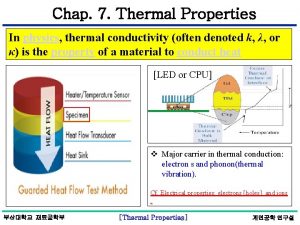
PROPERTIES OF MATTER DENSITY THERMAL AND ELECTRICAL CONDUCTIVITY








- Slides: 16

PROPERTIES OF MATTER: DENSITY, THERMAL AND ELECTRICAL CONDUCTIVITY SC. 8. P. 8. 4 Classify and compare substances on the basis of characteristic physical properties that can be demonstrated or measured: for example, density; thermal or electrical conductivity; solubility; magnetic properties; melting and boiling points; and know that these properties are independent of the amount of the sample. (Also assesses SC. 8. P. 8. 3. ) ESSENTIAL QUESTION: How can the physical properties of a substance help us reliably identify unknown substances?

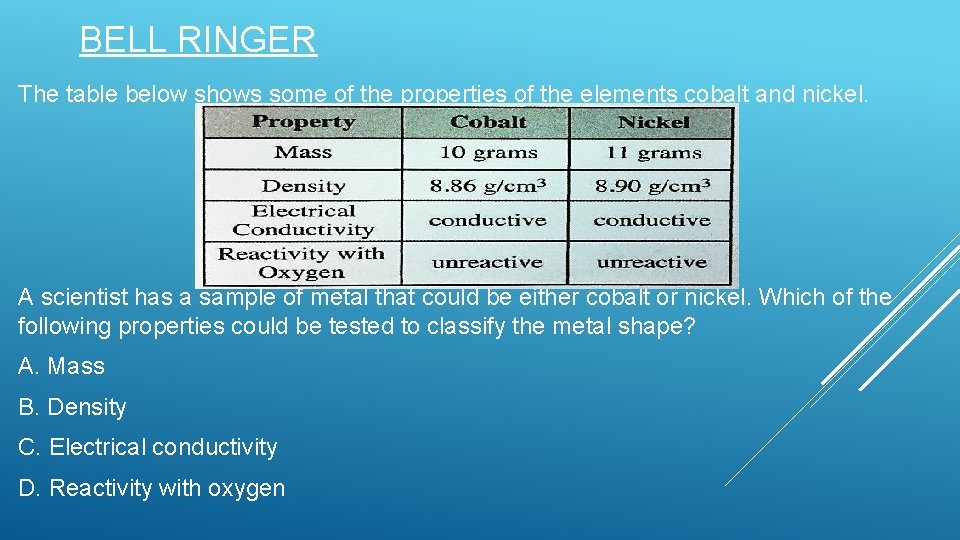
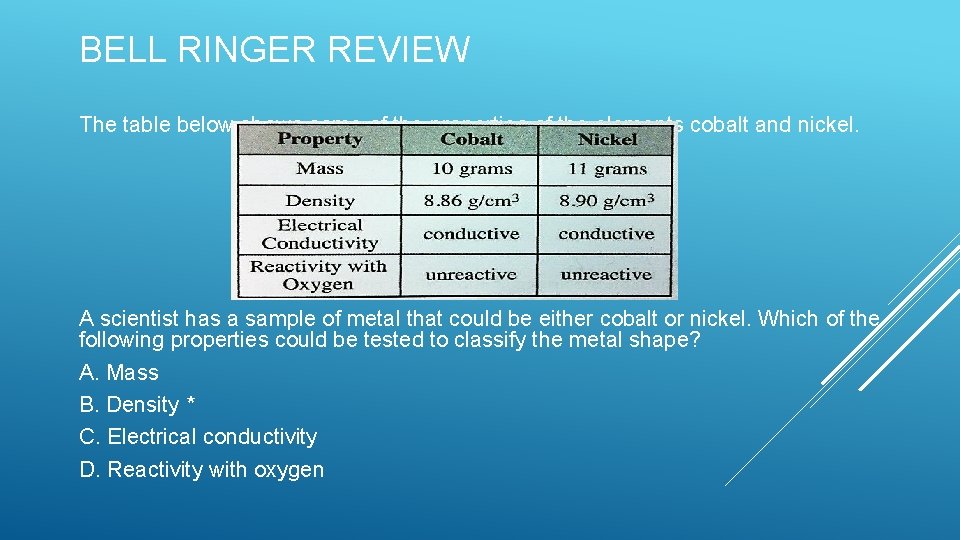
BELL RINGER The table below shows some of the properties of the elements cobalt and nickel. A scientist has a sample of metal that could be either cobalt or nickel. Which of the following properties could be tested to classify the metal shape? A. Mass B. Density C. Electrical conductivity D. Reactivity with oxygen

