Matter Property and Changes g Properties of Matter






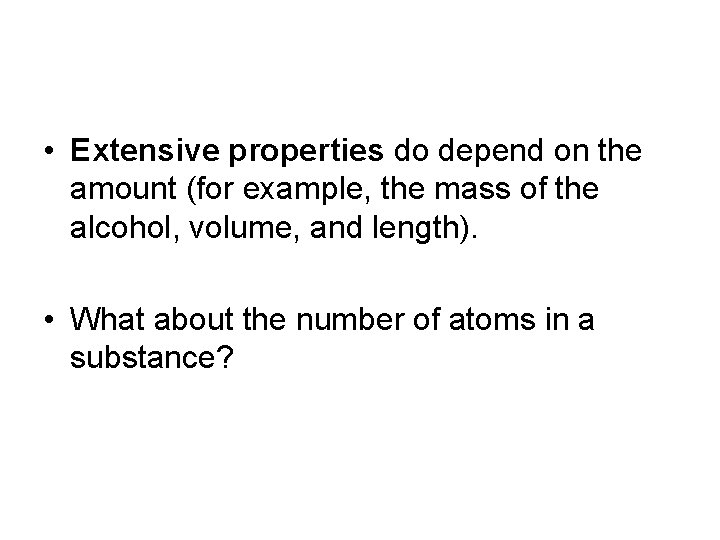





































- Slides: 65

Matter Property and Changes

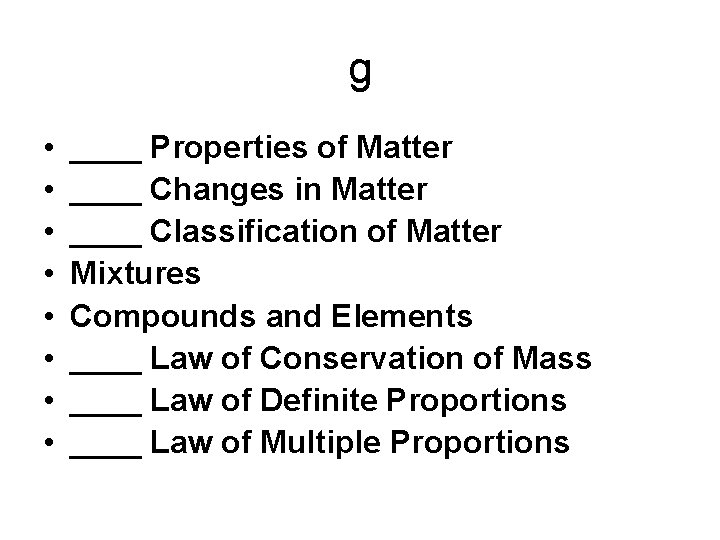
g • • ____ Properties of Matter ____ Changes in Matter ____ Classification of Matter Mixtures Compounds and Elements ____ Law of Conservation of Mass ____ Law of Definite Proportions ____ Law of Multiple Proportions

• • • Define the bold face words. Be sure you know table 3 -17 on page 71. Read Section 3 -1 Properties of Matter Take notes on the following terms: substance, solids, liquids, gases, physical properties, chemical properties, intensive properties, and extensive properties. Know the Law of Conservation of Mass. It is a good idea to leave space between each concept so that you can add any additional information in class. Read Section 3 -2 – Read this and takes notes on the following ideas: physical and chemical changes and the signs of a chemical reaction (fig 3 -8 and 3 -9) Read Section 3 -3. Read this and take notes on the following ideas: mixtures, heterogeneous, homogeneous. Separating mixtures. Having definitions and examples would be good. How are compounds different from mixtures? Read Section 3 -4 Elements and compounds. Know the difference between a compound a mixture. Become familiar with the Periodic Table of the Elements. Know The Law of Definite Proportions.

