdf (Accessed 8 September 2016) Bulgaria 25

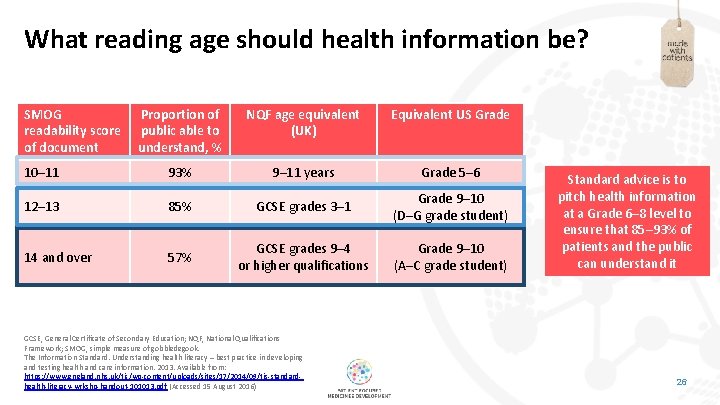
What reading age should health information be? SMOG readability score of document Proportion of public able to understand, % NQF age equivalent (UK) Equivalent US Grade 10– 11 93% 9– 11 years Grade 5– 6 12– 13 85% GCSE grades 3– 1 Grade 9– 10 (D–G grade student) 14 and over 57% GCSE grades 9– 4 or higher qualifications Grade 9– 10 (A–C grade student) GCSE, General Certificate of Secondary Education; NQF, National Qualifications Framework; SMOG, simple measure of gobbledegook. The Information Standard. Understanding health literacy – best practice in developing and testing health and care information. 2013. Available from: https: //www. england. nhs. uk/tis/wp-content/uploads/sites/17/2014/09/tis-standardhealth-literacy-wrkshp-handout-101013. pdf (Accessed 15 August 2016) Standard advice is to pitch health information at a Grade 6– 8 level to ensure that 85– 93% of patients and the public can understand it 26

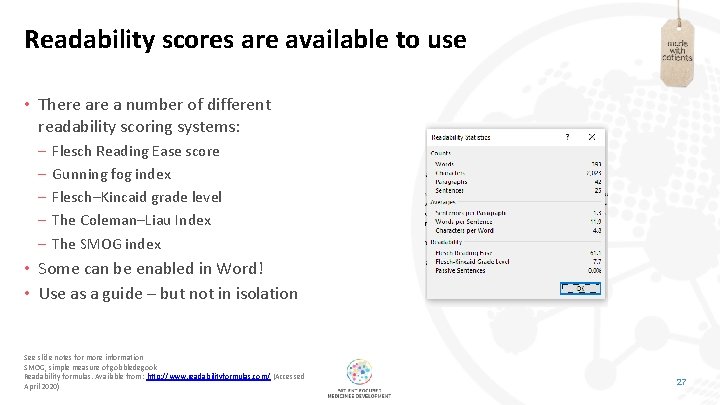
Readability scores are available to use • There a number of different readability scoring systems: – – – Flesch Reading Ease score Gunning fog index Flesch–Kincaid grade level The Coleman–Liau Index The SMOG index • Some can be enabled in Word! • Use as a guide – but not in isolation See slide notes for more information SMOG, simple measure of gobbledegook Readability formulas. Available from: http: //www. readabilityformulas. com/ (Accessed April 2020) 27

Readability scoring SMOG, simple measure of gobbledegook Readability formulas. Available from: http: //www. readabilityformulas. com/ (Accessed April 2020) 28

