Lecture 3 Chemistry of Life Lecture 3 Chemistry




















































- Slides: 108

Lecture 3: Chemistry of Life

Lecture 3: Chemistry of Life Goals: 1. Sprint through General Chemistry 2. Whisper past Organic Chemistry 3. Approach Biochemistry cautiously 4. Apply chemistry overview and relate biological chemistry to this course and your life in general Key Terms: Charge, proton, neutron, electron, radioisotope, tracer, chemical bonds a)ionic, b)covalent, c)hydrogen, atom, molecule, p. H scale, buffer, basic, acidic, hydrophobic, hydrophillic, acidosis, alkalosis, solute, polar, non-polar. http: //pearl 1. lanl. gov/periodic/default. htm http: //www. chemsoc. org/viselements/pages/pertable_fla. htm

Elements • Fundamental forms of matter • Can’t be broken apart by normal means • 92 occur naturally on Earth Less than 12 occur on the exam

Most Common Elements in Living Organisms CHON Carbon Hydrogen Oxygen Nitrogen

What Are Atoms? • Smallest particles that retain properties of an element • Made up of subatomic particles: – Protons (+) – Electrons (-) – Neutrons (no charge)

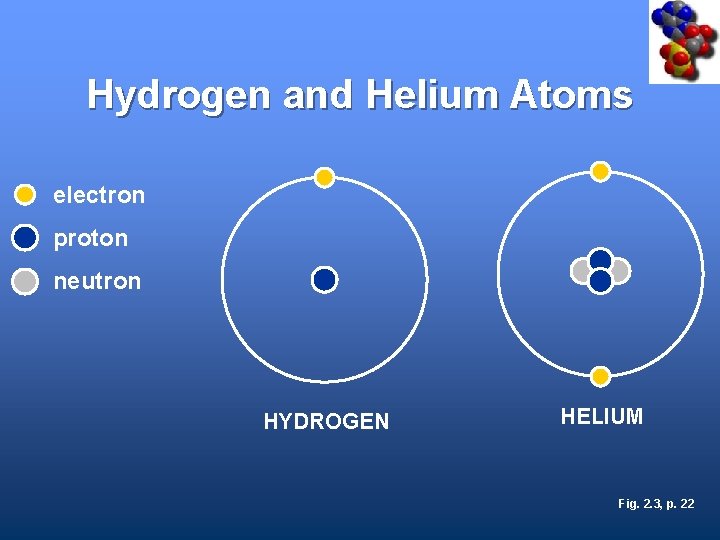
Hydrogen and Helium Atoms electron proton neutron HYDROGEN HELIUM Fig. 2. 3, p. 22

Atomic Number • Number of protons • All atoms of the same element have the same atomic number • Atomic number of hydrogen = 1 • Atomic number of carbon = 6

Mass Number of protons + Number of neutrons Isotopes vary in mass number (not atomic number or they would be something else)

Atomic Mass Isotopes Radioisotopes • Atoms of an element with different numbers of neutrons (different mass numbers) • Carbon 12 has 6 protons, 6 neutrons • Carbon 14 has 6 protons, 8 neutrons • Have an unstable nucleus that emits energy and particles • Radioactive decay transforms radioisotope into a different element • Decay occurs at a fixed rate

Radioisotopes as Tracers • Example: Tracer Drug Study – How long does a drug stay in the patient? – Determine dose guidelines • Compound synthesized with a radioisotope • Emissions from the tracer can be detected with special devices – Track levels in the blood, urine and feces • Following movement of tracers is useful in many areas of biology

High Sensitivity Very Low Dose

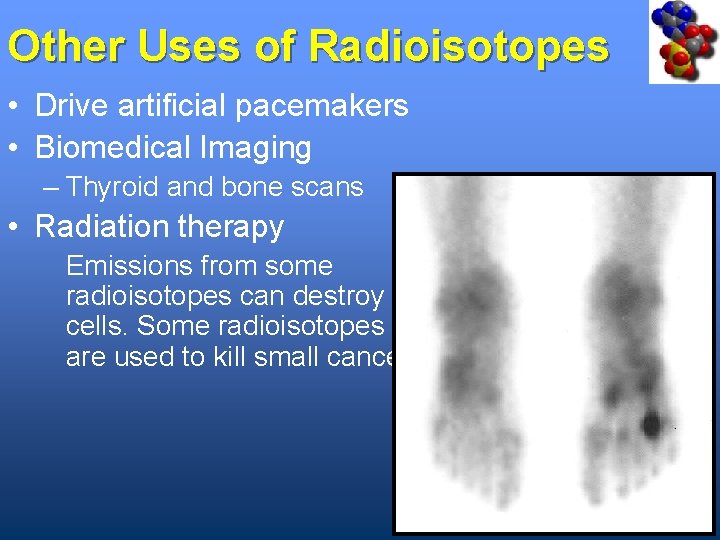
Other Uses of Radioisotopes • Drive artificial pacemakers • Biomedical Imaging – Thyroid and bone scans • Radiation therapy Emissions from some radioisotopes can destroy cells. Some radioisotopes are used to kill small cancers.

What Determines Whether Atoms Will Interact? The most general of General Chemistry

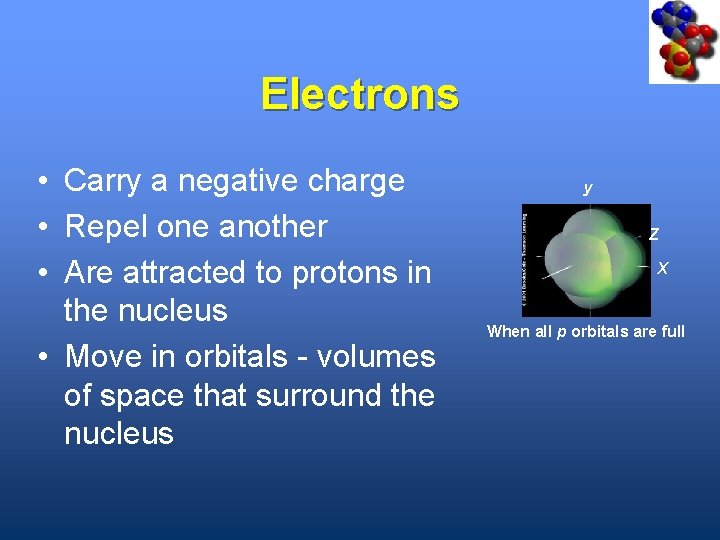
Electrons • Carry a negative charge • Repel one another • Are attracted to protons in the nucleus • Move in orbitals - volumes of space that surround the nucleus y Z X When all p orbitals are full

Electron Orbitals • Orbitals can hold up to two electrons • Atoms differ in the number of occupied orbitals • Orbitals closest to nucleus are lower energy and are filled first

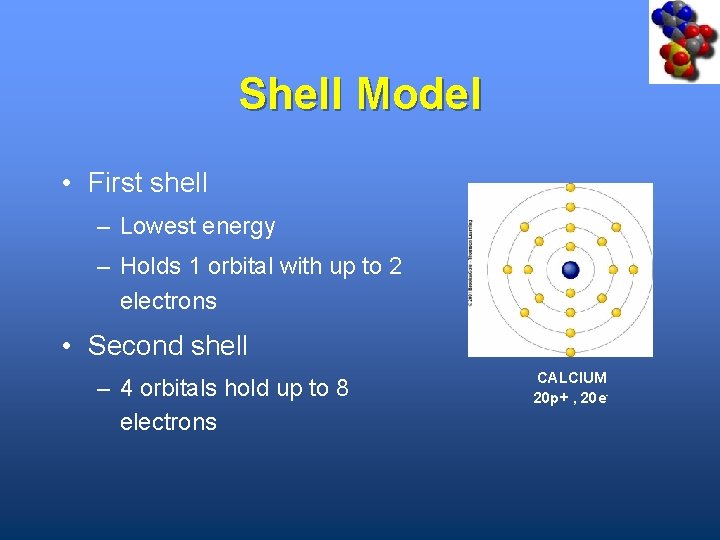
Shell Model • First shell – Lowest energy – Holds 1 orbital with up to 2 electrons • Second shell – 4 orbitals hold up to 8 electrons CALCIUM 20 p+ , 20 e-

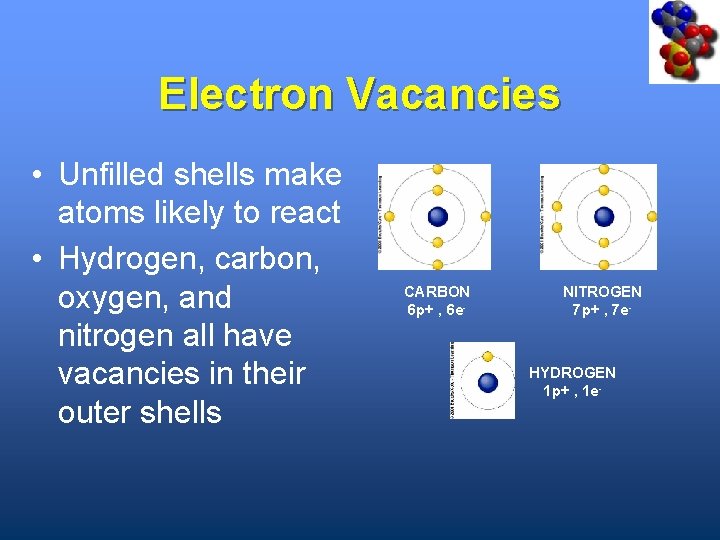
Electron Vacancies • Unfilled shells make atoms likely to react • Hydrogen, carbon, oxygen, and nitrogen all have vacancies in their outer shells CARBON 6 p+ , 6 e- NITROGEN 7 p+ , 7 e- HYDROGEN 1 p+ , 1 e-

Chemical Bonds, Molecules, & Compounds • Bond is union between electron structures of atoms • Atoms bond to form molecules • Molecules may contain atoms of only one element - O 2 • Molecules of compounds contain more than one element - H 2 O