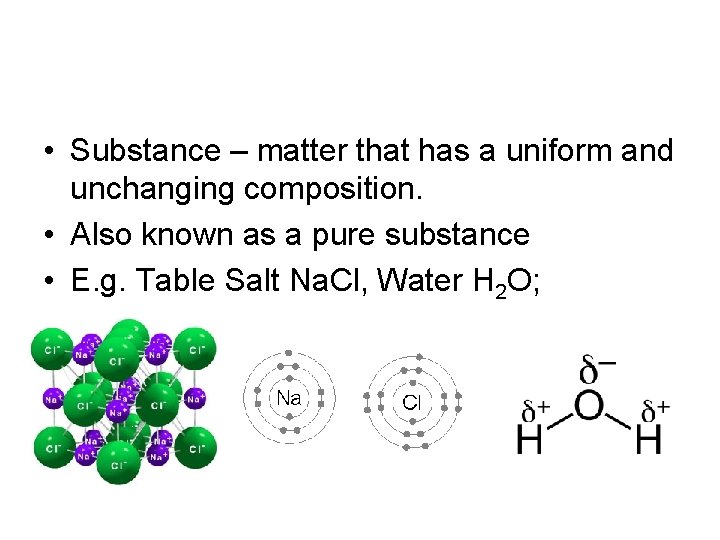
• Substance – matter that has a uniform and unchanging composition. • Also known as a pure substance • E. g. Table Salt Na. Cl, Water H 2 O;

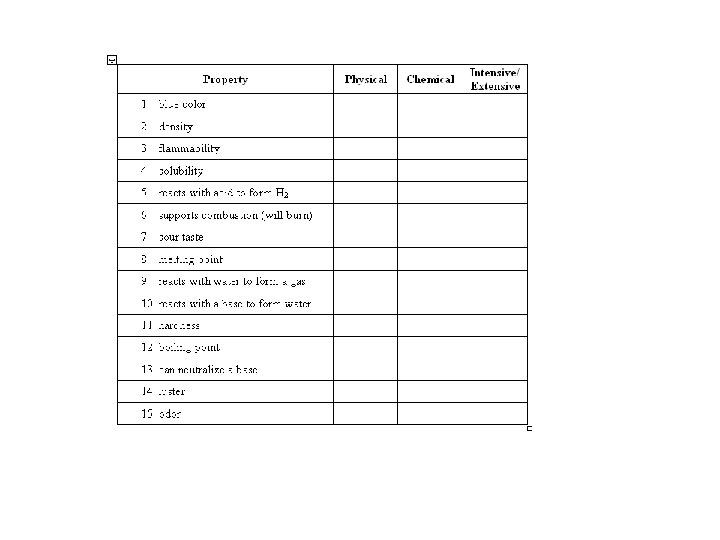
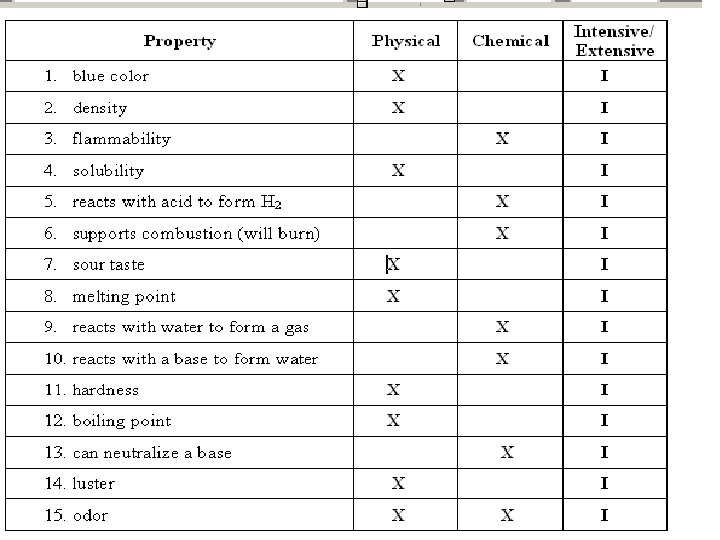
Physical Property • A physical property is observed with the senses and can be determined without destroying the object. For example, color, shape, mass, length, and odor are all examples of physical properties


chemical property • A chemical property indicates how a substance reacts with something else. The original substance is fundamentally changed in observing a chemical property. For example, the ability of iron to rust is a chemical property. The iron has reacted with oxygen, and the original iron metal is changed. It now exists as iron oxide, a completely different substance.