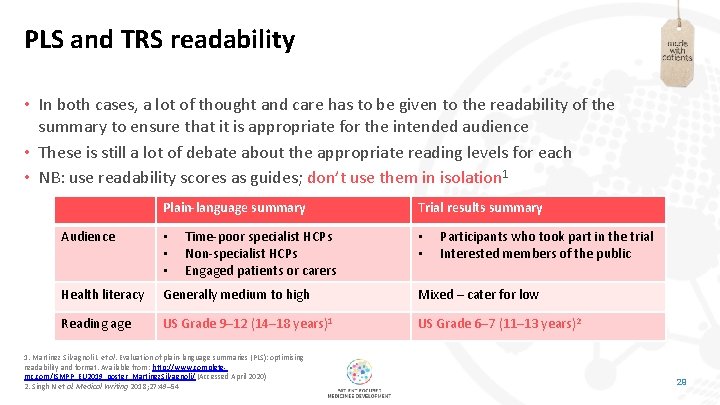
PLS and TRS readability • In both cases, a lot of thought and care has to be given to the readability of the summary to ensure that it is appropriate for the intended audience • These is still a lot of debate about the appropriate reading levels for each • NB: use readability scores as guides; don’t use them in isolation 1 Plain-language summary Time-poor specialist HCPs Non-specialist HCPs Engaged patients or carers Trial results summary Audience • • • Health literacy Generally medium to high Mixed – cater for low Reading age US Grade 9– 12 (14– 18 years)1 US Grade 6– 7 (11– 13 years)2 1. Martínez Silvagnoli L et al. Evaluation of plain-language summaries (PLS): optimising readability and format. Available from: http: //www. completemc. com/ISMPP_EU 2019_poster_Martinez. Silvagnoli/ (Accessed April 2020) 2. Singh N et al. Medical Writing 2018; 27: 49– 54 • • Participants who took part in the trial Interested members of the public 29

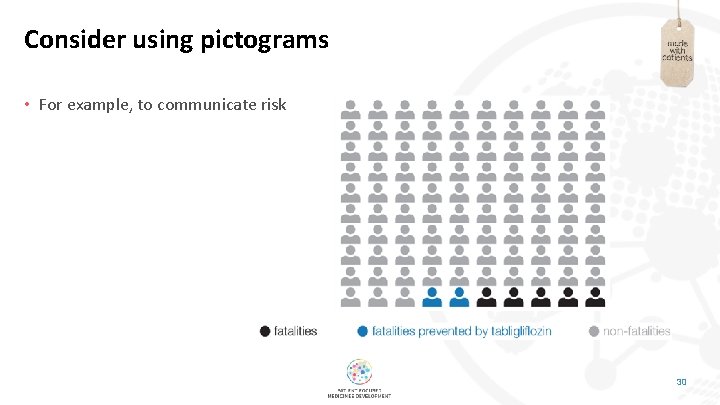
Consider using pictograms • For example, to communicate risk 30

Example: making it easier for patients to adhere to treatment • Understanding of common prescriptions and adherence to medication is reported to be as low as 50%1, 2 • Providing patients with calendars listing when to take their medications can improve adherence and treatment outcomes 3 • Prescription timetable for a commonly misunderstood prescription can help (chloramphenicol eye drops) 1. Davis TC et al. Ann Intern Med 2006; 145: 887– 94; 2. World Health Organization. Adherence to long-term therapies: evidence for action. 2003. Available from: http: //www. who. int/chp/knowledge/publications/adherence_full_report. pdf (Accessed 11 May 2018); 3. Zullig LL et al. Patient Educ Couns 2014; 95: 288– 91 31

Part 3: writing a summary – top tips Jan Seal-Roberts, Adis | Springer Healthcare Sarah Griffiths, Oxford Pharma. Genesis

