La cardiotossicit da farmaci prevenzione diagnosi e terapia







































- Slides: 70

La cardiotossicità da farmaci: prevenzione, diagnosi e terapia Daniela Cardinale Unità di Cardiologia Istituto Europeo di Oncologia - Milano Catania, 6 Novembre 2008

Definition of cardiotoxicity

Cardiotoxicity ¬One of the major factors limiting the use of chemotherapy ¬Acute or Subacute within 2 weeks ¬Chronic (more frequent); ü Early Onset ü Late Onset within 1 year > 1 year q dose dependent q dilated/hypokinetic cardiomyopathy q poor prognosis

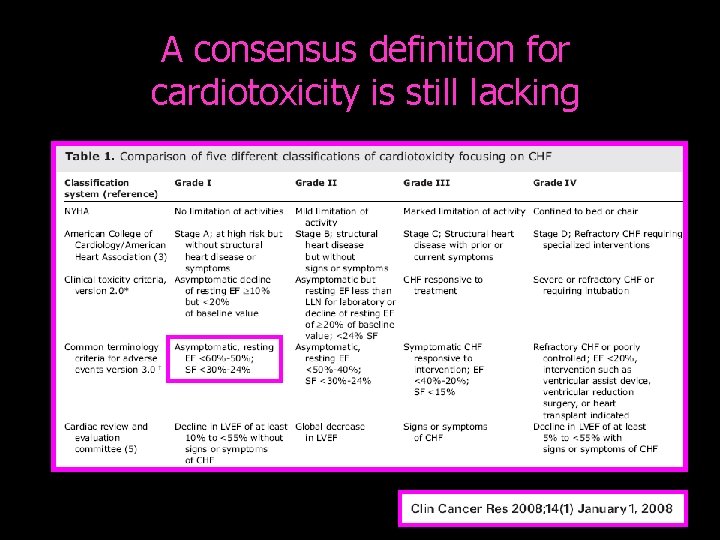
A consensus definition for cardiotoxicity is still lacking

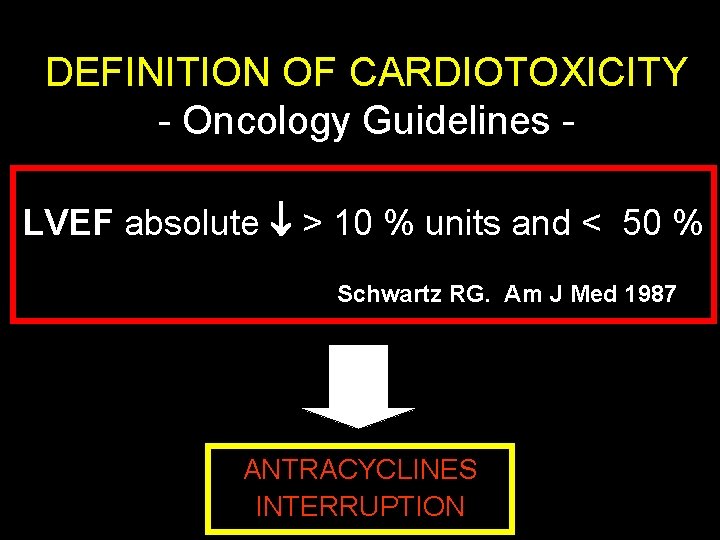
DEFINITION OF CARDIOTOXICITY - Oncology Guidelines LVEF absolute > 10 % units and < 50 % Schwartz RG. Am J Med 1987 ANTRACYCLINES INTERRUPTION

CARDIOTOXICITY ONCOLOGICAL IMPLICATIONS CARDIOLOGICAL IMPLICATIONS

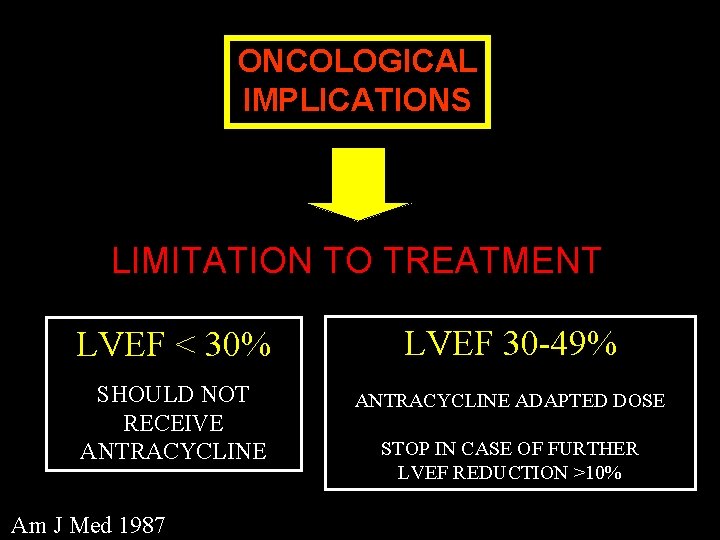
ONCOLOGICAL IMPLICATIONS LIMITATION TO TREATMENT LVEF < 30% LVEF 30 -49% SHOULD NOT RECEIVE ANTRACYCLINE ADAPTED DOSE Am J Med 1987 STOP IN CASE OF FURTHER LVEF REDUCTION >10%