• Intensive properties do not depend on the amount of substance (for example, alcohol is flammable, also density, hardness, temperature). • What about color?
• Extensive properties do depend on the amount (for example, the mass of the alcohol, volume, and length). • What about the number of atoms in a substance?



http: //www. youtube. com/watch? v=C 4 p. QQQNwy 30

Phases of Matter • Solid- Liquid- Gas- Plasma-BEC

Phases of Matter • Solid- Liquid- Gas- Plasma-BEC

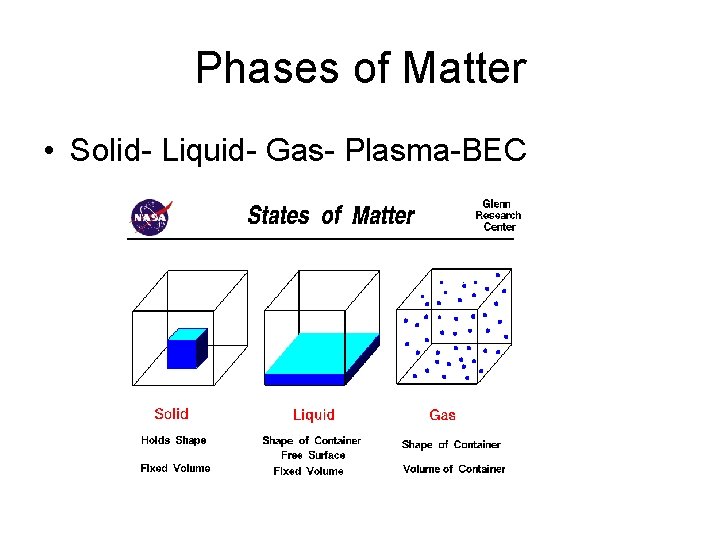
Phases of Matter • Solid- form of matter that has a definite shape and volume

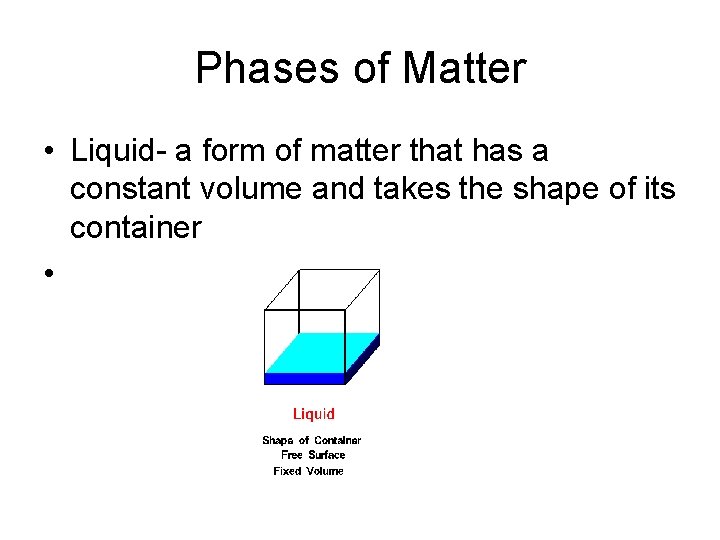
Phases of Matter • Liquid- a form of matter that has a constant volume and takes the shape of its container •