INTERACTIVE JOURNAL RIGHT SIDE

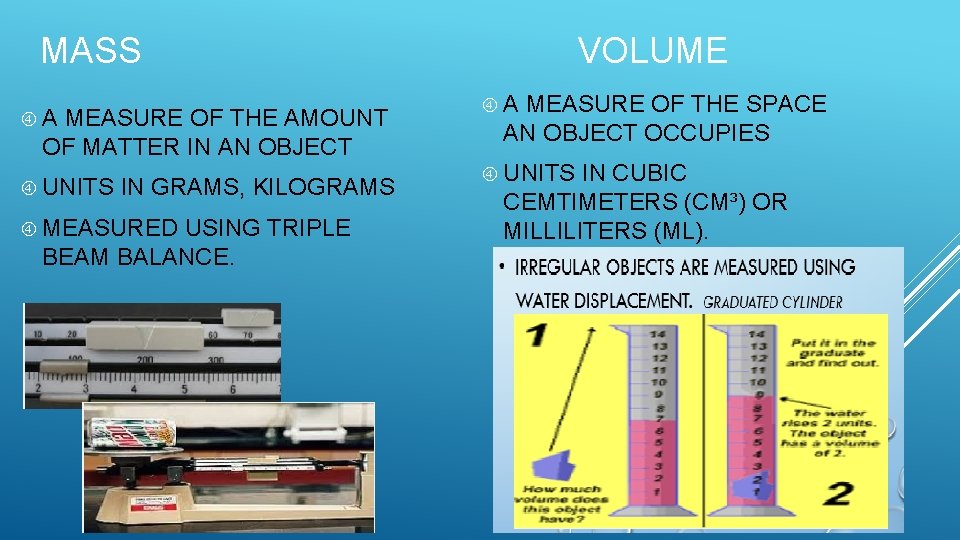
VOLUME MASS A MEASURE OF THE AMOUNT OF MATTER IN AN OBJECT UNITS IN GRAMS, KILOGRAMS MEASURED USING TRIPLE BEAM BALANCE. A MEASURE OF THE SPACE AN OBJECT OCCUPIES UNITS IN CUBIC CEMTIMETERS (CM³) OR MILLILITERS (ML).

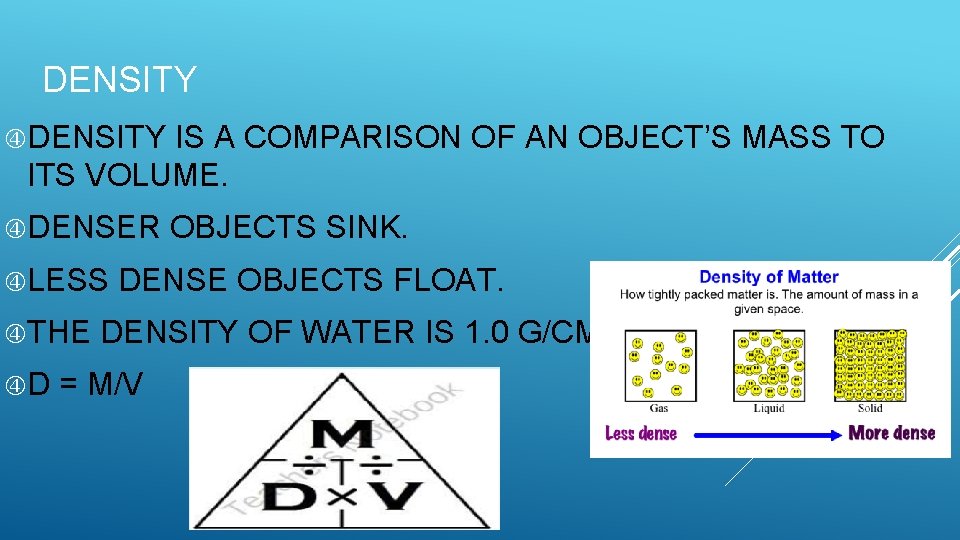
DENSITY IS A COMPARISON OF AN OBJECT’S MASS TO ITS VOLUME. DENSER LESS THE D OBJECTS SINK. DENSE OBJECTS FLOAT. DENSITY OF WATER IS 1. 0 G/CM³ = M/V

Density Sample Question: What is the density of a piece of metal if the mass of the metal is 562 grams, and it occupies 44. 9 m. L of space? mass volume What is the density of the metal?