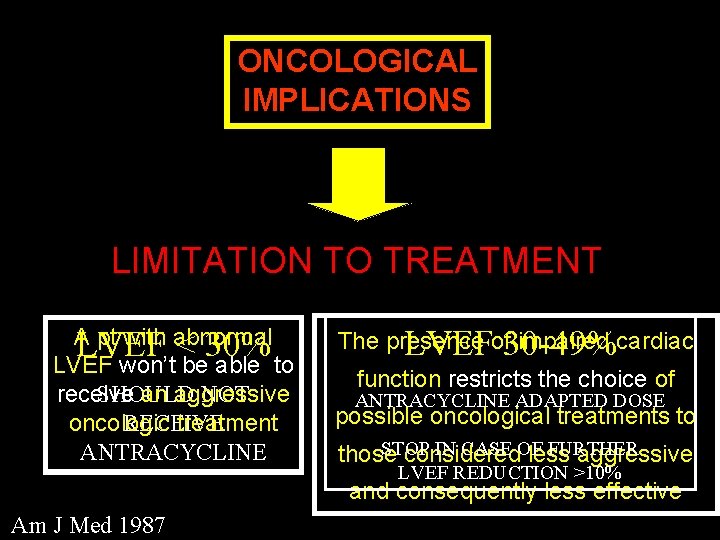
ONCOLOGICAL IMPLICATIONS LIMITATION TO TREATMENT A pt with abnormal LVEF < 30% LVEF won’t be able to SHOULD NOT receive an aggressive RECEIVE oncologic treatment ANTRACYCLINE The presence impaired cardiac LVEFof 30 -49% function restricts the choice of ANTRACYCLINE ADAPTED DOSE possible oncological treatments to STOP IN CASE OF FURTHER those considered less aggressive LVEF REDUCTION >10% and consequently less effective Am J Med 1987

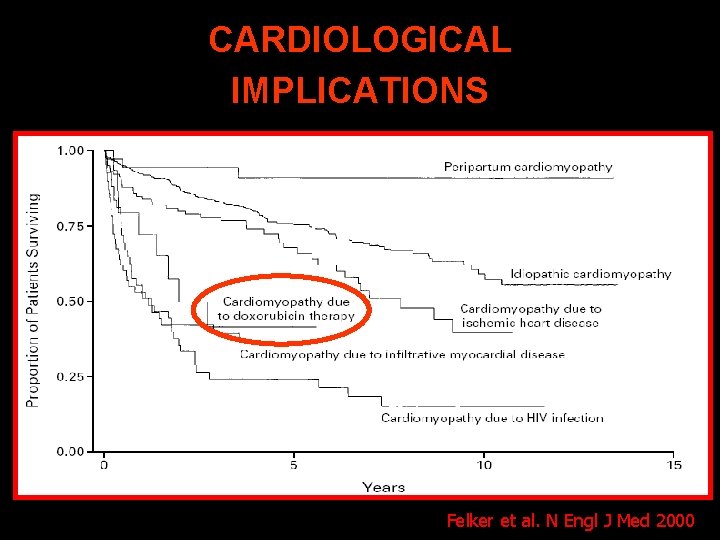
CARDIOLOGICAL IMPLICATIONS Felker et al. N Engl J Med 2000

Cardiac implications of chemoterapy Semin Oncol 1998



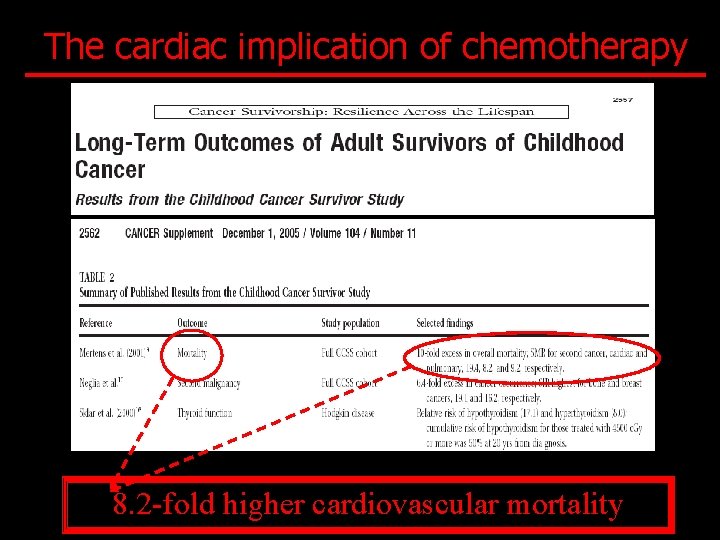
The cardiac implication of chemotherapy 8. 2 -fold higher cardiovascular mortality

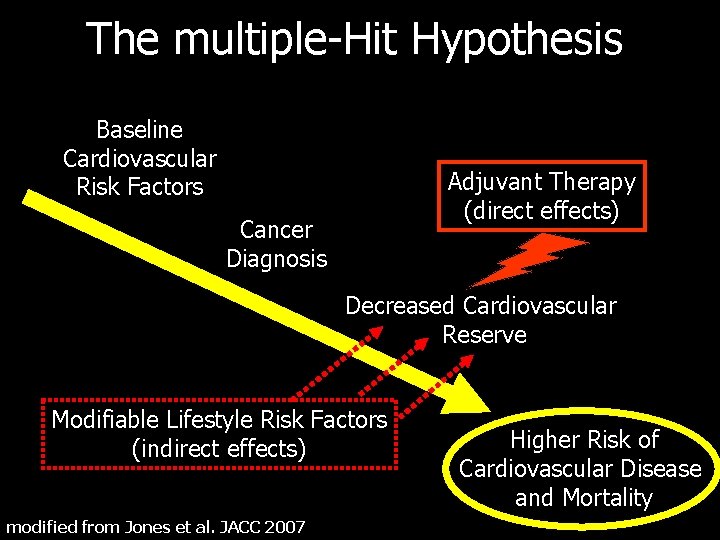
The multiple-Hit Hypothesis Baseline Cardiovascular Risk Factors Adjuvant Therapy (direct effects) Cancer Diagnosis Decreased Cardiovascular Reserve Modifiable Lifestyle Risk Factors (indirect effects) modified from Jones et al. JACC 2007 Higher Risk of Cardiovascular Disease and Mortality