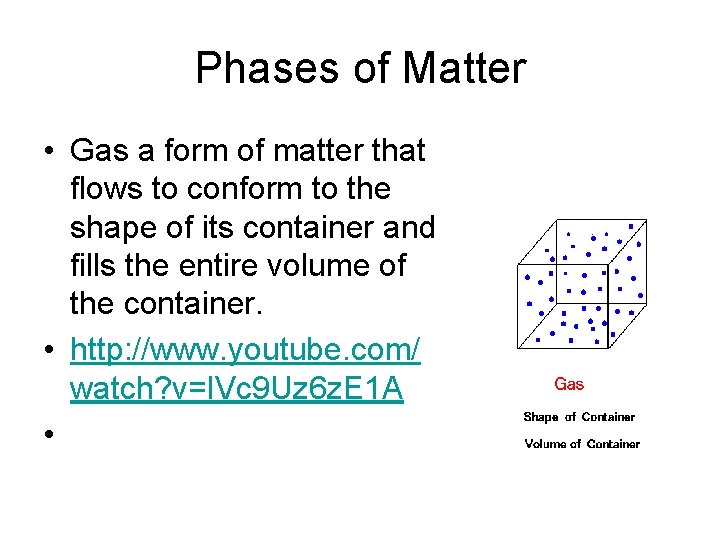
Phases of Matter • Gas a form of matter that flows to conform to the shape of its container and fills the entire volume of the container. • http: //www. youtube. com/ watch? v=IVc 9 Uz 6 z. E 1 A •

Phases of Matter • Solid- form of matter that has a definite shape and volume • Liquid- a form of matter that has a constant volume and takes the shape of its container • Gas a form of matter that flows to conform to the shape of its container and fills the entire volume of the container. • http: //www. youtube. com/watch? v=IVc 9 Uz 6 z. E 1 A

Physical vs. Chemical Changes In a physical change, the original substance still exists; it has only changed in form.

Physical vs. Chemical Changes In a chemical change, a new substance is produced. Energy changes always accompany chemical changes.

Physical vs. Chemical Changes In a chemical change, a new substance is produced. Energy changes always accompany chemical changes.


• • • • • Sodium hydroxide dissolves in water. Hydrochloric acid reacts with potassium hydroxide to produce salt, water, and heat. A pellet of sodium is sliced in two. Water is heated and changed to steam. Potassium chlorate decomposes into potassium chloride and oxygen gas. Iron rusts. When placed in water, a sodium pellet catches on fire as hydrogen gas is liberated and sodium hydroxide forms. Evaporation. Ice melting. Milk sours. Sugar dissolves in water. Wood rotting. Pancakes cooking on a griddle. Grass growing in a lawn. A tire is inflated with air. Food is digested in the stomach. Water is absorbed by a paper towel.

• Can it be separated by ordinary physical means? Yes. Classification of matter. All Matter. No____________Is its composition uniform? Can it be broken down by ordinary chemical means? Yes. No. No__________________________

Matter—Properties and Change: Additional Concepts Categories of Matter


Basic Assessment Questions Warm-Up Identify each of the following as an example of a chemical change or a physical change. A. Moisture in the air forms beads of water on a cold windowpane. B. An electric current changes water into hydrogen and oxygen. C. Yeast cells in bread dough make carbon dioxide an ethanol from sugar.

Matter—Properties and Change: Additional Concepts Categories of Matter

Matter—Properties and Change: Additional Concepts Mixtures • A mixture is a combination of two or more pure substances in which each pure substance retains its individual chemical properties. • The composition of mixtures is variable, and the number of mixtures that can be created by combining substances is infinite.

Matter—Properties and Change: Additional Concepts Mixtures • Although much of the focus of chemistry is the behavior of substances, it is important to remember that most everyday matter occurs as mixtures. • Substances tend naturally to mix; it is difficult to keep things pure.