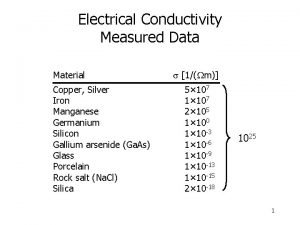
ELECTRICAL CONDUCTIVITY A material that allows electricity to pass through it easily.

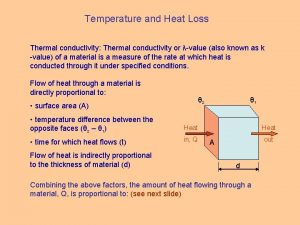
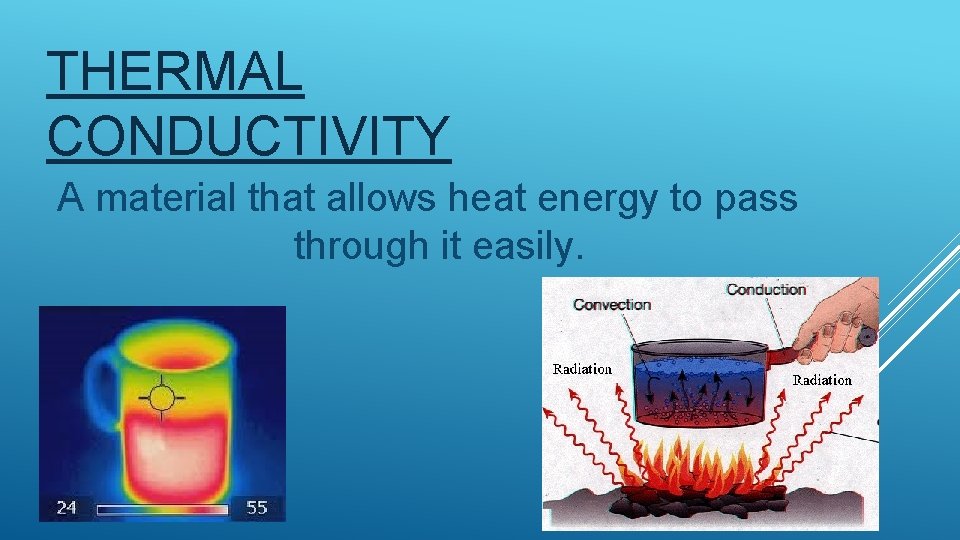
THERMAL CONDUCTIVITY A material that allows heat energy to pass through it easily.

COMMON CONDUCTORS METALS are excellent conductors of heat and electricity!

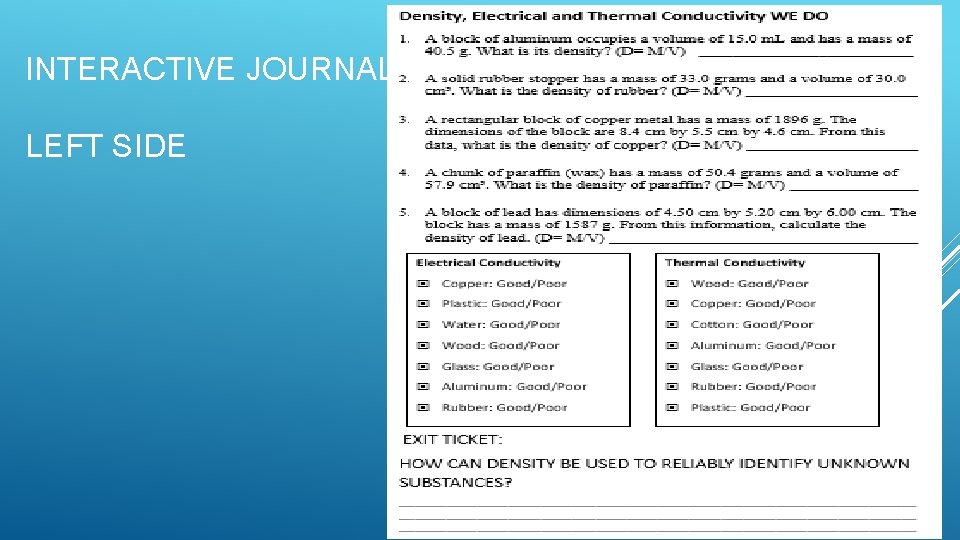
INTERACTIVE JOURNAL LEFT SIDE

BELL RINGER REVIEW The table below shows some of the properties of the elements cobalt and nickel. A scientist has a sample of metal that could be either cobalt or nickel. Which of the following properties could be tested to classify the metal shape? A. Mass B. Density * C. Electrical conductivity D. Reactivity with oxygen

YOU DO TIME!