Identificazione precoce della cardiotossicità

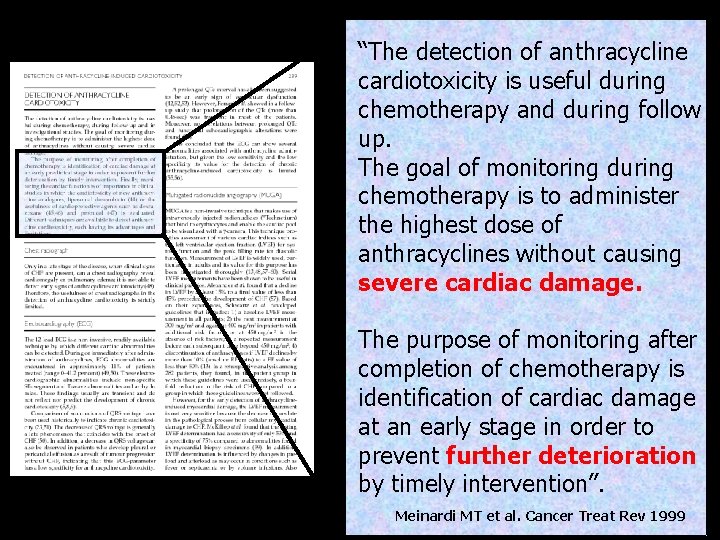
“The detection of anthracycline cardiotoxicity is useful during chemotherapy and during follow up. The goal of monitoring during chemotherapy is to administer the highest dose of anthracyclines without causing severe cardiac damage. The purpose of monitoring after completion of chemotherapy is identification of cardiac damage at an early stage in order to prevent further deterioration by timely intervention”. Meinardi MT et al. Cancer Treat Rev 1999

Late Diagnosis Functional impairment CT Early detection of cardiac damage Functional recovery Evolution toward to hypokinetic/dilated CMP

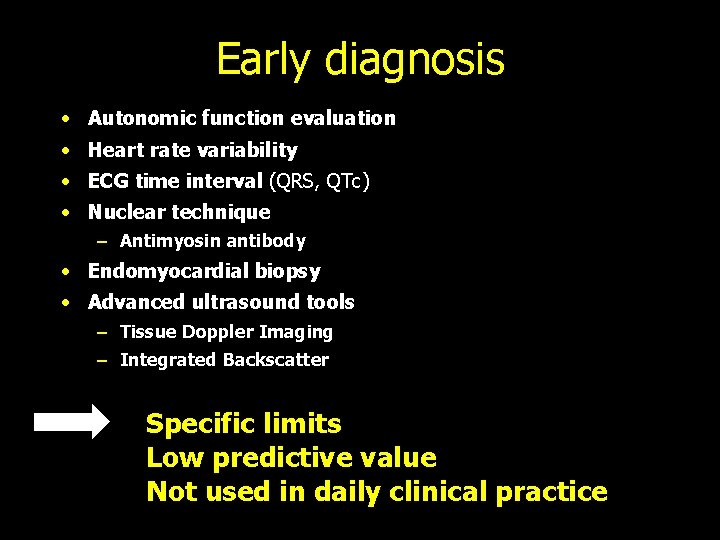
Early diagnosis · Autonomic function evaluation • Heart rate variability • ECG time interval (QRS, QTc) • Nuclear technique – Antimyosin antibody • Endomyocardial biopsy • Advanced ultrasound tools – Tissue Doppler Imaging – Integrated Backscatter Specific limits Low predictive value Not used in daily clinical practice

Cardiac markers Is there any possible application in the early detection of CT-induced cardiac damage? ? ?

• 204 patients (661 cycles of high-dose CT) • 39 males e 165 females (age 45± 10 years). • Poor-prognosis cancer diseases: – advanced or primary-resistant breast cancer – refractory ovarian carcinoma – small-cell lung cancer – high- grade non Hodgkin’s lymphoma – refractory Hodgkin’s disease • High-dose chemotherapy J Am Coll Cardiol 2000

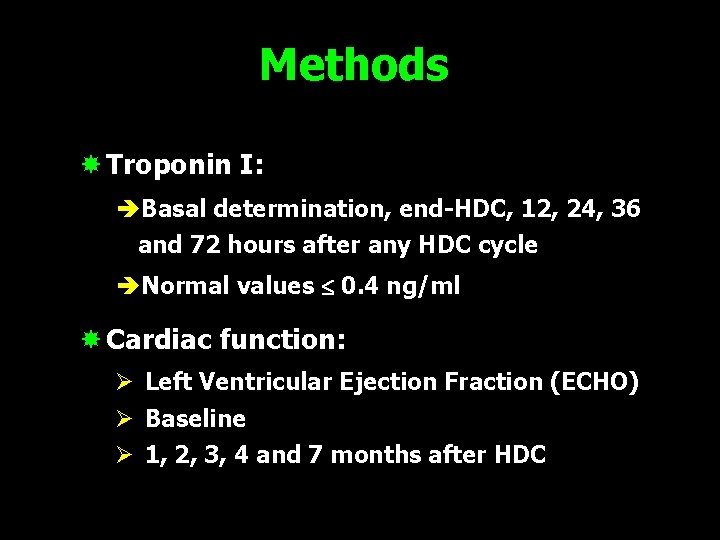
Methods Troponin I: èBasal determination, end-HDC, 12, 24, 36 and 72 hours after any HDC cycle èNormal values 0. 4 ng/ml Cardiac function: Ø Left Ventricular Ejection Fraction (ECHO) Ø Baseline Ø 1, 2, 3, 4 and 7 months after HDC