Only a few atoms, even fewer Chemical Bonds Ionic bonds Between metallic and non metallic atoms Easily dissolved by water Covalent Share at least one pair of electrons Polar and non-polar bonds Tight (high energy) bond Hydrogen bonds A hydrogen between atoms Not so tight (low energy) bond: 1/10 th covalent

1. Ionic Bonding • One atom loses electrons, becomes positively charged ion • Another atom gains these electrons, becomes negatively charged ion • Charge difference attracts the two ions to each other

Ion Formation • Atom has equal number of electrons and protons - no net charge • Atom loses electron(s), becomes positively charged ion • Atom gains electron(s), becomes negatively charged ion

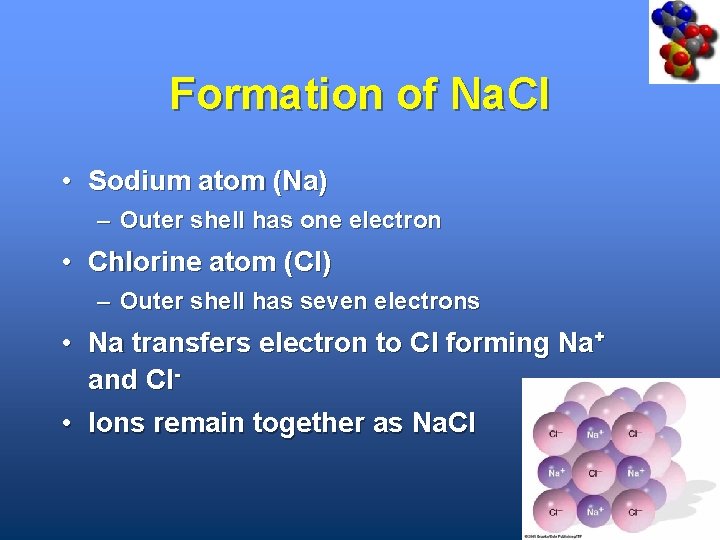
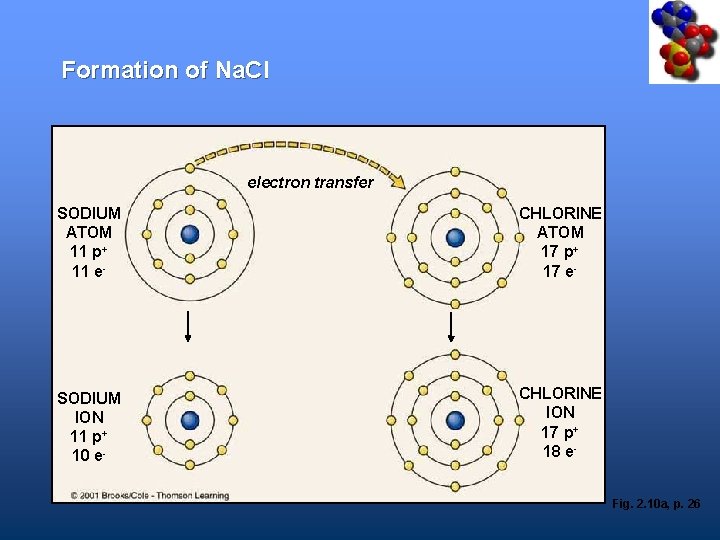
Formation of Na. Cl • Sodium atom (Na) – Outer shell has one electron • Chlorine atom (Cl) – Outer shell has seven electrons • Na transfers electron to Cl forming Na+ and Cl • Ions remain together as Na. Cl

Formation of Na. Cl 7 mm electron transfer SODIUM ATOM 11 p+ 11 e- CHLORINE ATOM 17 p+ 17 e- SODIUM ION 11 p+ 10 e- CHLORINE ION 17 p+ 18 e. Fig. 2. 10 a, p. 26

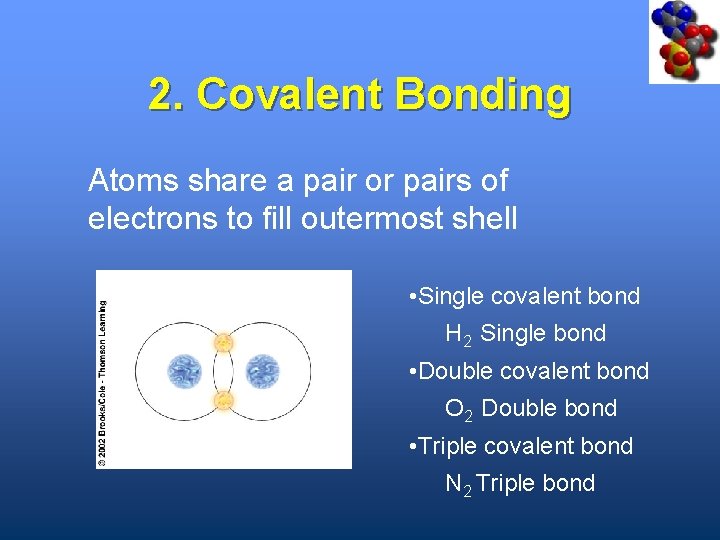
2. Covalent Bonding Atoms share a pair or pairs of electrons to fill outermost shell • Single covalent bond H 2 Single bond • Double covalent bond O 2 Double bond • Triple covalent bond N 2 Triple bond

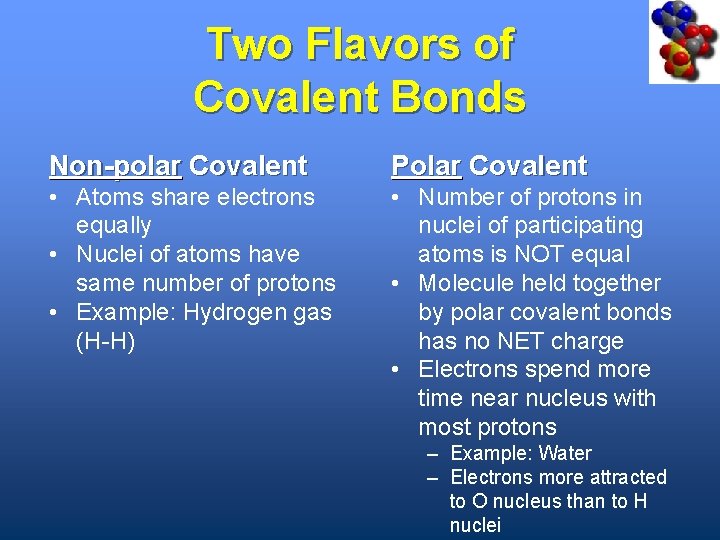
Two Flavors of Covalent Bonds Non-polar Covalent Polar Covalent • Atoms share electrons equally • Nuclei of atoms have same number of protons • Example: Hydrogen gas (H-H) • Number of protons in nuclei of participating atoms is NOT equal • Molecule held together by polar covalent bonds has no NET charge • Electrons spend more time near nucleus with most protons – Example: Water – Electrons more attracted to O nucleus than to H nuclei

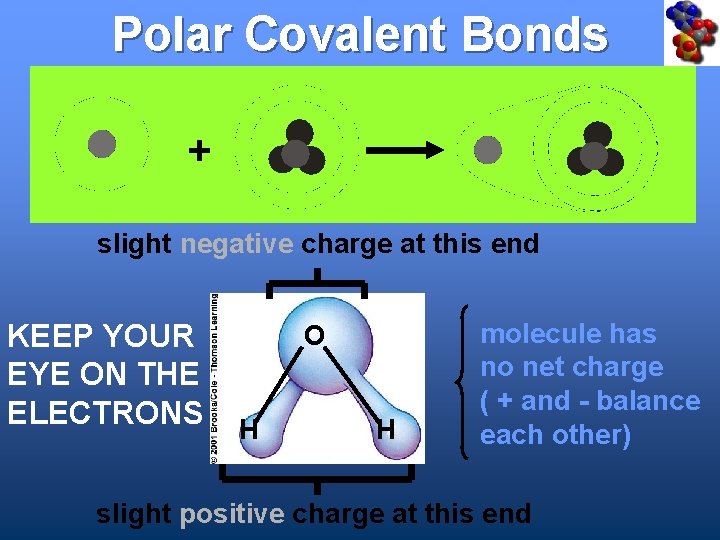
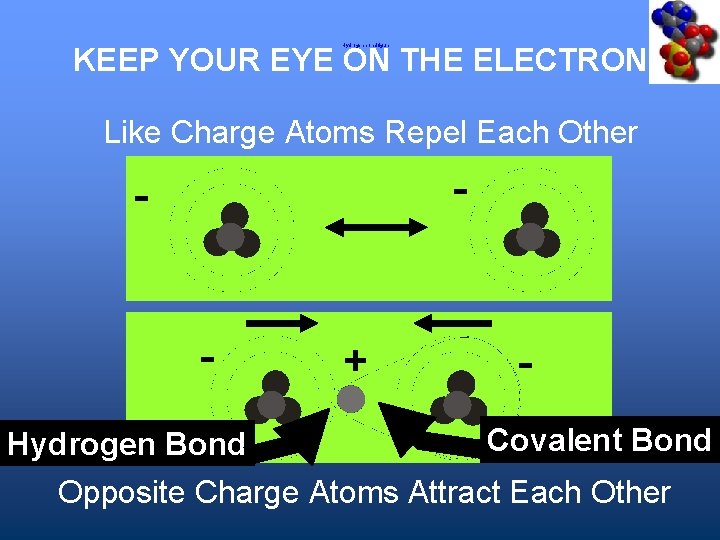
Polar Covalent Bonds + slight negative charge at this end KEEP YOUR EYE ON THE ELECTRONS O H H molecule has no net charge ( + and - balance each other) slight positive charge at this end

Hydrogen Bonding A bond by Hydrogen between two atoms • Important for O and N • Lets two electronegative atoms interact – The H gives one a net + and the other one that is still – is attracted to it. • The H proton becomes “naked” because its electron gets pulled away.