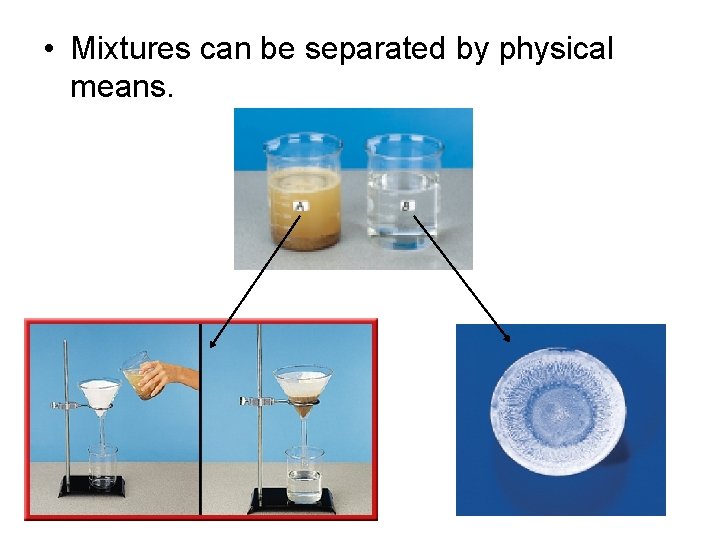
Matter—Properties and Change: Additional Concepts Mixtures • Two mixtures, sand water, and table salt and water, are shown. • You know water to be a colorless liquid


Matter—Properties and Change: Additional Concepts Types of Mixtures • Mixtures themselves are classified as either heterogeneous or homogeneous. • A heterogeneous mixture is one that does not blend smoothly throughout and in which the individual substances remain distinct. • The sand water mixture is an example of a heterogeneous mixture.

• A homogeneous mixture has constant composition throughout

• Mixtures can be separated by physical means.

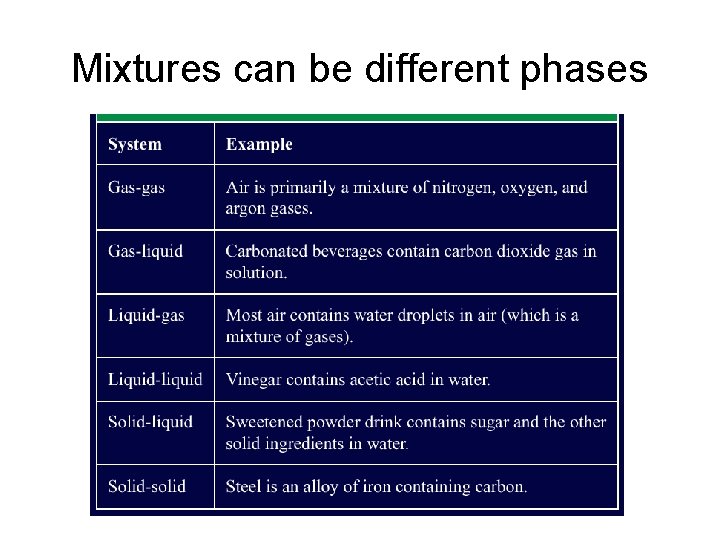
Mixtures can be different phases

• Chromotography- The separation occurs because the various components of the ink spread through the paper ant different rates.

Other means of physical separation • centrifuges/centrifuging * • chromatography (paper/thin layer) * * crystallisation * • decanting/decantation * • distillation (simple/fractional) * * evaporation * filtration * * magnet * mixture * molecule * * * precipitation * * * purification * * • sand/salt separation * • separating funnel * separating mixtures * *

• . filtration 2. mechanical separation 3. flotation 4. centrifugation 5. distillation 6. crystallization 7. chromatography 8. boiling 9. freezing 10. decantation 11. sublimation 12. evaporation 13. magnetic separation 14. scooping 15. sedimentation

Matter—Properties and Change: Additional Concepts Compounds • A compound is a combination of two or more different elements that are combined chemically. • Compounds have a unique composition and formula. • Water H 20, table salt Na. Cl, table sugar C 12 H 22 O 11 , and aspirin C 9 H 8 O 4 are examples of common compounds.