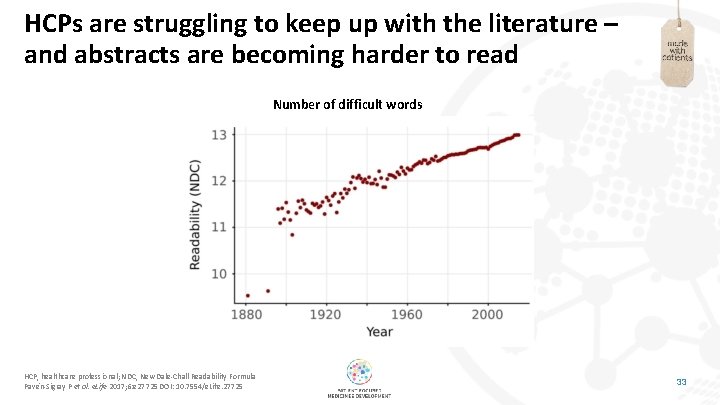
HCPs are struggling to keep up with the literature – and abstracts are becoming harder to read Number of difficult words HCP, healthcare professional; NDC, New Dale-Chall Readability Formula Pavén-Sigray P et al. e. Life 2017; 6: e 27725 DOI: 10. 7554/e. Life. 27725 33

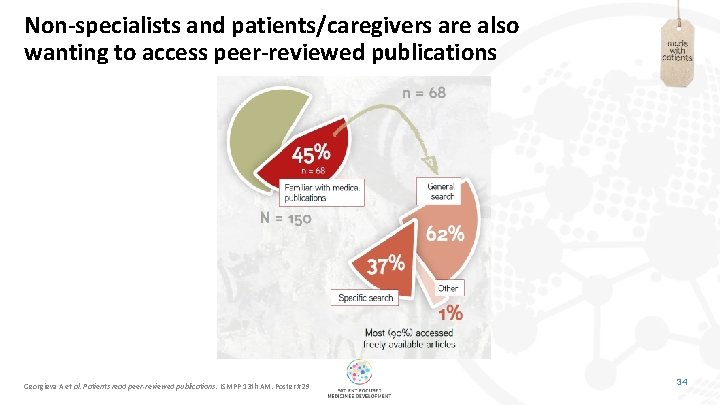
Non-specialists and patients/caregivers are also wanting to access peer-reviewed publications Georgieva A et al. Patients read peer-reviewed publications. ISMPP 13 th AM. Poster #29 34

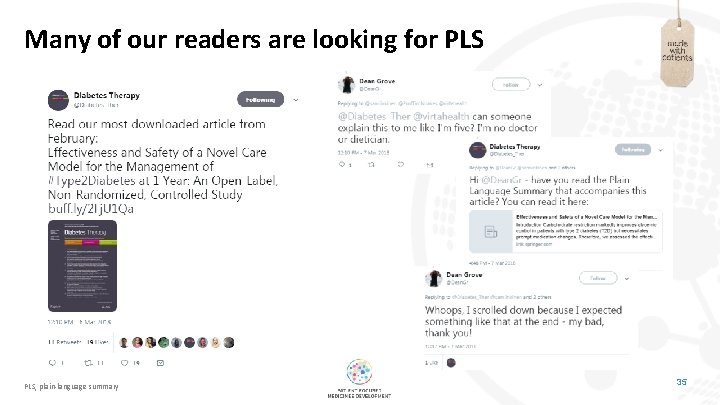
Many of our readers are looking for PLS, plain-language summary 35

And research indicates that plain-language summaries are useful tools Value of Developing Plain Language Summaries of Scientific and Clinical Articles: A Survey of Patients and Physicians – published in Therapeutic Innovation & Regulatory Science, Nov 17: Daphnee S. Pushparajah (UCB) et al. • • 74 patients with chronic disease diagnosis and 90 randomly selected neurologists in the USA participated in online surveys Surveyed patients reported that they routinely sought health-related information online – Articles in scientific journals were ranked the third-most important source in the survey (47%) – after general Internet searches (61%) and patient-specific websites (57%) – Patients stressed importance of knowledge and the sense of empowerment this engenders • Surveyed doctors were more equivocal – 46% rated lay summaries as valuable, 46% as neutral, and 8% as not valuable (oops!) – However, 60% reported that they would use them – especially important in patient dialogue – Saves doctors’ time – and improves patient communication Pushparajah DS et al. Value of developing plain language summaries of scientific and clinical articles: a survey of patients and physicians. Available from: Http: //journals. sagepub. com/doi/abs/10. 1177/2168479017738723 (Accessed April 2020) 36

