CHEMICAL AND PHYSICAL PROPERTIES AND CHANGES https youtu


























- Slides: 28

CHEMICAL AND PHYSICAL PROPERTIES AND CHANGES

https: //youtu. be/37 pir 0 ej_SE

Is a New substance forming? (copy into your Science journal!) Write your prediction for each- YES or NO 1. Wood burns 2. ice cube melts 3. iron nail turns dark brown 4. piece of chalk is crushed

Physical Properties that can be observed or measured without changing the identity of the matter. Descriptions

Chemical Properties Characteristics that determine how a substance reacts with other substances.

Examples of Physical Properties State of Matter Taste Mass Volume Size Boiling Point Shape Freezing Point

Examples of Chemical Properties Ability to Burn Reaction with Oxygen (Oxidation) Reaction with other chemicals

Physical Changes Chemical Changes

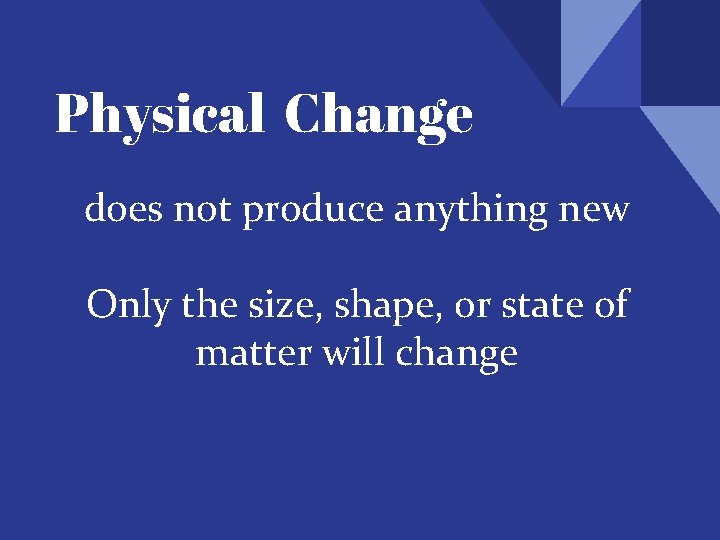
Physical Change does not produce anything new Only the size, shape, or state of matter will change

Physical Change 1. Change of state of matter (solid, liquid, or gas) melting, boiling 2. Change in size (cutting, chopping, breaking, shredding, glueing) 3. Change in shape (bending, crushing, twisting, stretching) 4. Dissolving (“sugar” in water) Still the SAME substance!

Examples of Physical Change 1. Splitting a wood log into kindling Change in size and shape

Examples of Physical Change 2. Ice cream melting in a dish Change in state of matter

Examples of Physical Change 3. Wadding a blanket into a ball Change in shape

Examples of Physical Change 4. Water freezing to make ice Change in state of matter

Examples of Physical Change 5. Dissolving sugar in a glass of tea The sugar is just all spread out – it is still sugar

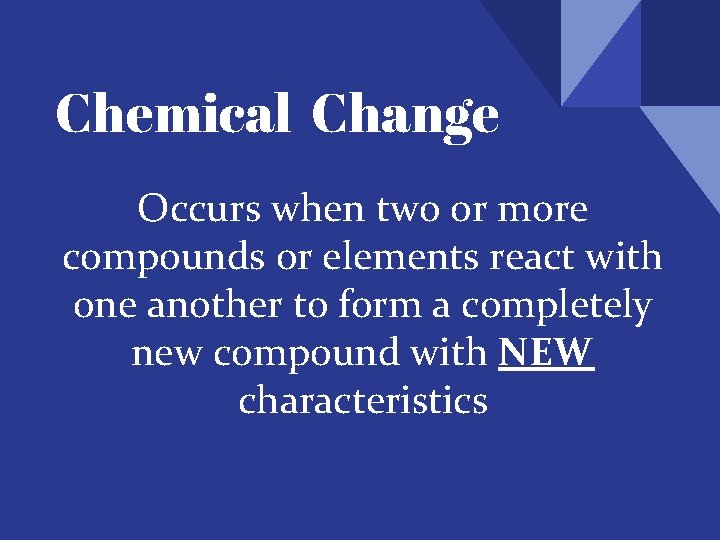
Chemical Change Occurs when two or more compounds or elements react with one another to form a completely new compound with NEW characteristics

Cool Chemical Reactions https: //youtu. be/Y 10 Oq_we 5 Yo

Indicators of a Chemical Change 1. A new color 2. Heat is Released (gets Hot) 3. Heat is Absorbed (gets Cold) 4. Production of Gas 5. Production of Light 6. Precipitate (solid is formed) 7. Odor 8. Rust (Oxidation) or Tarnish 9. Digesting 10. Baking

DEMO #1 A reaction between Calcium Chloride and Water What indicator of a chemical reaction do you observe? Heat is Released

Demo #2 Sodium Nitrate and H 2 O What indicator of a chemical reaction do you observe? Heat is Absorbed (gets Cold) https: //www. youtube. com/watch? v=h. Vh-bp. Av 4_E

DEMO #3 A reaction between Lead Nitrate and Potassium Iodide What indicator of a chemical reaction do you observe? Color change and production of precipitate

Law of Conservation of Mass cannot be created nor destroyed but only changes forms. The total mass always remains the same.

Is a New substance forming? (copy into your Science journal!) Write your prediction for each- YES or NO 1. Wood burns - YES 2. ice cube melts - No 3. iron nail turns dark brown -YES 4. piece of chalk is crushed- No

Vocabulary Solubility- the ability of a substance to be dissolved Solution- a mixture of two or more substances. A solution is a physical change.

Precipitate- the creation of a solid from a solution. When the reaction occurs in a liquid solution, the solid formed is called the 'precipitate'. The chemical that causes the solid to form is called the 'precipitant'.

Photosynthesis Chemical OR Physical Change?

Chemical Change? Label chemical or physical change & list the evidence of the change. 1. Water boils in a pot on the stove _ 2. Gasoline is combusted in a car engine ___ 3. Salt dissolves in water ____ 4. Two liquids are mixed together and a white solid forms and sinks to the bottom ____ 5. A piece of metal dissolves in acid and bubbles form in the solution ____ 6. Photosynthesis is _______ 7. An aluminum can is crushed and recycled ____

Brain Pop https: //www. brainpop. com/science/matterandchemistry/propertyc hanges/