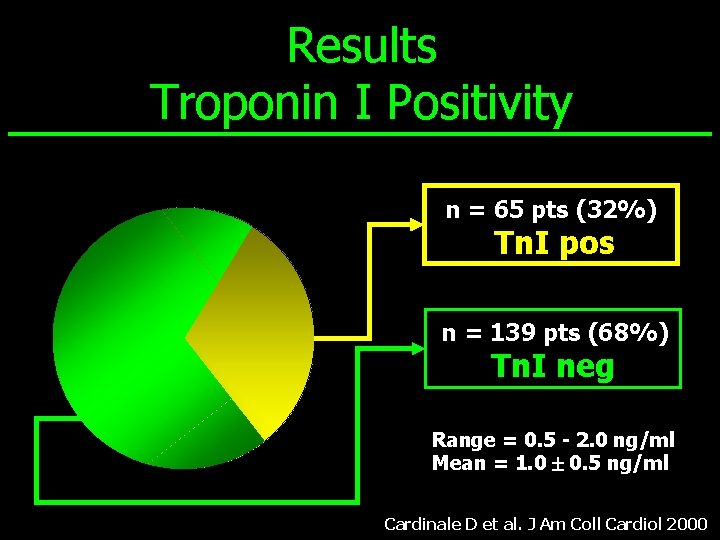
Results Troponin I Positivity n = 65 pts (32%) Tn. I pos n = 139 pts (68%) Tn. I neg Range = 0. 5 - 2. 0 ng/ml Mean = 1. 0 0. 5 ng/ml Cardinale D et al. J Am Coll Cardiol 2000

J Am Coll Cardiol 2000

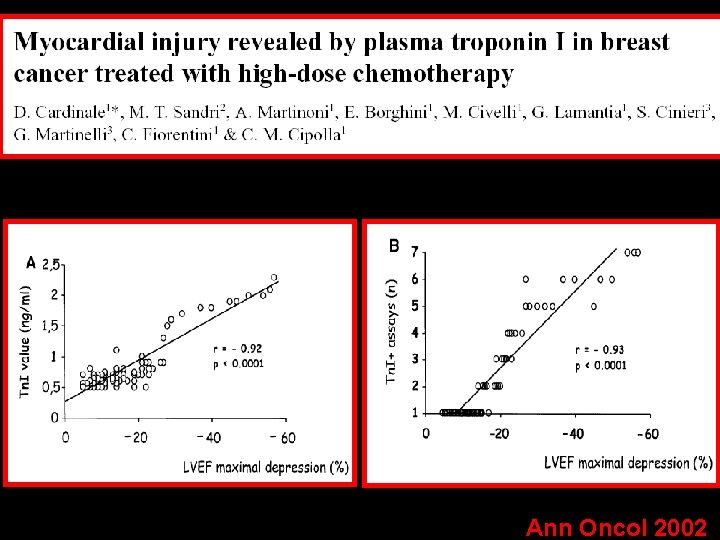
Ann Oncol 2002

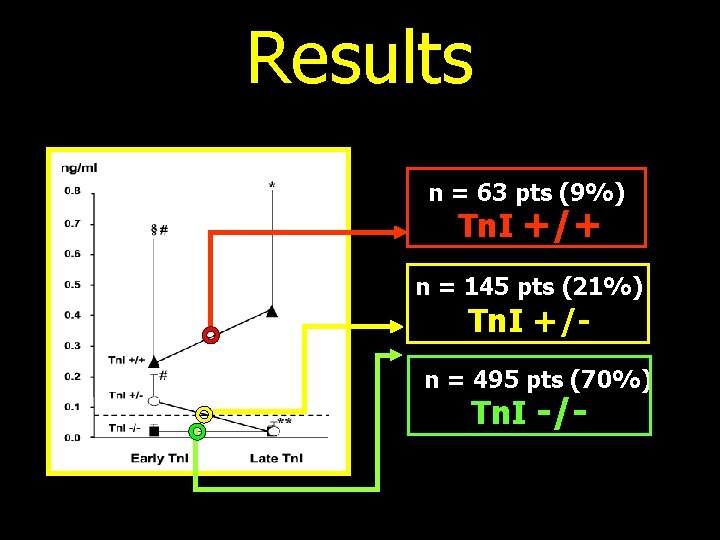
¬ 703 patients (216 males) ¬ age 47± 12 years ¬ treated with HDC ¬ poor prognosis malignancies ♫ Follow-up = 48 months ♫ MACE incidence ü Tn. I serum determination: l Baseline = before HDC l Early = soon after HDC (0, 12, 24, 36, 72 hours) l Late = 1 month after HDC Circulation 2004

Results n = 63 pts (9%) Tn. I +/+ n = 145 pts (21%) Tn. I +/- n = 495 pts (70%) Tn. I -/-

Left ventricular ejection fraction (%) 65 Tn. I -/- 60 55 50 * 45 * * 40 0 1 3 * = p<0. 01 vs. Tn. I -/-; 6 * * * §* §* §* 12 18 Tn. I +/+ 48 months § = p<0. 01 vs. Tn. I +/-. Cardinale et al. Circulation 2004

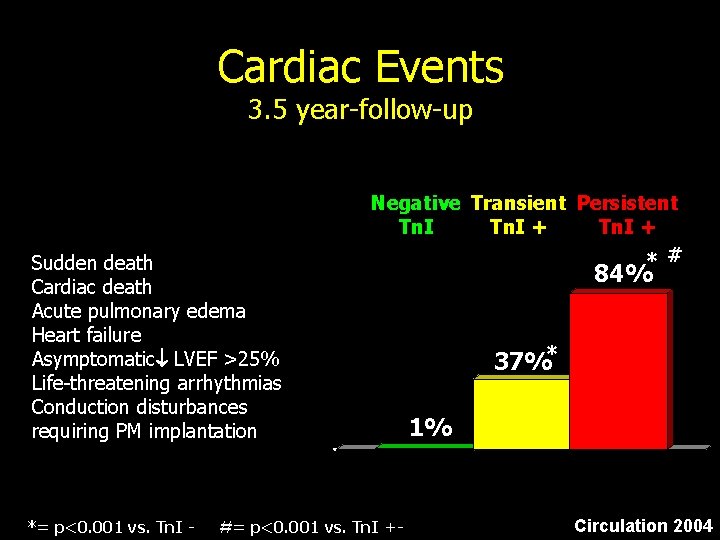
Cardiac Events 3. 5 year-follow-up Sudden death Cardiac death Acute pulmonary edema Heart failure Asymptomatic LVEF >25% Life-threatening arrhythmias Conduction disturbances requiring PM implantation *= p<0. 001 vs. Tn. I - Negative Transient Persistent Tn. I + * # #= p<0. 001 vs. Tn. I +- 84% 37%* 1% Circulation 2004