So what makes a good plain-language summary? Format and content • Need to focus on the significance of the research (the ‘so what? ’) rather than the ‘how? ’ • Need to consider what’s appropriate for intended audience (likely to depend on therapeutic area) – but common non-expert jargon and abbreviations are acceptable 1 • Lots of tools available to simplify writing style (e. g. Microsoft Word) and online readability tools (e. g. Readable. Pro 2 – provides readability and keyword density analysis; Readability Formulas 3 – checks for reading levels) • Where possible, consider generalising numbers (e. g. to ‘half’, one-third’ etc. ) to improve clarity and readability – but care is needed not to change overall meaning 1 • Need to ensure that all visuals used are necessary and actually support the text – Too many visuals or stock photos can be confusing and/or distracting The skills and experience of trained writers are likely to be very useful! 1. Martínez Silvagnoli L et al. Evaluation of plain-language summaries (PLS): optimising readability and format. Available from: http: //www. completemc. com/ISMPP_EU 2019_poster_Martinez. Silvagnoli/ (Accessed April 2020) 2. Readable. Test your readability. Available from: https: //readable. com/text/? gclid=EAIa. IQob. Ch. MI 3 Pzl 6 Pmk 4 QIVDMDICh 1 Ing. MFEAAY ASAAEg. JYUv. D_Bw. E (Accessed April 2020); 3. Readability formulas. Available 37 from: http: //www. readabilityformulas. com/ (Accessed April 2020)

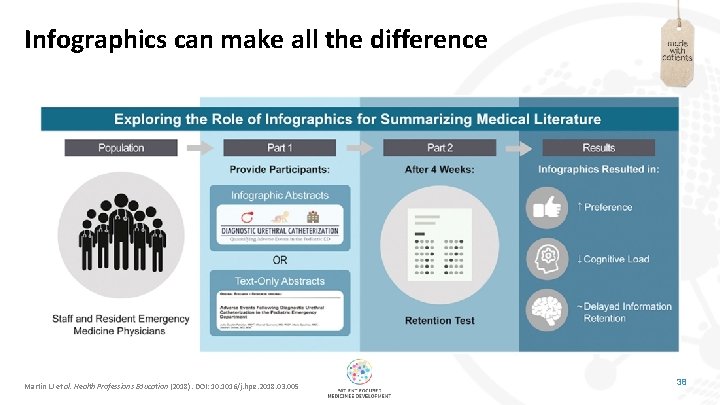
Infographics can make all the difference Martin LJ et al. Health Professions Education (2018). DOI: 10. 1016/j. hpe. 2018. 03. 005 38

Top tips for a good plain-language summary 1 • Avoid ‘expert-only’ jargon, excessive adverbs, and the passive voice • Focus on the ‘so what? ’ • Use short sentences and aim for clarity – don’t just take out a few complex • • • words from the standard abstract to produce a ‘dumbed down’ version But: don’t aim too low either – this is likely to annoy readers (remember to aim for 14– 18 years) Consider use of subheadings (framed as questions) if journal allows Infographics can be helpful if journal allows – but only if adding real clarity (e. g. results or methodology) Consider using non-specialist peers and lay readers to review and provide objective feedback – but be prepared to be open-minded Ensure the PLS content remains faithful to the original article 1. Martínez Silvagnoli L et al. Evaluation of plain-language summaries (PLS): optimising readability and format. Available from: http: //www. completemc. com/ISMPP_EU 2019_poster_Martinez. Silvagnoli/ (Accessed April 2020) At every stage, think: end-user! 39