Matter—Properties and Change: Additional Concepts Compounds can be broken down into simpler substances by chemical means. To separate a compound into its elements often requires external energy such as heat or electricity.

• The properties of a compound are different from those of its component elements. The example of water illustrates this fact. • Water is a stable compound that is liquid at room temperature.

• When water is broken down into its components, it is obvious that hydrogen and oxygen are dramatically different than the liquid they form when combined. http: //www. youtube. com/watch? v=OTEX 38 b Q-2 w

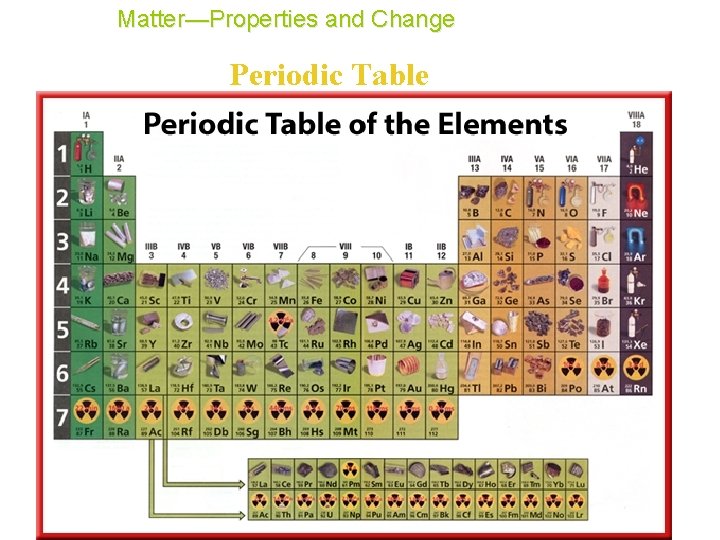
Matter—Properties and Change Periodic Table


Matter—Properties and Change: Additional Concepts Categories of Matter

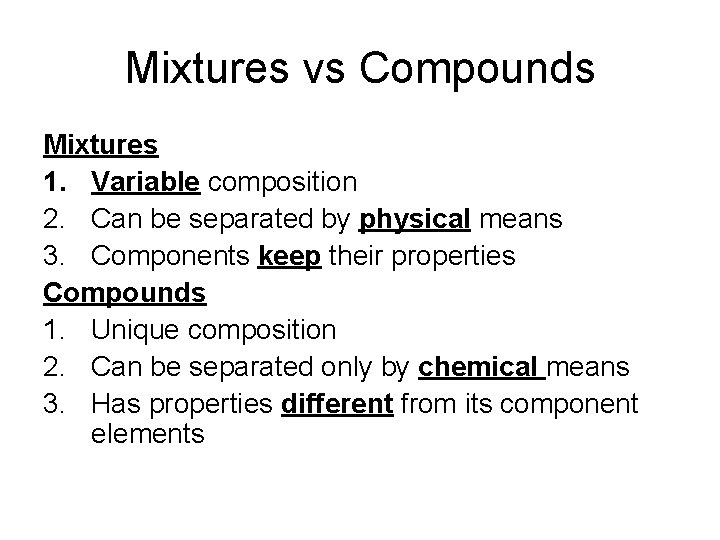
Mixtures vs Compounds Mixtures 1. Variable composition 2. Can be separated by physical means 3. Components keep their properties Compounds 1. Unique composition 2. Can be separated only by chemical means 3. Has properties different from its component elements