Tn. I positivity/different schedule CONVENTIONAL DOSE 50 40 % = preceded by AC AC CEF 30 20 20% 21% CMF 24% ACOD 19% 10 0 Doxorubicin Cyclophosphamide Epirubicin Metotrexate Cyclophosphamide 5 -Fluorouracil Vincristine Dexamethasone

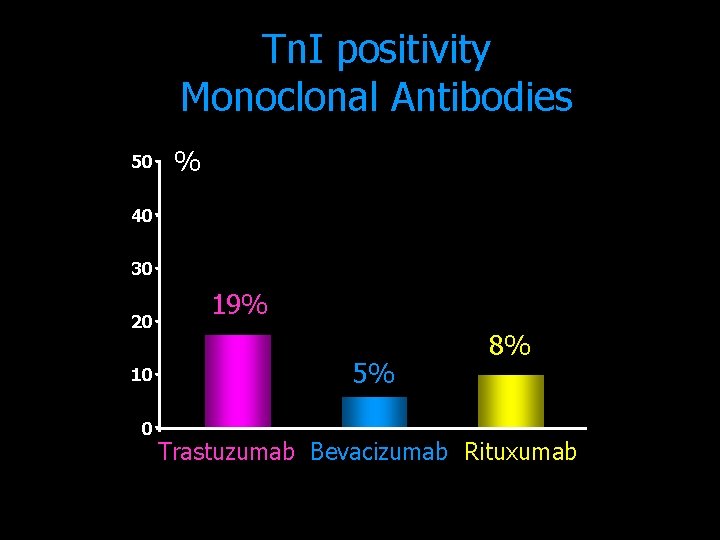
Tn. I positivity Monoclonal Antibodies 50 % 40 30 20 10 0 19% 5% 8% Trastuzumab Bevacizumab Rituxumab

Prevenzione della cardiotossicità

Possibili strategie di prevenzione della cardiotossicità ◙ ◙ ◙ limitazione della dose totale di antracicline; utilizzo di analoghi della antracicline utilizzo di chemioterapici meno cardiotossici; utilizzo di agenti cardioprotettori durante CT; stretto monitoraggio cardiologico; l possibile compromissione del successo clinico della CT l alti costi l scarso potere predittivo n considerati tutti i pazienti sottoposti a CT n rapporto costo-beneficio sfavorevole

Possible strategies 2 Cardiological preventive therapy in selected high-risk patients = Tn. I positivity

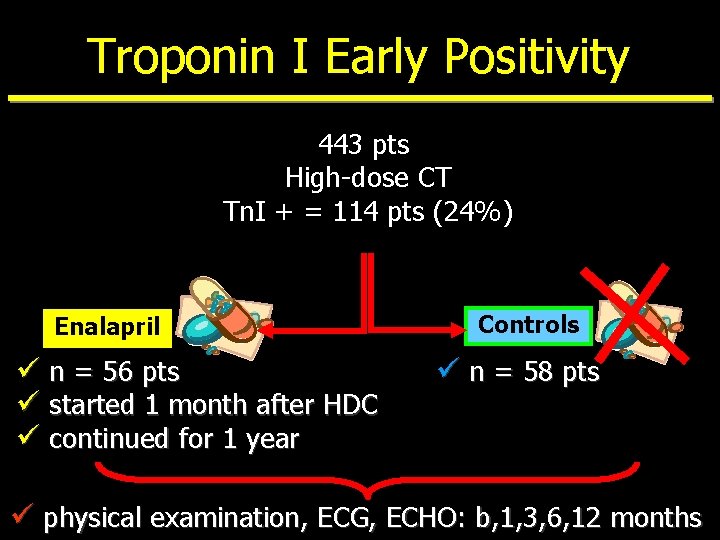
Troponin I Early Positivity 443 pts High-dose CT Tn. I + = 114 pts (24%) Enalapril ü n = 56 pts ü started 1 month after HDC ü continued for 1 year Controls ü n = 58 pts ü physical examination, ECG, ECHO: b, 1, 3, 6, 12 months

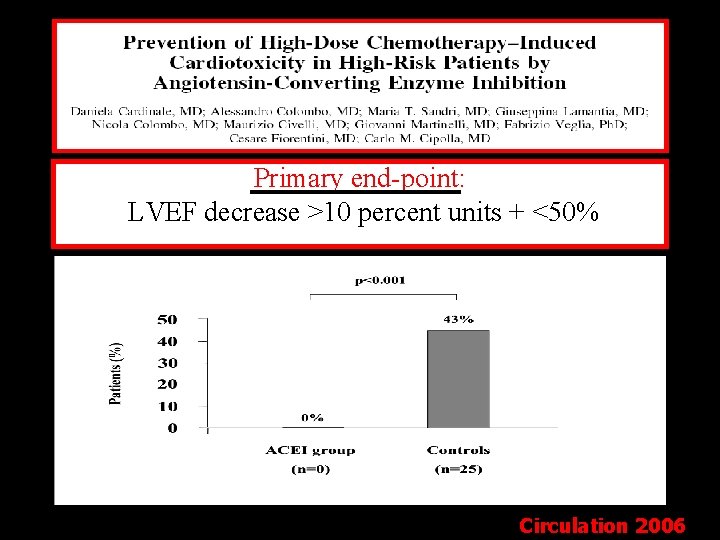
Primary end-point: LVEF decrease >10 percent units + <50% Circulation 2006