KEEP YOUR EYE ON THE ELECTRONS Hydrogen bond figure Like Charge Atoms Repel Each Other - - - + - Covalent Bond Hydrogen Bond Opposite Charge Atoms Attract Each Other

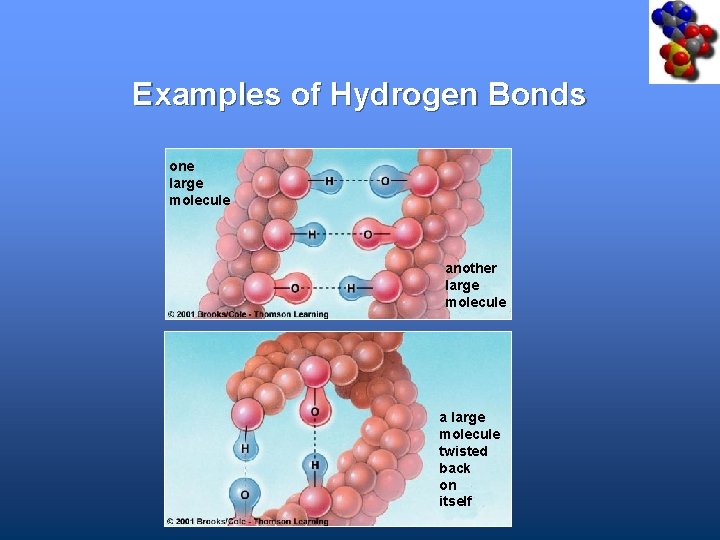
Examples of Hydrogen Bonds one large molecule another large molecule a large molecule twisted back on itself

Properties of Water • Polarity • Temperature-Stabilizing • Cohesive • Solvent

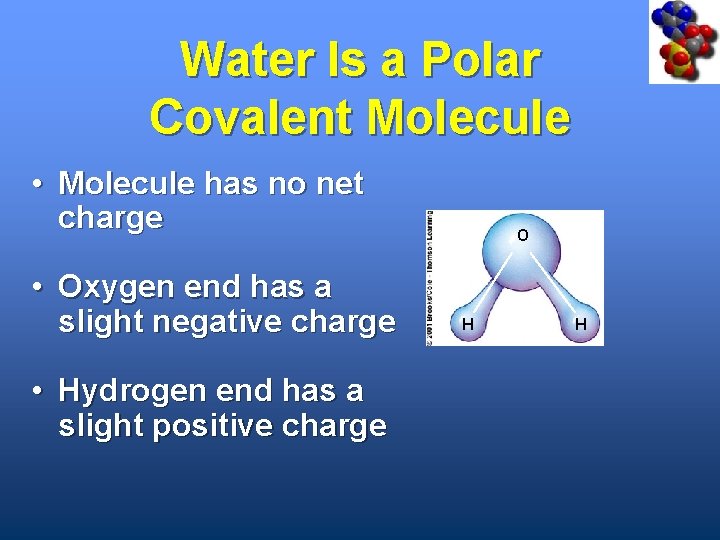
Water Is a Polar Covalent Molecule • Molecule has no net charge • Oxygen end has a slight negative charge • Hydrogen end has a slight positive charge O H H

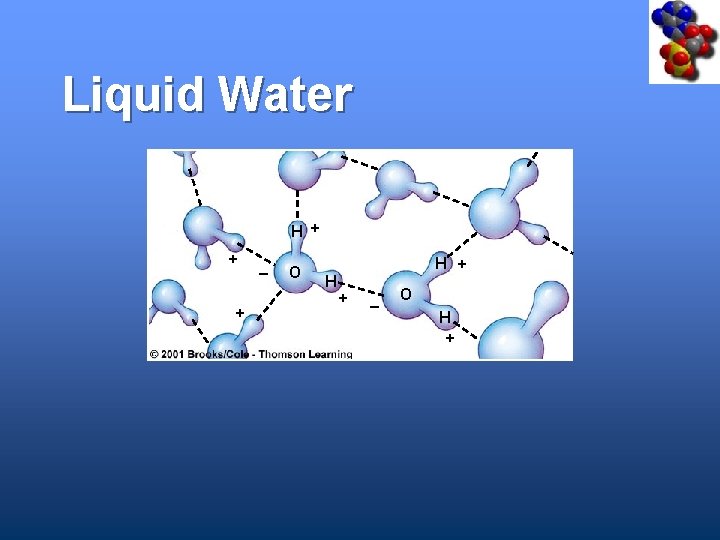
Liquid Water H + + + _ O H H + + _ O H +

Hydrophilic & Hydrophobic Substances • Hydrophilic substances – Polar – Hydrogen bond with water – Example: sugar • Hydrophobic substances – Non-polar – Repelled by water – Example: oil

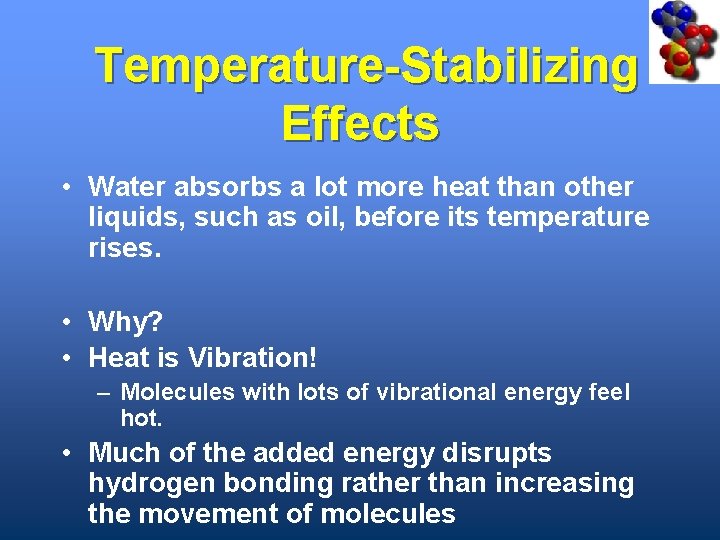
Temperature-Stabilizing Effects • Water absorbs a lot more heat than other liquids, such as oil, before its temperature rises. • Why? • Heat is Vibration! – Molecules with lots of vibrational energy feel hot. • Much of the added energy disrupts hydrogen bonding rather than increasing the movement of molecules

Evaporation of Water • Large energy input can cause individual molecules of water to break free into air • As molecules break free, they carry away some energy (lower temperature) • Evaporative water loss is used by mammals to lower body temperature

Why Ice Floats • In ice, hydrogen bonds lock molecules in a lattice • Water molecules in lattice are spaced farther apart then those in liquid water • Ice is less dense than water

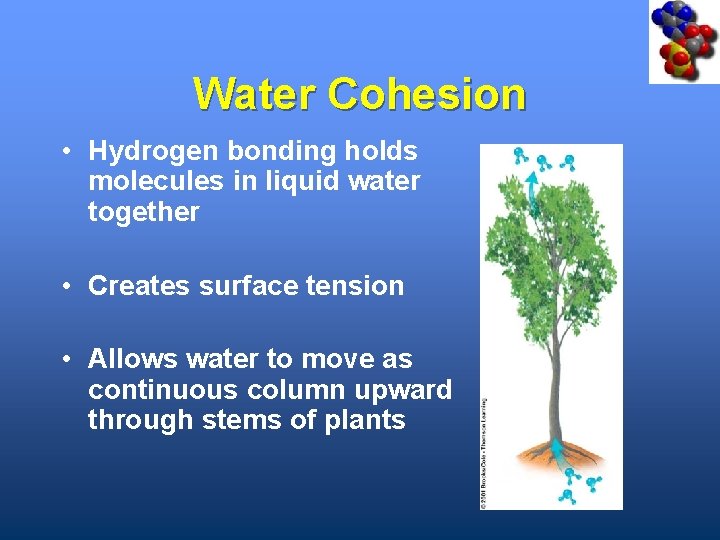
Water Cohesion • Hydrogen bonding holds molecules in liquid water together • Creates surface tension • Allows water to move as continuous column upward through stems of plants

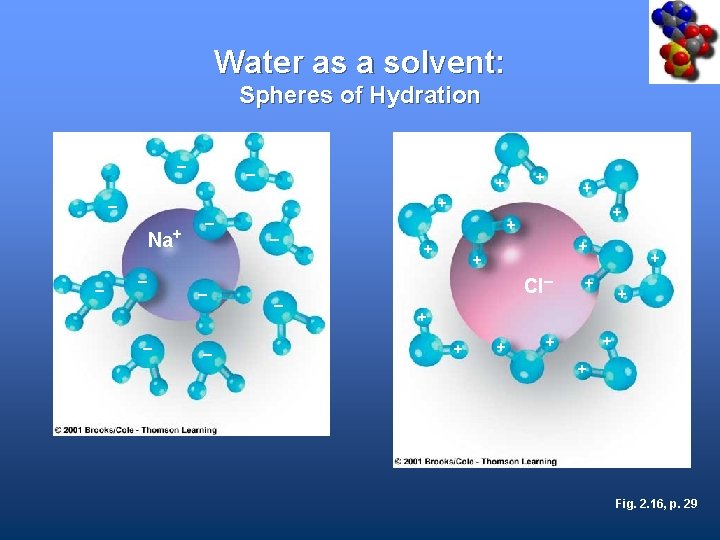
Water Is a Good Solvent • Ions and polar molecules dissolve easily in water • When solute dissolves, water molecules cluster around its ions or molecules and keep them separated

Water as a solvent: Spheres of Hydration – + + – Na+ – – – – – + + + Cl– + + + + + Fig. 2. 16, p. 29

Water • Solvent- polar – Keeps ions in solution – Doesn’t dissolve membranes • Heat management – – – Loosing heat Holding heat Density Changes

If it wasn’t ugly enough already: Hydrogen Ions: + H • Unbound protons • Have important biological effects • Form when water ionizes