Evidence of a chemical reaction

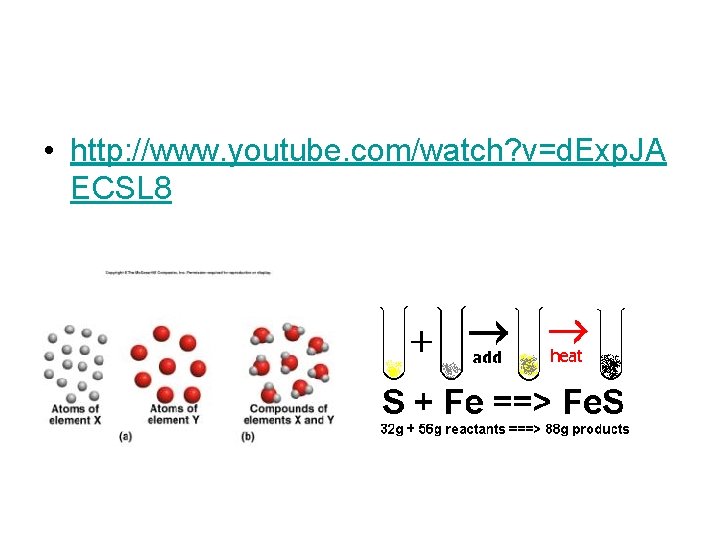
• The law of Conservation of Mass- mass is neither created nor destroyed during a chemical reaction; it is conserved Mass Reactants = Mass Products


• http: //www. youtube. com/watch? v=d. Exp. JA ECSL 8








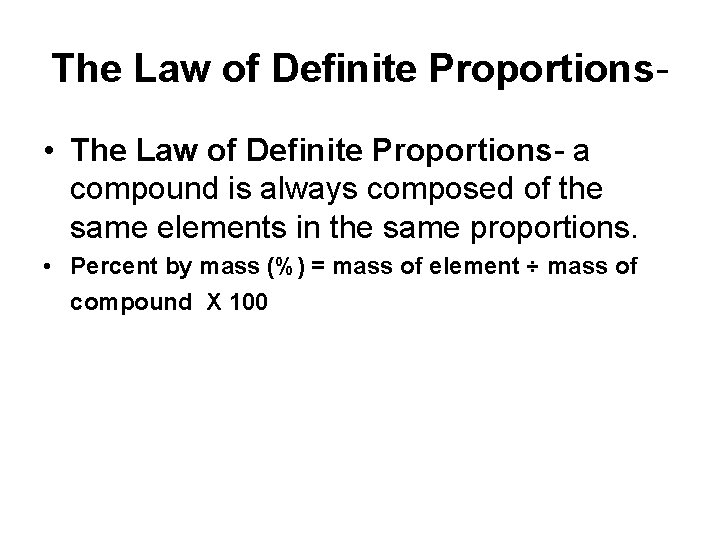
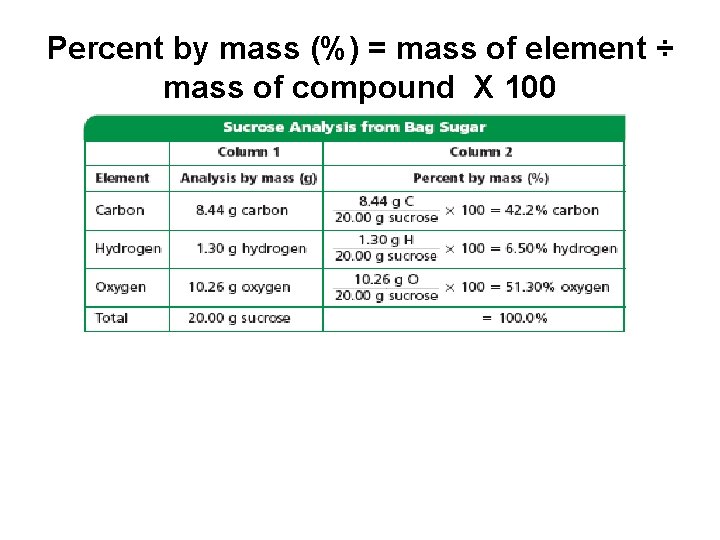
The Law of Definite Proportions • The Law of Definite Proportions- a compound is always composed of the same elements in the same proportions. • Percent by mass (%) = mass of element ÷ mass of compound X 100