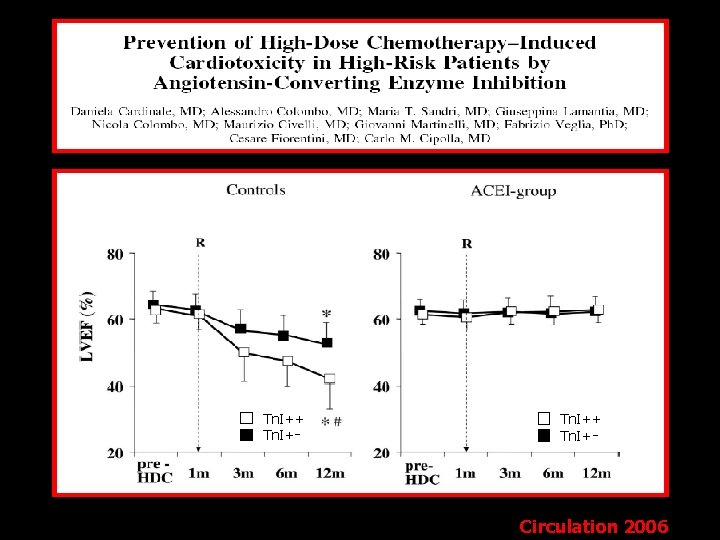
Tn. I++ Tn. I+ - - Circulation 2006

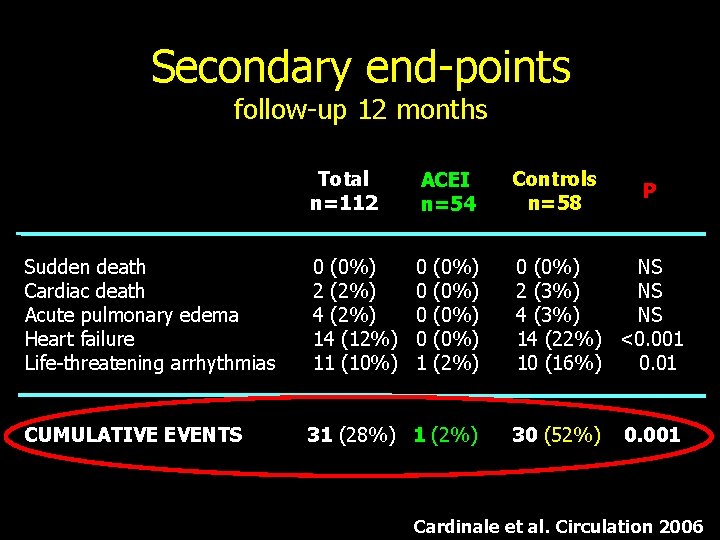
Secondary end-points follow-up 12 months Sudden death Cardiac death Acute pulmonary edema Heart failure Life-threatening arrhythmias CUMULATIVE EVENTS Total n=112 ACEI n=54 Controls n=58 0 (0%) 2 (2%) 4 (2%) 14 (12%) 11 (10%) 0 0 1 0 (0%) NS 2 (3%) NS 4 (3%) NS 14 (22%) <0. 001 10 (16%) 0. 01 (0%) (2%) 31 (28%) 1 (2%) 30 (52%) P 0. 001 Cardinale et al. Circulation 2006

Tn. I positivity during follow-up Enalapril ACEI- group Controls Circulation 2006

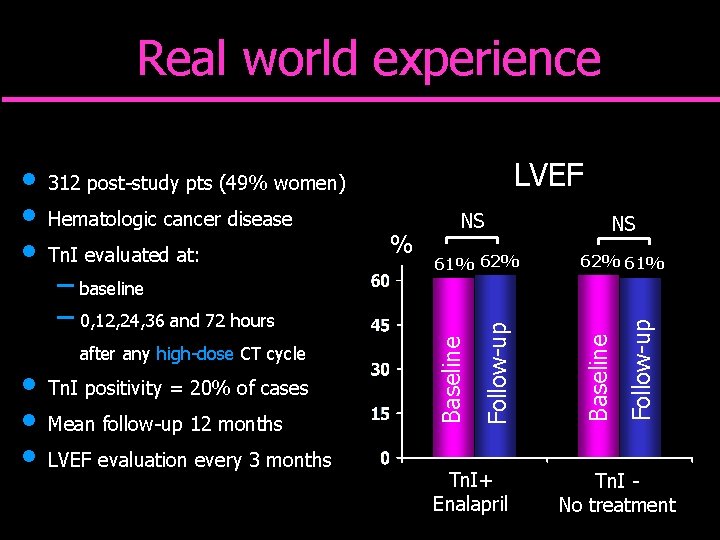
Real world experience – baseline – 0, 12, 24, 36 and 72 hours after any high-dose CT cycle Tn. I positivity = 20% of cases Mean follow-up 12 months LVEF evaluation every 3 months % 61% 62% Tn. I+ Enalapril NS 62% 61% Follow-up Tn. I evaluated at: NS Baseline Hematologic cancer disease Follow-up • • • LVEF 312 post-study pts (49% women) Baseline • • • Tn. I No treatment

Trattamento della cardiotossicità

CT-induced CMP ¥ Typically, these patients have been excluded from large randomized trials evaluating the efficacy of recommended HF therapy. ¥ Historically, CT-induced CMP is believed to be refractory to conventional therapy; but much of this data are anecdotal and are based on reports of small number of patients.