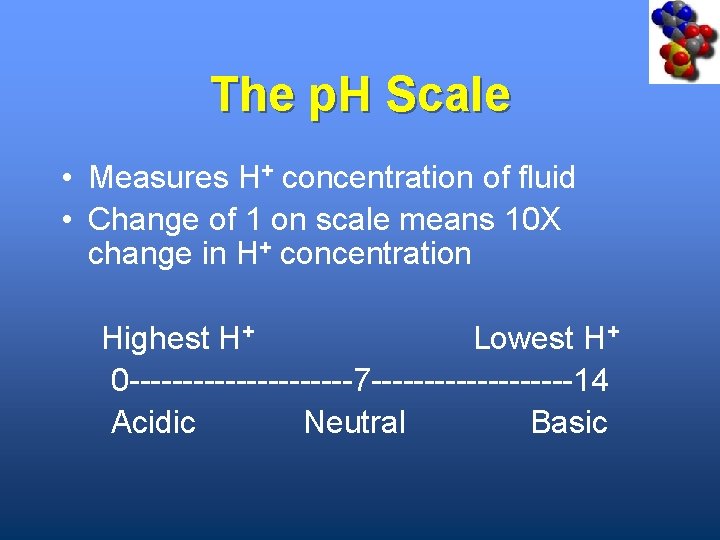
The p. H Scale • Measures H+ concentration of fluid • Change of 1 on scale means 10 X change in H+ concentration Highest H+ Lowest H+ 0 -----------7 ----------14 Acidic Neutral Basic

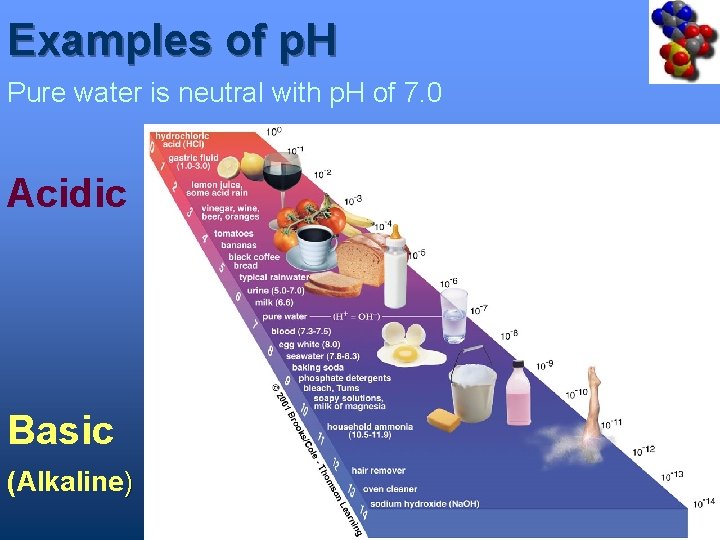
Examples of p. H Pure water is neutral with p. H of 7. 0 Acidic Basic (Alkaline)

Acids & Bases • Acids – Donate H+ when dissolved in water – Acidic solutions have p. H < 7 • Bases – Accept H+ when dissolved in water – Acidic solutions have p. H > 7

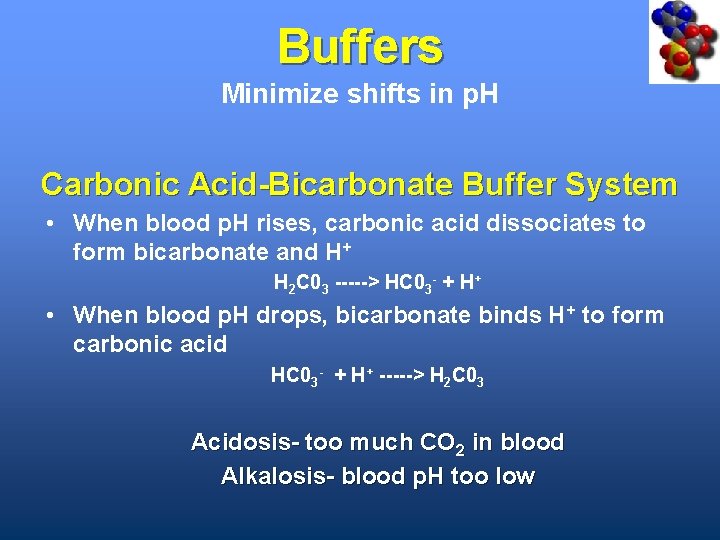
Buffers Minimize shifts in p. H Carbonic Acid-Bicarbonate Buffer System • When blood p. H rises, carbonic acid dissociates to form bicarbonate and H+ H 2 C 03 -----> HC 03 - + H+ • When blood p. H drops, bicarbonate binds H+ to form carbonic acid HC 03 - + H+ -----> H 2 C 03 Acidosis- too much CO 2 in blood Alkalosis- blood p. H too low

Lecture 2: Chemistry of Life Part 2 Feeling a little burnt out?

Demonstration of Chemical Bonds Tests: 1. Water as a solvent 2. Bond strength Predictions: Covalent bonds Ionic bonds Hydrogen bonds Hydrophilic interactions Hydrophobic interactions

Hydrogen Bonds Aliphatic Resin, PVA and Elmer Why does glue work? 1. Mechanical component 2. Chemical component Process 1. Infiltrate wood fibers 2. Allow tight contact 3. Remove water (solvent) Demonstration of Hydrogen bond strength

Hydrogen Bonds Aliphatic Resin, PVA and Elmer • Bond Strength: – 3, 500 pounds per square inch • Hydrogen bonds form between the wood and glue as the water leaves • Conclusion:

Organic Compounds • Hydrogen and other elements covalently bonded to carbon • Major Classes of Biological Molecules – Carbohydrates – Lipids – Proteins – Nucleic Acids

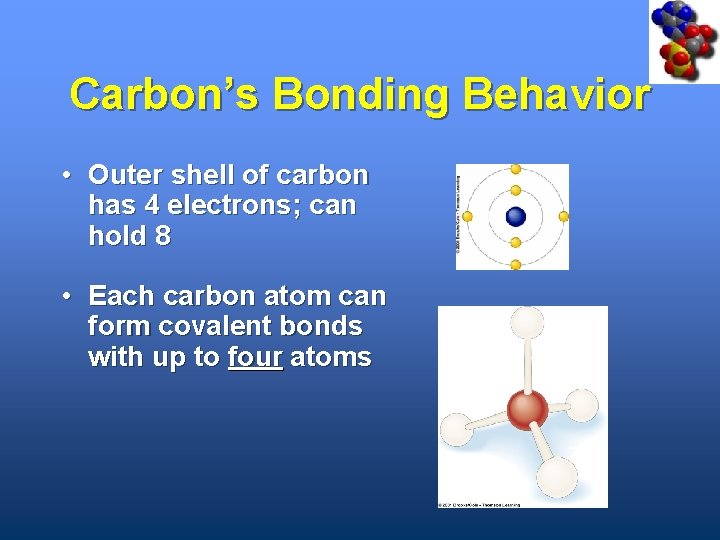
Carbon’s Bonding Behavior • Outer shell of carbon has 4 electrons; can hold 8 • Each carbon atom can form covalent bonds with up to four atoms

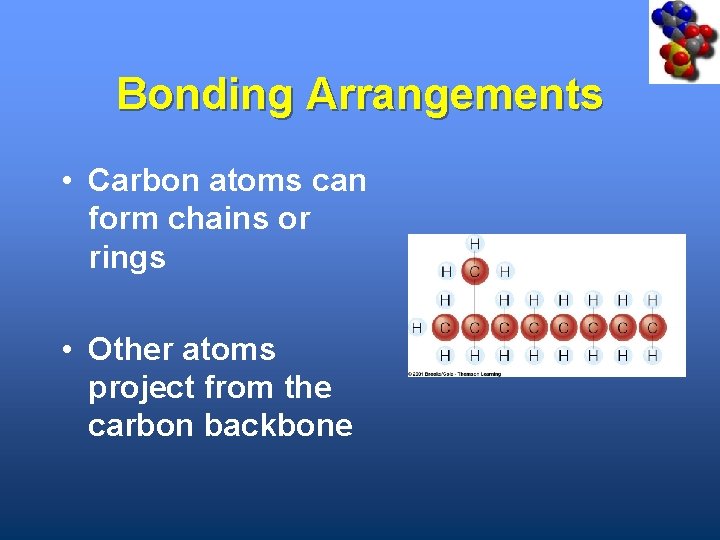
Bonding Arrangements • Carbon atoms can form chains or rings • Other atoms project from the carbon backbone

Functional Groups • Atoms or clusters of atoms that are covalently bonded to carbon backbone • Give organic compounds their different properties

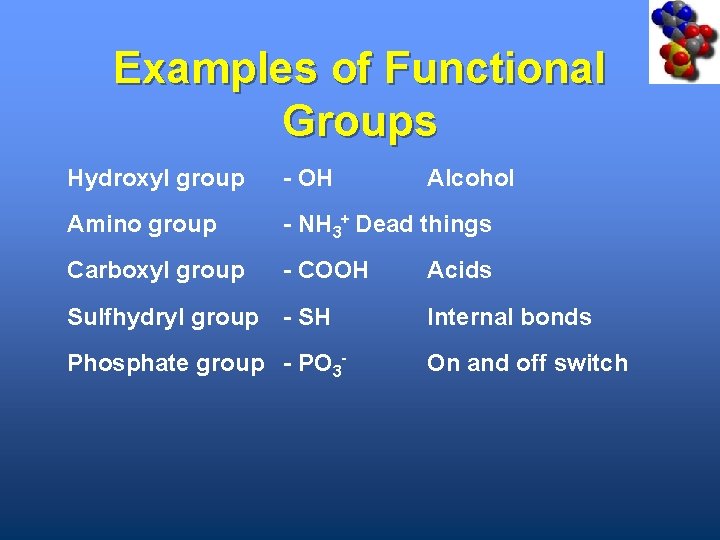
Examples of Functional Groups Hydroxyl group - OH Amino group - NH 3+ Dead things Carboxyl group - COOH Acids Sulfhydryl group - SH Internal bonds Phosphate group - PO 3 - Alcohol On and off switch

Types of Reactions Functional group transfer Electron transfer Rearrangement x Condensation Cleavage Hydrolysis

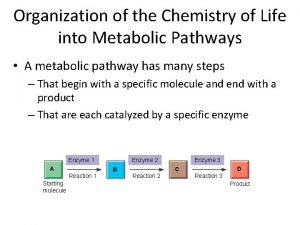
Condensation Reactions • Form polymers from subunits • Enzymes remove -OH from one molecule, H from another, form bond between two molecules • Discarded atoms can join to form water

Condensation -ie. Water condenses on the inside of my window when the air conditioner is on full blast Or. . Water forms ….