But patients also want to find out the results of clinical studies – especially their own! • Most patients want to know the results of their study – In 2017, a survey of 12 427 clinical study participants, 72% of respondents were most interested in receiving information after participating in a clinical study 1 – 91% rated receiving a study summary after participation as being very important • But few had actually been told about the study findings – 53% of these participants had never been notified of the results • Improving participant communication is likely to improve participation in clinical trials 2 – Most participants would not participate in future clinical trials (272 [68%]) if they were not informed about the results 2 – Trial participants feel they are “no longer valued” by researchers 3 1. Center for Information and Study on Clinical Research Participation. Perceptions and Insights Study. Available from: https: //www. ciscrp. org/services/researchservices/perceptions-and-insights-study/ (Accessed April 2020); 2. Sood A et al. Mayo Clinic Proceedings 2009; 84: 243– 7; 3. Getz KA. The Monitor 2008: 17– 21 40

Part 4: plain-language summaries – development and publication Jan Seal-Roberts, Adis | Springer Healthcare

Just to recap Plain-language summaries sit alongside published articles, aiming to summarise in clear language what has been presented in a peer-reviewed paper • Journals are increasingly offering authors the opportunity to include these • Provide a quick entrée for the time-poor expert – and also non-specialists (e. g. crossdiscipline specialists, GPs, nurses, etc. ) and non-native speakers • Also useful for other interested parties (e. g. payer, tax payers, etc. ) • But can also be very useful for patients/carers who wish to be better informed on their treatment options – but this is not the primary purpose of the peer-reviewed literature – NB: it can raise concerns re ‘inappropriate promotion’ if patients are regarded as a key intended audience for the peer-reviewed medical literature Overall aim: to achieve greater transparency and impact of published outcomes via increased readability, understanding and a wider audience reach 42

Who is responsible for creating PLS? • PLS are the responsibility of the authors submitting the paper • But are often written by a specialist writer (it takes a special skill set to get these right) • Most publishers accepting PLS require these to be submitted at the same time as the paper (but a • • few allow these to follow – e. g. Adis Rapid+ journals) PLS should always be peer reviewed – for accuracy and to ensure they represent only what is in the paper (and make no other claims) These usually sit just below the main abstract, but may additionally be hosted elsewhere (e. g. Fig. Share) – although Pub. Med’s policy in including these currently seems ‘variable’ For hybrid journals any PLS should ideally always be available open access for easy reading prior to downloading the full article But it’s early days – and the quality of PLS in the published literature is currently inconsistent PLS, plain-language summaries 43

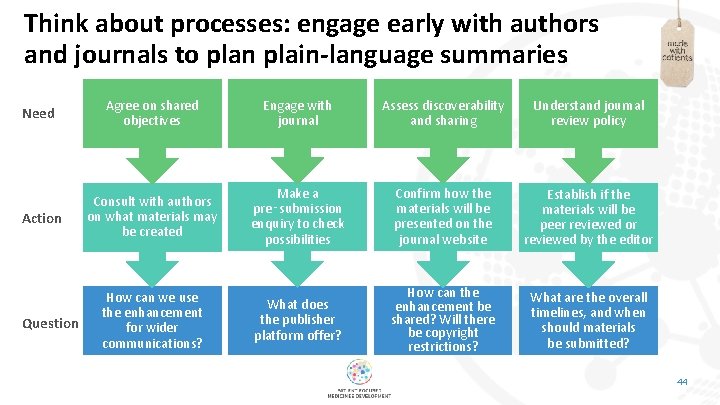
Think about processes: engage early with authors and journals to plan plain-language summaries Need Agree on shared objectives Engage with journal Assess discoverability and sharing Understand journal review policy Action Consult with authors on what materials may be created Make a pre‑submission enquiry to check possibilities Confirm how the materials will be presented on the journal website Establish if the materials will be peer reviewed or reviewed by the editor How can we use the enhancement for wider communications? What does the publisher platform offer? How can the enhancement be shared? Will there be copyright restrictions? What are the overall timelines, and when should materials be submitted? Question 44