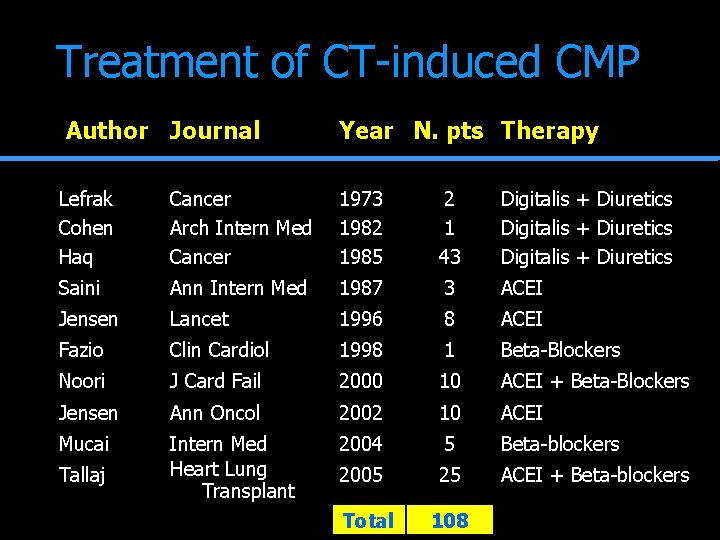
Treatment of CT-induced CMP Author Journal Year N. pts Therapy Lefrak Cohen Haq Cancer Arch Intern Med Cancer 1973 1982 1985 2 1 43 Digitalis + Diuretics Saini Ann Intern Med 1987 3 ACEI Jensen Lancet 1996 8 ACEI Fazio Clin Cardiol 1998 1 Beta-Blockers Noori J Card Fail 2000 10 ACEI + Beta-Blockers Jensen Ann Oncol 2002 10 ACEI Mucai Intern Med Heart Lung Transplant 2004 5 Beta-blockers 2005 25 ACEI + Beta-blockers Tallaj Total 108

What data can we base our therapy on? • There isn’t evidence whether the use of ACEI and BB agents, recommended by international cardiologic guidelines for treatment of heart failure can be directly transferred with similar long-term benefits to this particular setting. • As a consequence, evidence-based recommendations for management of cancer patients with asymptomatic and symptomatic CT-induced cardiomyopathy are still lacking and no definite guidelines are currently adopted.

……. is whether or not to treat cancer patients with asymptomatic LV dysfunction. Silber et al. J Clin Oncol 2004

CT-induced CMP § § § § 201 patients (148 women) Mean age 53± 12 years LVEF < 45% (range 20 -45%) Enrolled since 1998 Treatment: ACEI first BB added to ACEI (if tolerated) Follow-up: > 1 year

DEFINITION OF CARDIOTOXICITY - Oncology Guidelines LVEF absolute > 10 % units and < 50 % Schwartz RG. Am J Med 1987 ANTRACYCLINES INTERRUPTION

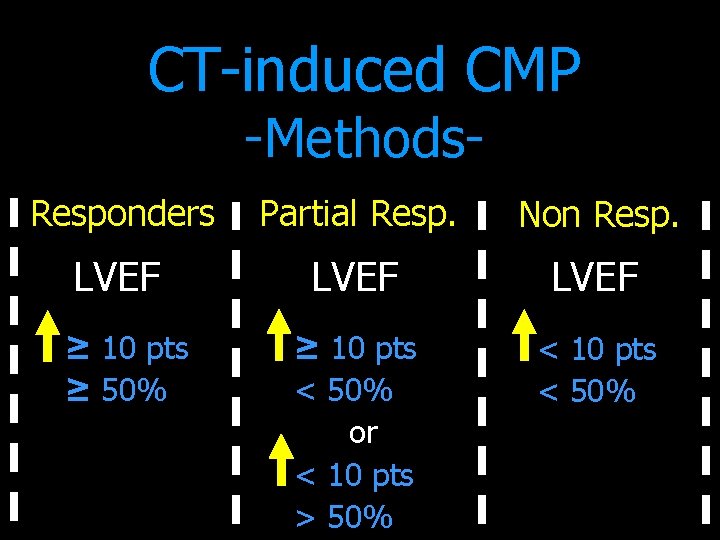
CT-induced CMP -Methods- Responders Partial Resp. Non Resp. LVEF ≥ 10 pts < 50% or < 10 pts > 50% < 10 pts < 50% ≥ 10 pts ≥ 50%

Results Partial NON Responders n = 67 n = 44 n = 90 AC TOT DOSE (mg/m 2) NYHA Class III-IV (%) ACEI + BB (%) CARDIAC EVENTS (n) TIME TO TREATMENT (months) P 263± 153 293± 161 311± 168 NS 13% 45% 27% <0. 01 73% 70% 54% 0. 03 2 10 26 <0. 01 2± 1 4± 3 24± 22 <0. 001

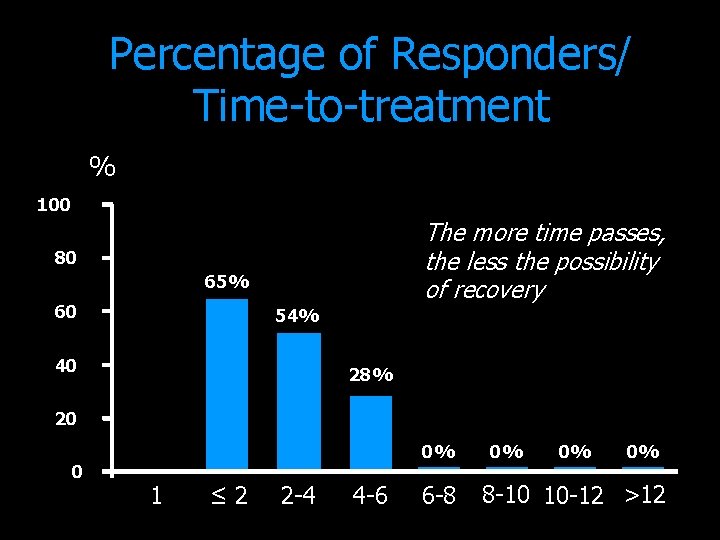
Percentage of Responders/ Time-to-treatment % 100 The more time passes, the less the possibility of recovery 80 65% 60 54% 40 28% 20 0 0% 1 ≤ 2 2 -4 4 -6 6 -8 0% 0% 0% 8 -10 10 -12 >12

Conclusioni I • La cardiotossicità è una complicanza frequente e potenzialmente severa dopo CT. • La sua rilevanza nella moderna pratica clinica è in aumento parallelamente all’incremento del numero di pz oncologici trattati e alla sempre maggiore complessità ed aggressività dei trattamenti CT.