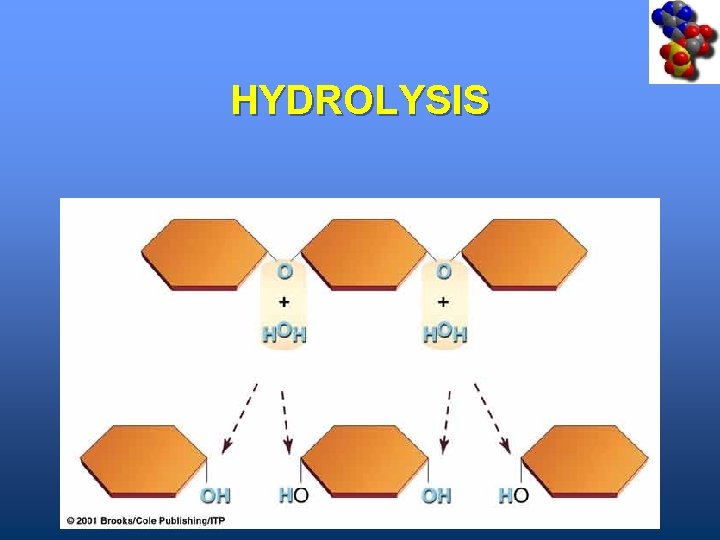
Hydrolysis • A type of cleavage reaction • Breaks polymers into smaller units • Enzymes split molecules into two or more parts • An OH group and an H atom derived from water are attached at exposed sites

HYDROLYSIS

Carbohydrates Monosaccharides (simple sugars) Oligosaccharides (short-chain carbohydrates) Polysaccharides (complex carbohydrates)

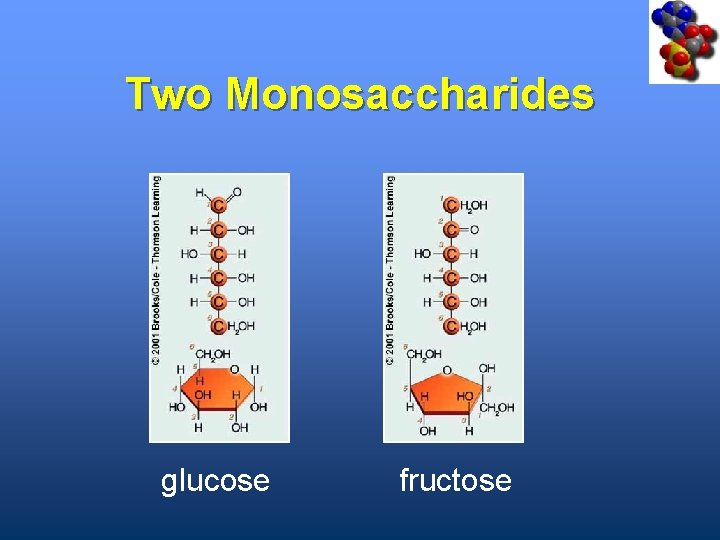
Monosaccharides • Simplest carbohydrates • Most are sweet tasting, water soluble • Most have 5 - or 6 -carbon backbone Glucose (6 C) Fructose (6 C) Ribose (5 C) Deoxyribose (5 C)

Two Monosaccharides glucose fructose

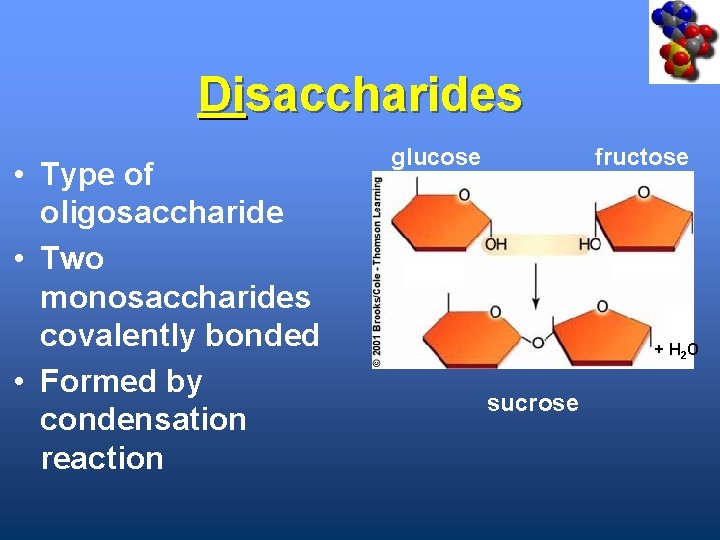
Disaccharides • Type of oligosaccharide • Two monosaccharides covalently bonded • Formed by condensation reaction glucose fructose + H 2 O sucrose

Polysaccharides • Straight or branched chains of many sugar monomers • Most common are composed entirely of glucose – Cellulose – Starch (such as amylose) – Glycogen

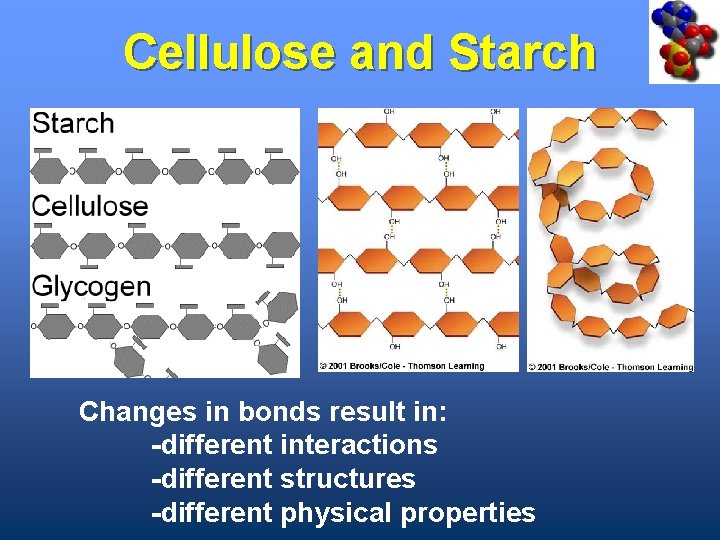
Cellulose & Starch • Differ in bonding patterns between monomers (type of linkage) • Cellulose - tough, indigestible, structural material in plants • Starch - easily digested, storage form in plants

Cellulose and Starch Changes in bonds result in: -different interactions -different structures -different physical properties

Glycogen • Sugar storage form in animals • Large stores in muscle and liver cells • When blood sugar decreases, liver cells degrade glycogen, release glucose

Chitin • Polysaccharide • Nitrogen-containing groups attached to glucose monomers • Found in insects, worms, and fungi (not humans) • Structural material for hard parts of invertebrates, cell walls of many fungi

Lipids • Most include fatty acids – Fats – Phospholipids – Waxes • Sterols and their derivatives have no fatty acids • Tend to be insoluble in water

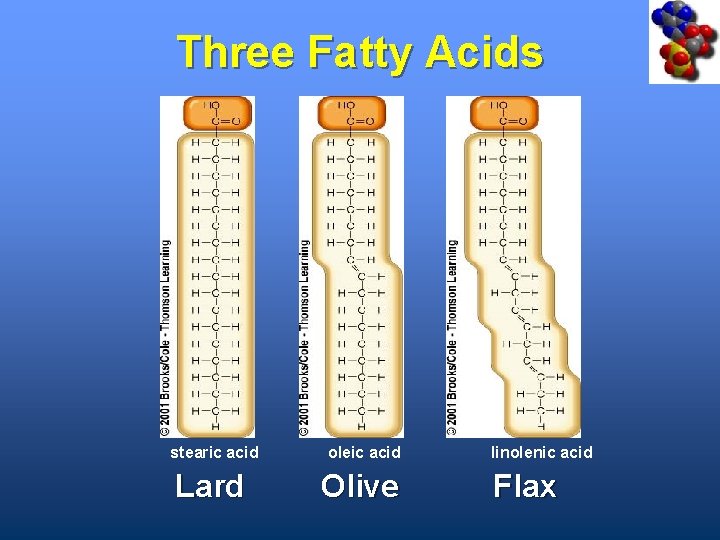
Fatty Acids • Carboxyl group (-COOH) at one end • Carbon backbone (up to 36 C atoms) – Saturated - Single bonds between carbons – Unsaturated - One or more double bonds

Three Fatty Acids stearic acid oleic acid Lard Olive linolenic acid Flax

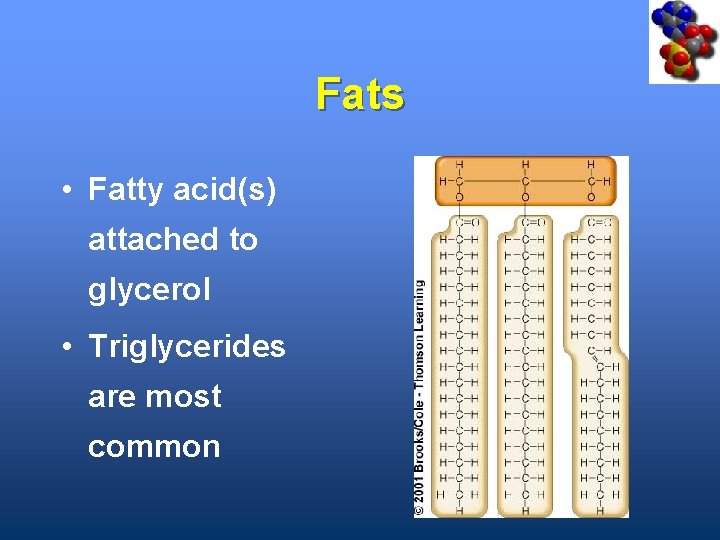
Fats • Fatty acid(s) attached to glycerol • Triglycerides are most common