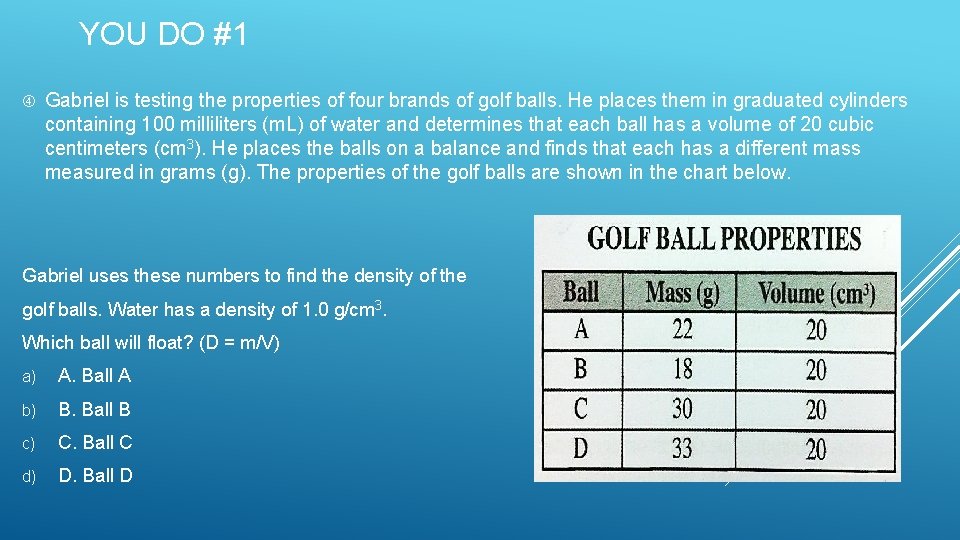
YOU DO #1 Gabriel is testing the properties of four brands of golf balls. He places them in graduated cylinders containing 100 milliliters (m. L) of water and determines that each ball has a volume of 20 cubic centimeters (cm 3). He places the balls on a balance and finds that each has a different mass measured in grams (g). The properties of the golf balls are shown in the chart below. Gabriel uses these numbers to find the density of the golf balls. Water has a density of 1. 0 g/cm 3. Which ball will float? (D = m/V) a) A. Ball A b) B. Ball B c) C. Ball C d) D. Ball D

YOU DO #2 Latrice is given an unknown substance and is asked to identify it based on its properties. She measures its mass as 237. 6 g. The cube’s length, width and height are 3 cm. What is the unknown substance? a) Aluminum Substance: Density: Aluminum 2. 70 g/cm³ b) Iron c) Nickel Iron 7. 80 g/cm³ d) Wood Nickel 8. 80 g/cm³ Wood 0. 69 g/cm³

YOU DO #3 The table shows groups of different materials based on physical characteristics. Groups of Different Materials Group 1 Group 2 Iron Skillet Cotton Towel Aluminum Foil Rubber Spatula Copper Kettle Plastic Bowl Based on the classification of items in the table, into which group would you place a wooden spoon? a) Group 1, because they are good electrical conductors. b) Group 1, because they have relatively low melting points. c) Group 2, because they are poor thermal conductors. d) Group 2, because they have relatively high melting points

EXIT TICKET HOW CAN DENSITY BE USED TO RELIABLY IDENTIFY UNKNOWN SUBSTANCES?
 Conductivity
Conductivity Electrical conductivity of acids and bases
Electrical conductivity of acids and bases Thermal conductivity of styrofoam
Thermal conductivity of styrofoam Example
Example Thermal conductivity detector
Thermal conductivity detector Physical properties of dental material
Physical properties of dental material Thermal conductivity detector
Thermal conductivity detector Enhancing thermal conductivity of fluids with nanoparticles
Enhancing thermal conductivity of fluids with nanoparticles Unit of thermal diffusivity is
Unit of thermal diffusivity is Reometer
Reometer Heat loss symbol
Heat loss symbol Thermal conductivity formula
Thermal conductivity formula We transferü
We transferü Fundamentals of thermal-fluidsciences chapter 2 problem 24p
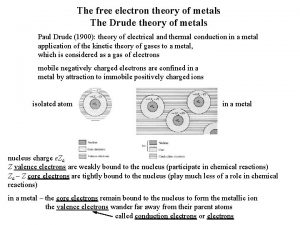
Fundamentals of thermal-fluidsciences chapter 2 problem 24p Drude theory of metals
Drude theory of metals Electrical conductivity soil definition
Electrical conductivity soil definition Electric field and resistivity
Electric field and resistivity