Where can plain-language summaries be published? • • Currently rather patchy, but PLS are now becoming a lot more commonplace – depends on publisher, individual journal policy, and often on therapeutic area Journals within therapeutic areas recognised as having higher levels of engagement generally seem to be showing most interest in offering this facility (e. g. neurology, dermatology, rheumatology) All Adis journals have been accepting PLS since mid 2017 A few journals (including PLOS Medicine) are now mandating these – PLOS Medicine requests an ‘Author Summary’: two or three sentences covering the following: – – Why was this study done? What did the researchers do and find? What do these findings mean? Many publishers are now happy to accept PLS – just ask! If not, consider using an infographic instead (e. g. for complex methodology or outcomes) “Bullet points should be objective, brief, succinct, specific, accurate, and avoid technical language. ” These are requested at revised-proof stage 1 1. PLOS Medicine. Revising your manuscript. Available from: https: //journals. plos. org/plosmedicine/s/revising-your-manuscript (Accessed April 2020) 45

Some of the journals and organisations currently accepting or requiring PLS* Journal/society What are they called? Length? Patient summaries 500– 1000 words Lay abstracts 250 words max. N/A Variable, generally around 500– 700 words Plain-language summaries 400– 700 words e. Life digests 200– 400 words Author summaries 6– 9 single-sentence bullet points Significance statements ~120 words Annals of the Rheumatic Diseases Autism British Psychological Society Cochrane Library e. Life PLOS Medicine Proceedings of the National Academy of Sciences *Taken from: Shailes S. Plain-language summaries of research: something for everyone. Available from: https: //elifesciences. org/articles/25411 (Accessed April 2020) 46

But ‘big’ journals still have limited options for PLS Journal Open access options Journal-generated enhancements • Delayed OA after 6‑month embargo No APC • Quick Take Videos – one featured article every week Available for industry‑funded research • Some papers selected for audio interview with journal • • Author-generated enhancements Plain-language summaries • • ‘Research in context’ section could be adapted as PLS • ‘Publication highlights’ section could be adapted as PLS, or submitted as supplementary material • • • All research articles available to read immediately on JN Reader app and 6 months after publication on JAMA website No APC • Immediate OA available (CC BY-NC) ~US$5000 APC applied APC, article processing charge A variety of content, including videos, considered as supplementary material Peer reviewed • • Visual abstracts for most clinical studies Some papers selected for video/audio interview with journal Supplementary material should complement and not repeat the information in the paper • Visual abstracts • Video abstracts with paper and on BMJ You. Tube channel Not peer reviewed Other content as supplementary material • • 47

Publisher platforms dictate what is possible for their journals Elsevier • All journals accept: Audio. Slides (slideshow with narration) • Some journals may also accept: visual and video abstracts, PLS, interactive questions Springer Nature • All 33 Adis journals accept enhanced content (visual abstracts, videos, PLS, infographics, animations, e-learning, etc. ) • Some Bio. Med Central titles accept PLS and video abs Wiley • Some journals may accept: video abstracts, video bytes (1 -minute videos for lay audience), PLS Informa/Taylor & Francis • All Dove Medical Press journals accept video abstracts • Other journals may accept video abstracts • Publishers often allow enhancements, including PLS and infographics, to be published alongside or in the supplementary material • Always check with the journal (NB: even if it is possible from a platform perspective, the journal may not allow it) Wolters Kluwer Health • Journals may accept video abstracts in the supplementary materials PLS, plain-language summaries 48