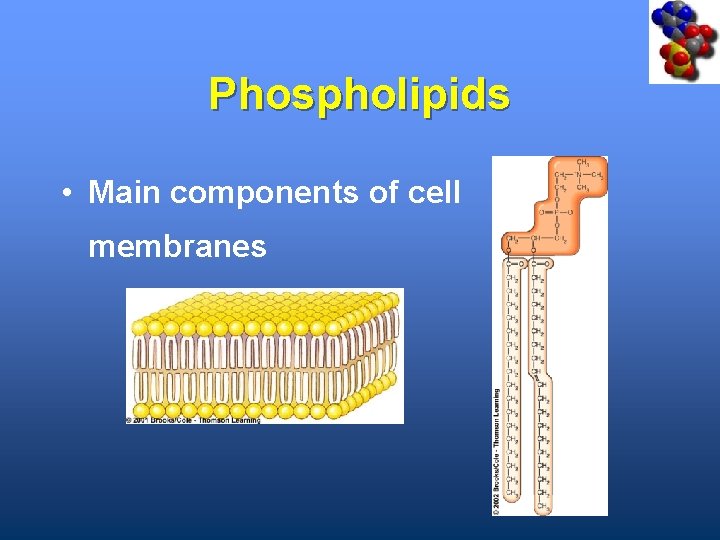
Phospholipids • Main components of cell membranes

Sterols and Derivatives • No fatty acids • Rigid backbone of four fused-together carbon rings • Cholesterol - most common type in animals

Waxes • Long-chain fatty acids linked to long chain alcohols or carbon rings • Firm consistency, repel water • Important in water-proofing • Size matters

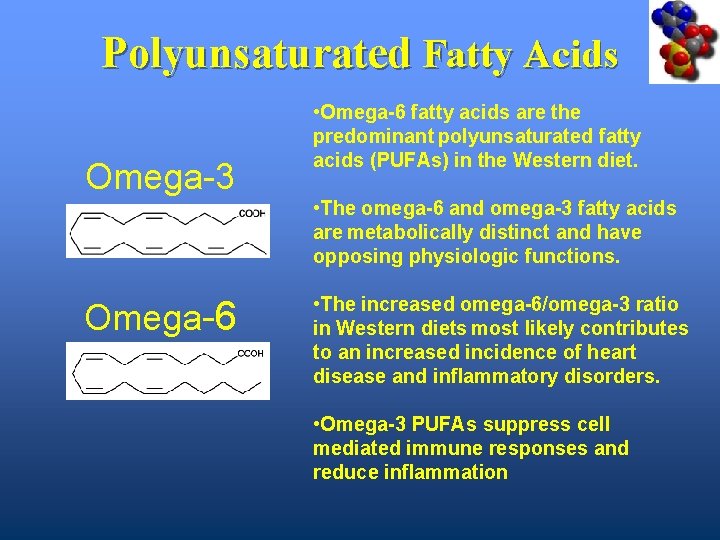
Polyunsaturated Fatty Acids Omega-3 • Omega-6 fatty acids are the predominant polyunsaturated fatty acids (PUFAs) in the Western diet. • The omega-6 and omega-3 fatty acids are metabolically distinct and have opposing physiologic functions. Omega-6 • The increased omega-6/omega-3 ratio in Western diets most likely contributes to an increased incidence of heart disease and inflammatory disorders. • Omega-3 PUFAs suppress cell mediated immune responses and reduce inflammation

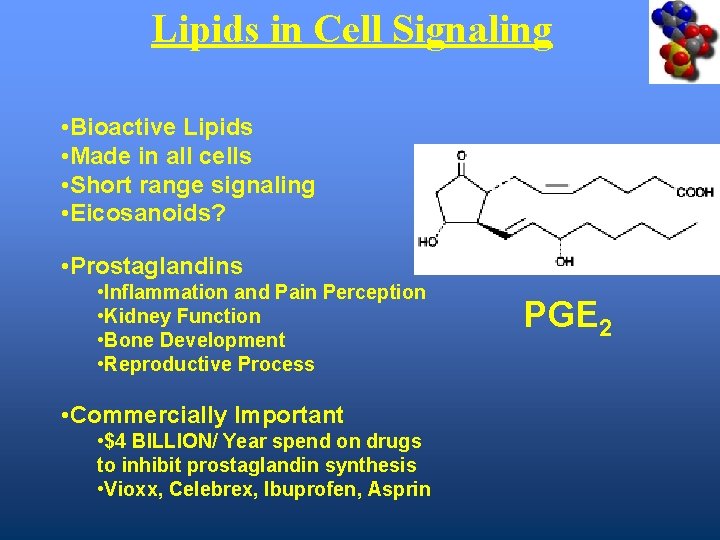
Lipids in Cell Signaling • Bioactive Lipids • Made in all cells • Short range signaling • Eicosanoids? • Prostaglandins • Inflammation and Pain Perception • Kidney Function • Bone Development • Reproductive Process • Commercially Important • $4 BILLION/ Year spend on drugs to inhibit prostaglandin synthesis • Vioxx, Celebrex, Ibuprofen, Asprin PGE 2

Amino Acid Structure carboxyl group amino group R group

Properties of Amino Acids • Determined by the “R group” • Amino acids may be: – Non-polar – Uncharged, polar – Positively charged, polar – Negatively charged, polar

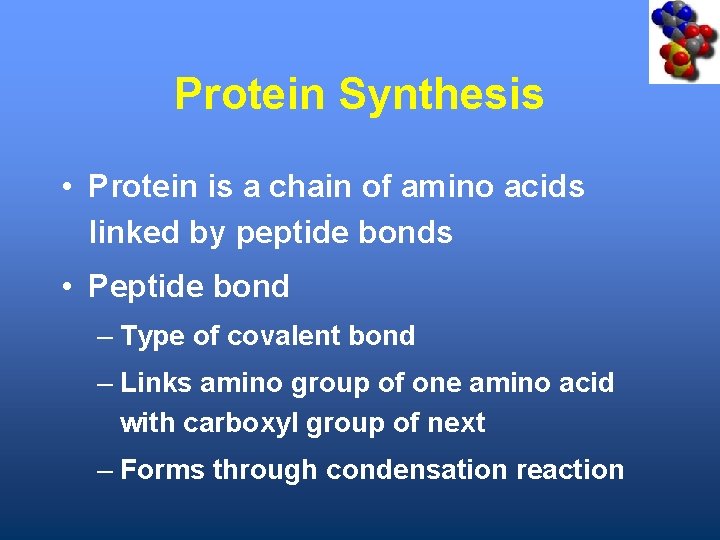
Protein Synthesis • Protein is a chain of amino acids linked by peptide bonds • Peptide bond – Type of covalent bond – Links amino group of one amino acid with carboxyl group of next – Forms through condensation reaction

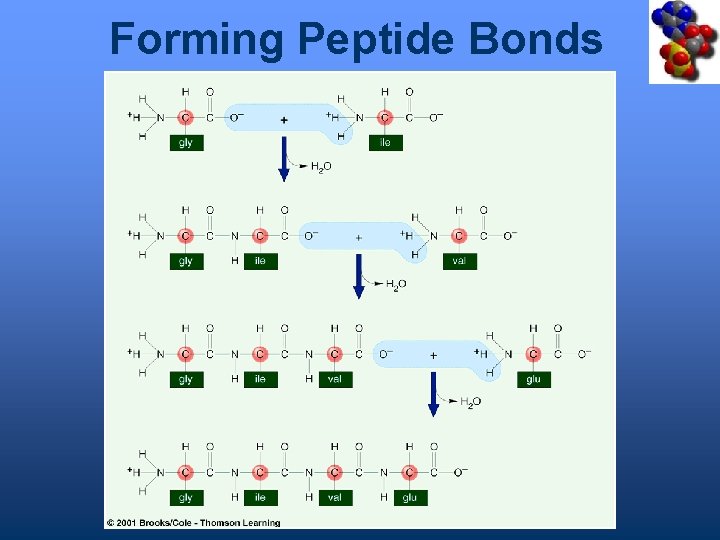
Forming Peptide Bonds

Primary Structure • Sequence of amino acids • Unique for each protein • Two linked amino acids = dipeptide • Three or more = polypeptide • Backbone of polypeptide has N atoms: -N-C-C-N-C-C-N-

Protein Shapes • Fibrous proteins – Polypeptide chains arranged as strands or sheets • Globular proteins – Polypeptide chains folded into compact, rounded shapes

Protein Structure • Primary- just the sequence (1 D) • Secondary- interactions on the chain (2 D) • Tertiary- interactions between parts of the chain. (3 D) • Quaternary- interactions with other chains

Primary Structure & Protein Shape • Primary structure influences shape in two main ways: – Allows hydrogen bonds to form between different amino acids along length of chain – Puts R groups in positions that allow them to interact

Secondary Structure • Hydrogen bonds form between different parts of polypeptide chain • These bonds give rise to coiled or extended pattern • Helix or pleated sheet

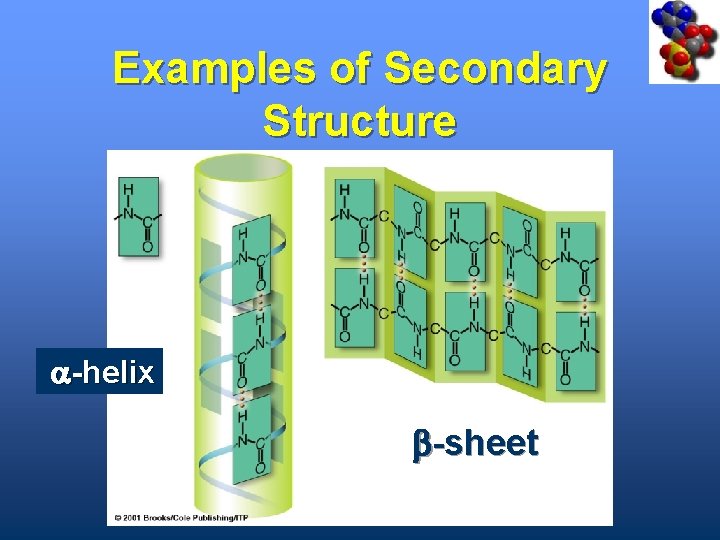
Examples of Secondary Structure a-helix b-sheet