Percent by mass (%) = mass of element ÷ mass of compound X 100




• http: //www. youtube. com/watch? v=ith. Qj 7 X eh. W 8&NR=1&feature=endscreen
 Properties and changes of matter worksheet
Properties and changes of matter worksheet Matter-properties and changes answer key
Matter-properties and changes answer key Which is a “big idea” for matter and change?
Which is a “big idea” for matter and change? Elizabeth mulroney
Elizabeth mulroney Chemical vs physical change
Chemical vs physical change Chemical properties and changes lesson 4
Chemical properties and changes lesson 4 Brainpop property changes
Brainpop property changes Definition of substance
Definition of substance True or false: chemical and physical changes alter matter.
True or false: chemical and physical changes alter matter. Classification of property law
Classification of property law Is smell a physical property
Is smell a physical property Changes in wave properties sorting activity
Changes in wave properties sorting activity Physical/chemical changes & properties color by number
Physical/chemical changes & properties color by number Thermodynamics intensive and extensive properties
Thermodynamics intensive and extensive properties Extensive vs intensive
Extensive vs intensive Physical change
Physical change 5 phases of matter
5 phases of matter Change in state of matter
Change in state of matter Types of change in matter
Types of change in matter Stp law
Stp law 6 common phase changes
6 common phase changes Concept map of states of matter
Concept map of states of matter Commutative and associative properties
Commutative and associative properties Classification and properties of matter
Classification and properties of matter Matter and its properties
Matter and its properties The study of composition structure and properties
The study of composition structure and properties Properties and characteristics of matter
Properties and characteristics of matter Example of useful and harmful materials at home
Example of useful and harmful materials at home Grade 7 natural science term 3 notes
Grade 7 natural science term 3 notes Common properties of matter
Common properties of matter The general properties of matter
The general properties of matter Electrical properties of matter
Electrical properties of matter What property of matter that describes a rusty anchor.
What property of matter that describes a rusty anchor. Hooke's law mathematical expression
Hooke's law mathematical expression General properties of solids
General properties of solids Physical and chemical properties of helium
Physical and chemical properties of helium White matter nervous system
White matter nervous system Brain falx
Brain falx Gray matter and white matter
Gray matter and white matter Rhinencephalon
Rhinencephalon Liquid state of matter properties
Liquid state of matter properties Properties of matter vocabulary
Properties of matter vocabulary Properties of matter concept map
Properties of matter concept map Objectives of properties of matter
Objectives of properties of matter Measurable properties examples
Measurable properties examples States of matter jeopardy
States of matter jeopardy Matter jeopardy
Matter jeopardy Volume properties of matter
Volume properties of matter Mixture matter graphic organizer
Mixture matter graphic organizer What are some chemical properties of matter
What are some chemical properties of matter Section 2 properties of matter
Section 2 properties of matter A material's ability to allow heat to flow is called _____.
A material's ability to allow heat to flow is called _____. Properties of matter vocabulary
Properties of matter vocabulary Matter and materials grade 7
Matter and materials grade 7 Big idea 8 properties of matter
Big idea 8 properties of matter Properties of water in matter
Properties of water in matter Physical properties of matter jeopardy
Physical properties of matter jeopardy Properties of matterwhat is matter?
Properties of matterwhat is matter? Physical properties of matter
Physical properties of matter Chapter 2 properties of matter answer key
Chapter 2 properties of matter answer key Bamboo basket properties of matter
Bamboo basket properties of matter Properties of matter elasticity
Properties of matter elasticity Qualitative and quantitative physical properties
Qualitative and quantitative physical properties Electrical properties of matter
Electrical properties of matter Properties of matter objectives
Properties of matter objectives What are matter waves
What are matter waves