What is the journal policy of Adis? • All Adis articles can be accompanied by a PLS 1 – PLS are intended for readers requiring a succinct, simplified overview of a manuscript (such as informed patients and caregivers, and scientists outside of the field who may not have an in-depth knowledge of the topic) – The aim of a PLS is to assist in understanding the scientific content and overall implications of the manuscript – While some prior understanding of the topic may be assumed, shorter sentences without ambiguous or unnecessarily complex terms are recommended, as well as use of the active voice – PLS should be up to 250 words in length, and be placed after the Abstract of the article under the heading: ‘Plain Language Summary’ – PLS should be submitted to the relevant journal alongside the respective article in order for the PLS to be published after the main abstract – but if submitted retrospectively, will be published as an accompaniment to the article via a link-out positioned underneath the abstract – All PLS are peer-reviewed, either at the same time as the submitted article or later if submitted separately PLS, plain-language summaries 1. https: //www. google. com/search? ie=UTF-8&oe=UTF-8&q=Adis+plainlanguage+summaries&btn. G=Google+Search&gws_rd=ssl 49

Open access and paywalls Don’t forget to check: • whether your intended journal accepts PLS • and if so, where the PLS will be hosted • and whether this PLS will be easily accessible, open access/discoverable • All Adis journals (both fully open access and hybrid journals) accept PLS • These appear just below the abstract (NB: outside of any paywall) • NB: Pub. Med policy is currently ‘variable’ – they currently seem happy to include PLS for some journals – but not for others … PLS, plain-language summaries 50

If not, don’t forget that there are other ways to add clarity to a paper … 51

And there also non-journal opportunities for providing summaries of the medical literature … https: //www. growkudos. com/ https: //www. scienceopen. com/ https: //theconversation. com/us/health https: //atlasofscience. org/ https: //figshare. com/ 52

In closing … Jan Seal-Roberts, Adis | Springer Healthcare Sarah Griffiths, Oxford Pharma. Genesis

Take-home messages • Plain-language summaries are becoming an increasingly popular option for inclusion in peer-reviewed biomedical literature – Provide summary for time-poor HCPs and also for non-experts (which may include patients) – Assist in information readability, assimilation and discovery/reach/impact of article – Many journals welcome plain-language summaries as useful enrichments as these aid understanding – but may also provide a means of boosting the impact of published research to a wider audience – And remember that plain-language summaries of peer-reviewed articles can also be hosted elsewhere • Trial results summaries for patients are now mandated for clinical trial postings – But serve a different purpose for a wider audience – And a much lower level of prior knowledge should be assumed In both cases, a good ‘non-expert’ summary pays close attention to style and structure It’s not just about ‘dumbing down’ and avoiding long words! 54

Further reading • • Singh N et al. Writing lay summaries: What medical writers need to know. Medical Writing 2018; 27: 49– 54. EU Guidance for writing clinical lay summaries. Available from: https: //ec. europa. eu/health/sites/health/files/ eudralex/vol 10/2017_01_26_summaries_of_ct_results_for_laypersons. pdf Silvagnoli LM et al. Evaluation of plain-language summaries (PLS): optimising readability and format. Poster 21 presented at ISMPP EU 2019: http: //www. completemc. com/ISMPP_EU 2019_poster_Martinez. Silvagnoli/ Elsevier Researcher Academy: How to write a lay summary. Available from: https: //researcheracademy. elsevier. com/communicating-research/social-impact/write-lay-summary Nunn E, Pinfield S. Lay summaries of open access journal articles: engaging with the general public on medical research. Learned Publ 2014; 27: 173– 84. Salita JT et al. Writing for lay audiences: A challenge for scientists. Medical Writing 24. 4 (2015): 183– 9. Rees T et al. Patient lay summary framework. PAREXEL Access 2017. Available from: http: //preview. parexelmms. com/ISMPP-2017/index. aspx Duke M. How to write a lay summary, a Digital Curation Centre ‘working level’ guide. Digital Curation Centre, 2011. Available from: http: //www. bath. ac. uk/marketing/publicengagement/assets/How. To. Write. Lay. Summaries. UKOLN. pdf 55