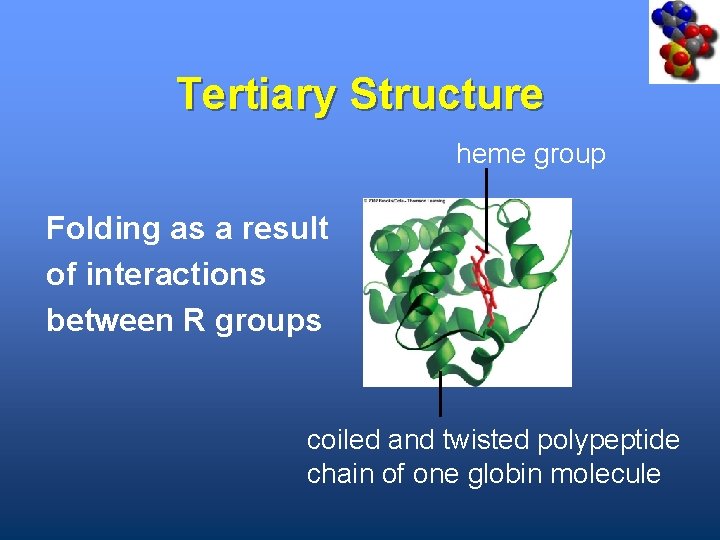
Tertiary Structure heme group Folding as a result of interactions between R groups coiled and twisted polypeptide chain of one globin molecule

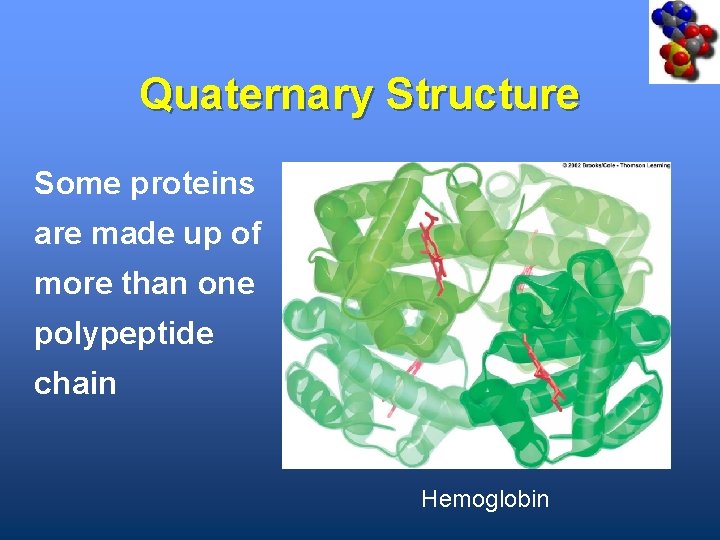
Quaternary Structure Some proteins are made up of more than one polypeptide chain Hemoglobin

Polypeptides With Attached Organic Compounds Nothing new, just more combinations • Lipoproteins – Proteins combined with cholesterol, triglycerides, phospholipids • Glycoproteins – Proteins combined with oligosaccharides

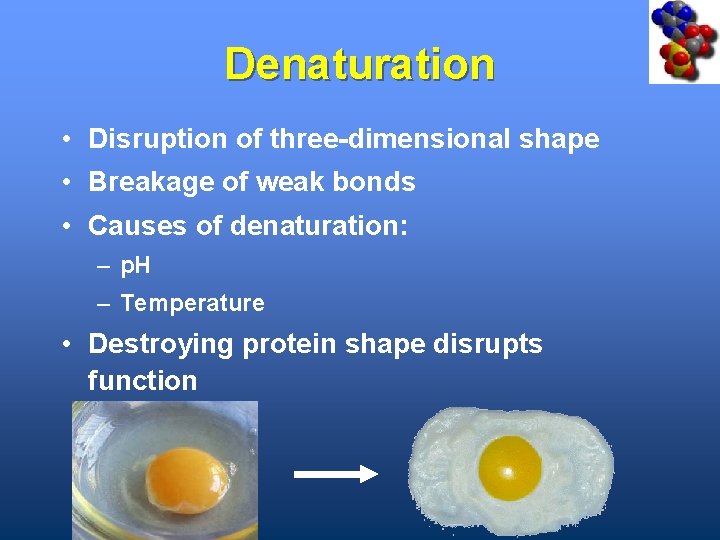
Denaturation • Disruption of three-dimensional shape • Breakage of weak bonds • Causes of denaturation: – p. H – Temperature • Destroying protein shape disrupts function

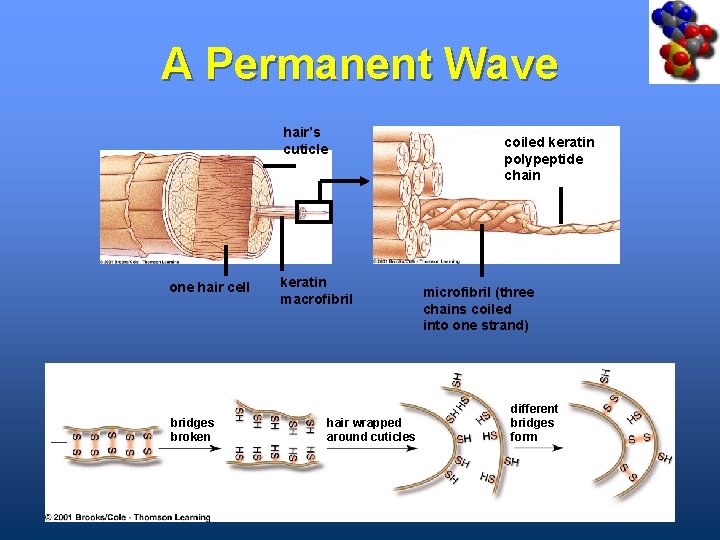
A Permanent Wave hair’s cuticle one hair cell bridges broken keratin macrofibril hair wrapped around cuticles coiled keratin polypeptide chain microfibril (three chains coiled into one strand) different bridges form

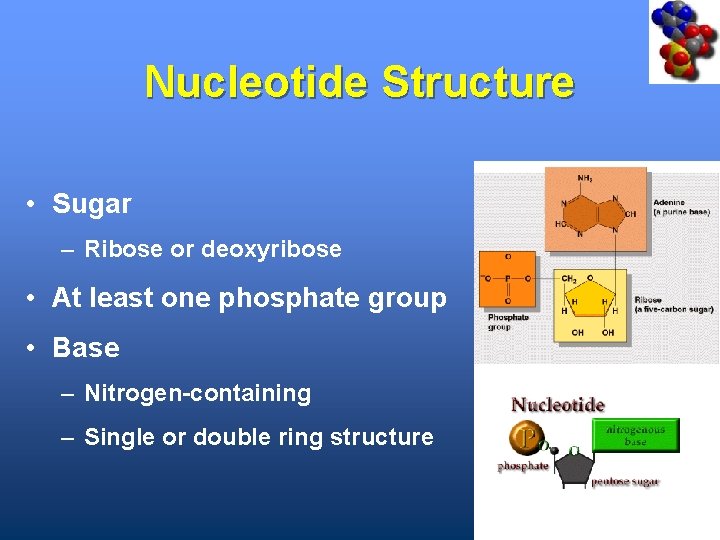
Nucleotide Structure • Sugar – Ribose or deoxyribose • At least one phosphate group • Base – Nitrogen-containing – Single or double ring structure

Nucleotide Functions • Energy carriers • Coenzymes • Chemical messengers • Building blocks for nucleic acids Careful: Nucleotide isn’t just DNA or RNA

ATP - A Nucleotide base three phosphate groups sugar

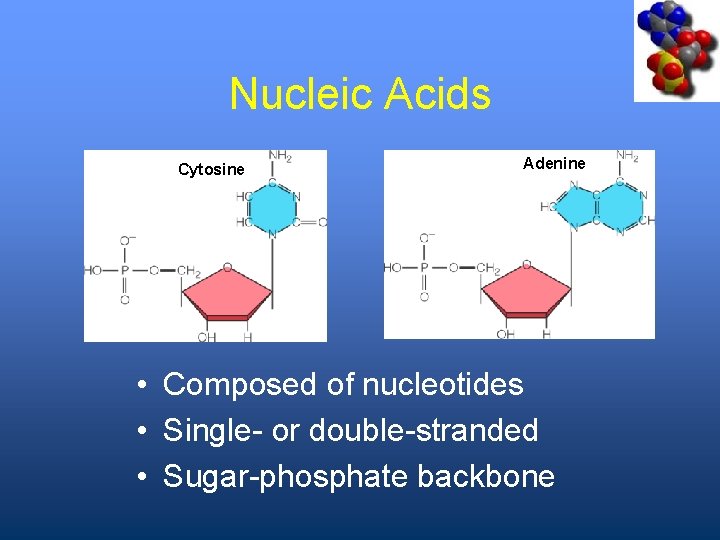
Nucleic Acids Cytosine Adenine • Composed of nucleotides • Single- or double-stranded • Sugar-phosphate backbone

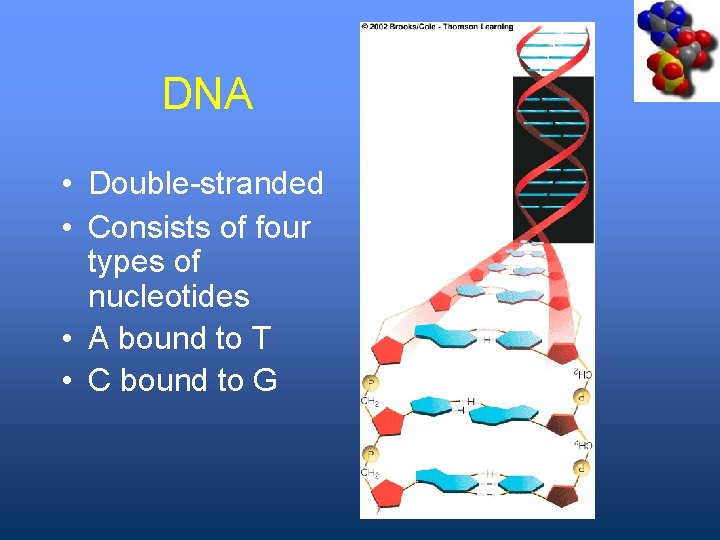
DNA • Double-stranded • Consists of four types of nucleotides • A bound to T • C bound to G

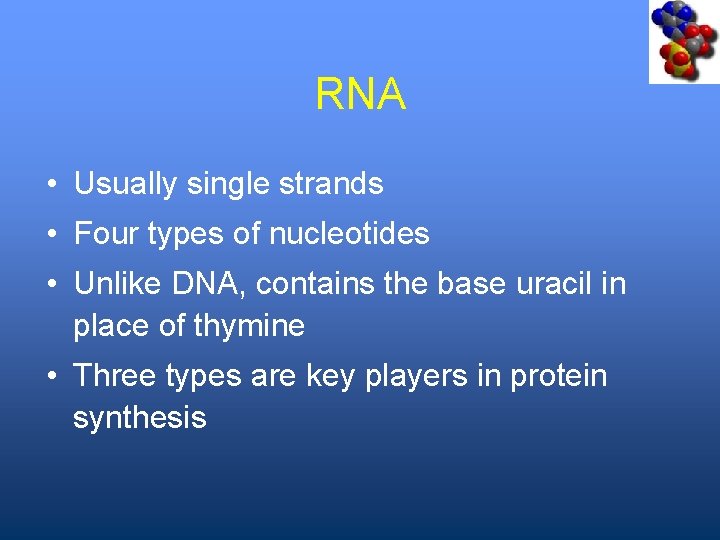
RNA • Usually single strands • Four types of nucleotides • Unlike DNA, contains the base uracil in place of thymine • Three types are key players in protein synthesis

Natural Toxins • Normal metabolic products of one species that can harm or kill a different species • Natural pesticides – Compounds from tobacco – Compounds from chrysanthemum

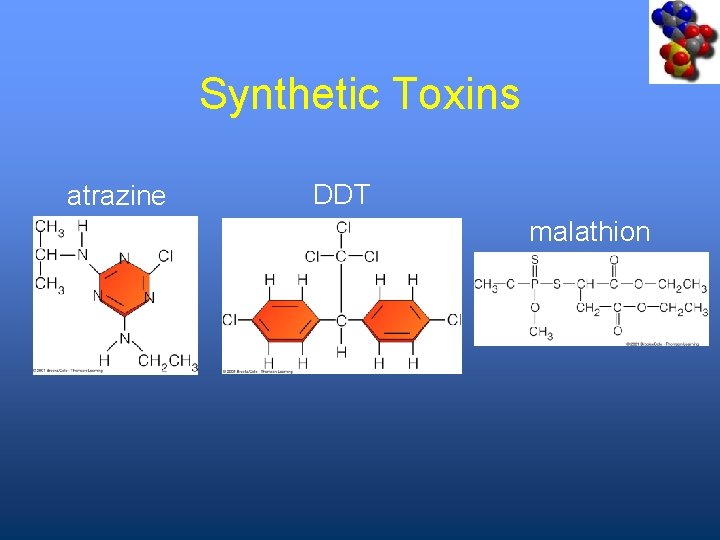
Synthetic Toxins atrazine DDT malathion

Negative Effects of Pesticides • May be toxic to predators that help fight pests • May be active for weeks to years • Can be accidentally inhaled, ingested, or absorbed by humans • Can cause rashes, headaches, allergic reactions

Producers Capture Carbon Using photosynthesis, plants and other producers turn carbon dioxide and water into carbon-based compounds

Atmospheric Carbon Dioxide • Researchers have studied concentration of CO 2 in air since the 1950 s • Concentration shifts with season – Declines in spring and summer when producers take up CO 2 for photosynthesis

CO 2 and Global Warming • Seasonal swings in CO 2 increasing • Spring decline starting earlier • Temperatures in lower atmosphere increasing • Warming may be promoting increased photosynthesis

Humans and Global Warming • Fossil fuels are rich in carbon • Use of fossil fuels releases CO 2 into atmosphere • Increased CO 2 may contribute to global warming

Chemical Benefits and Costs • Understanding of chemistry provides fertilizers, medicines, etc. • Chemical pollutants damage ecosystems

Bioremediation Use of living organisms to withdraw harmful substances from the environment

Thyroid Scan • Measures health of thyroid by detecting radioactive iodine taken up by thyroid gland normal thyroid enlarged cancerous
 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad Advanced inorganic chemistry lecture notes
Advanced inorganic chemistry lecture notes Lightning elves
Lightning elves Life lecture meaning
Life lecture meaning Ib chemistry functional groups
Ib chemistry functional groups Inorganic vs organic chemistry
Inorganic vs organic chemistry You light up my life lab answers
You light up my life lab answers Half life chemical kinetics
Half life chemical kinetics Chemistry
Chemistry Chapter 2 the chemistry of life section review 2-2
Chapter 2 the chemistry of life section review 2-2 Concept 2 chemistry of life
Concept 2 chemistry of life Concept 2 chemistry of life
Concept 2 chemistry of life Deficiency disease of protein
Deficiency disease of protein How is thermal energy generated
How is thermal energy generated Chemistry for life
Chemistry for life City and country life vocabulary
City and country life vocabulary City life vs country life
City life vs country life Daily life real life polynomial problems
Daily life real life polynomial problems Single life or married life which is better
Single life or married life which is better Life orientation skills
Life orientation skills Country life vs city life compare /contrast
Country life vs city life compare /contrast City life vs country life
City life vs country life Lessons from life of pi
Lessons from life of pi Boundaries meme
Boundaries meme The life that is truly life
The life that is truly life The idea life comes from life is
The idea life comes from life is Freetutorical.com harvest land
Freetutorical.com harvest land Project procurement management lecture notes
Project procurement management lecture notes Lecture about sport
Lecture about sport Healthy lifestyle wrap up lecture
Healthy lifestyle wrap up lecture Meaning of this
Meaning of this Randy pausch the last lecture summary
Randy pausch the last lecture summary Tensorflow lecture
Tensorflow lecture Theology proper lecture notes
Theology proper lecture notes Strategic management lecture
Strategic management lecture Geology lecture series
Geology lecture series Social psychology lecture
Social psychology lecture In text citation for a lecture
In text citation for a lecture Public sector accounting lecture notes pdf
Public sector accounting lecture notes pdf Lecture notes on project management
Lecture notes on project management Anchorage length eurocode
Anchorage length eurocode Electricity and magnetism lecture notes
Electricity and magnetism lecture notes Physics 111 lecture notes
Physics 111 lecture notes What is a harmonic wave in physics
What is a harmonic wave in physics Physical science lecture notes
Physical science lecture notes Power system dynamics and stability lecture notes
Power system dynamics and stability lecture notes Natural language processing nlp - theory lecture
Natural language processing nlp - theory lecture Microbial physiology and metabolism lecture notes
Microbial physiology and metabolism lecture notes Sensors and actuators lecture notes
Sensors and actuators lecture notes Ternology
Ternology Les objectifs de la lecture au primaire
Les objectifs de la lecture au primaire Instruction de lecture
Instruction de lecture Lecture carte aéronautique
Lecture carte aéronautique Lecture title
Lecture title Slidetodoc
Slidetodoc Money-time relationship and equivalence
Money-time relationship and equivalence Quasi saturation in power bjt
Quasi saturation in power bjt Software engineering lecture notes
Software engineering lecture notes Extension lecture meaning
Extension lecture meaning La situation de passage du dernier jour d'un condamné
La situation de passage du dernier jour d'un condamné Tegrity lecture capture
Tegrity lecture capture Why is sincerity so important in social business letters?
Why is sincerity so important in social business letters? Transistor lecture
Transistor lecture Examples of harvard referencing
Examples of harvard referencing How to write a recipe review
How to write a recipe review The parsec lecture tutorial answers
The parsec lecture tutorial answers Introduction to ofdm
Introduction to ofdm Semiconductor definition physics
Semiconductor definition physics Milieu citrate de simmons
Milieu citrate de simmons Land use planning '' lecture notes
Land use planning '' lecture notes Grille de lecture systémique
Grille de lecture systémique La double énonciation dans antigone
La double énonciation dans antigone Texte lecture pas à pas
Texte lecture pas à pas La lecture renoir
La lecture renoir Lecture automatique de document
Lecture automatique de document Les dictions
Les dictions La fiche de la ficelle
La fiche de la ficelle Comprhension
Comprhension Jane_ixx
Jane_ixx Project quality management lecture notes
Project quality management lecture notes Fuzzy logic lecture
Fuzzy logic lecture Fuzzy logic lecture
Fuzzy logic lecture Trois femmes puissantes lecture cursive
Trois femmes puissantes lecture cursive Lecture notes on homiletics
Lecture notes on homiletics Foundation engineering lecture notes
Foundation engineering lecture notes Forensic psychology lecture
Forensic psychology lecture Financial management lecture
Financial management lecture Image processing lecture notes
Image processing lecture notes Intermediate microeconomics lecture notes
Intermediate microeconomics lecture notes Divine principle 3 hour lecture
Divine principle 3 hour lecture Xxx
Xxx Computer security 161 cryptocurrency lecture
Computer security 161 cryptocurrency lecture It 101 introduction to computing
It 101 introduction to computing Crisis communication lecture
Crisis communication lecture Lecture à l'unisson
Lecture à l'unisson Les 4 dimensions de la lecture
Les 4 dimensions de la lecture Double lecture
Double lecture Parallel and distributed computing lecture notes
Parallel and distributed computing lecture notes Lecture about flowers
Lecture about flowers Bayesian decision theory lecture notes
Bayesian decision theory lecture notes Polynomial regression least squares
Polynomial regression least squares Psaume 23 comme lecture sainte cène
Psaume 23 comme lecture sainte cène Recording business transactions
Recording business transactions Bmfp lecture schedule
Bmfp lecture schedule Bilateral vs unilateral tolerance
Bilateral vs unilateral tolerance Gsm lecture
Gsm lecture Direct stiffness method truss
Direct stiffness method truss Slagle lecture
Slagle lecture Reservoir routing example
Reservoir routing example