Conclusioni II • La valutazione della Troponina I dopo CT consente di: – – – predire lo sviluppo di cardiotossicità stratificare il rischio cardiaco impostare strategie terapeutiche di prevenzione della cardiotossicità. • Il suo utilizzo dovrebbe essere incluso nella definizione moderna di cardiotossicità.

Conclusions III In patients with CT-induced CMP, complete LVEF recovery is achievable only when cardiac toxicity is early detected and a prompt treatment is carried out.

Conclusions II Don’t waste time!…. . Guidelines regarding cardiotoxicity should be revalued by oncologists and cardiologists together, to optimize the management of cancer patients.

www. coverweb. info/cardio 3/

Caso clinico - G. A. • • • Età: 36 anni Sesso: maschile Fattori di rischio cardiovascolare: ex fumatore • Diagnosi oncologica: LNH a grandi cellule B

Terapia oncologica • • • • 8/8/2003 29/8/2003 26/9/2003 21/10/2003 7/11/2003 2/12/2003 10/2/2004 20/5/2004 27/5/2004 4/8/2004 29/9/2004 28/10/2004 7/12/2004 ACOD CHOP CTX 7 gr/mq MTX 8 gr/mq VP 16 2 gr/mq Novantrone 60 mg/mq +Alkeran 180 mg/mq R-ACOD X 4 Ab anti-CD 20 Mabthera 250 mg/mq ACOD X 2 ESHAP BEAM (ultima terapia)

Caso clinico - G. A. • Dose totale antracicline: Età: 36 anni 532 mg/mq Sesso: maschile Fattori di rischio cardiovascolare: • Stato attuale (Ott. 2008): ex fumatore assenza di malattia • Diagnosi oncologica: LNH a grandi cellule B • • •

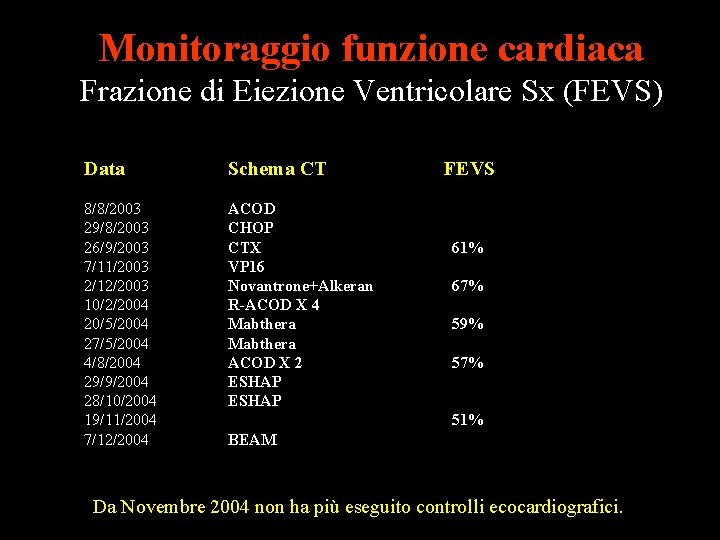
Monitoraggio funzione cardiaca Frazione di Eiezione Ventricolare Sx (FEVS) Data Schema CT 8/8/2003 29/8/2003 26/9/2003 7/11/2003 2/12/2003 10/2/2004 20/5/2004 27/5/2004 4/8/2004 29/9/2004 28/10/2004 19/11/2004 7/12/2004 ACOD CHOP CTX VP 16 Novantrone+Alkeran R-ACOD X 4 Mabthera ACOD X 2 ESHAP FEVS Tn. I 61% 67% 0. 01 0. 00 59% 0. 07 57% 0. 10 0. 16 0. 14 51% BEAM 0. 10 Da Novembre 2004 non ha più eseguito controlli ecocardiografici.

Follow-up cardiologico • Il 4 Luglio 2005 comparsa di dispnea a bassa soglia Ricovero per scompenso cardiaco: evidenza di cardiomiopatia ipocinetica con severa riduzione della FEVS (21%). • Inizia terapia cardiologica con: • A Settembre 2005 sospende gli ACE-inibitori per ipotensione. • Il 2 Gennaio 2006 nuovo ricovero per scompenso cardiaco (FEVS 25%) ACE-inibitori + Digitale + Diuretici

DOMANDA 1 • Dal punto di vista cardiologico, quali controlli aggiuntivi sarebbero stati utili in questo paziente per diagnosticare precocemente una CMP da CT dopo la fine del trattamento oncologico? 1) monitoraggio dei sintomi 2) monitoraggio della FEVS 3) esecuzione di ECG seriati

Monitoraggio funzione cardiaca Frazione di Eiezione Ventricolare Sx (FEVS) Data Schema CT 8/8/2003 29/8/2003 26/9/2003 7/11/2003 2/12/2003 10/2/2004 20/5/2004 27/5/2004 4/8/2004 29/9/2004 28/10/2004 19/11/2004 7/12/2004 ACOD CHOP CTX VP 16 Novantrone + Alkeran R-ACOD X 4 Mabthera ACOD X 2 ESHAP FEVS Tn. I 61% 67% 0. 01 0. 00 59% 0. 07 57% 0. 10 0. 16 0. 14 51% BEAM 0. 10 4/7/2005 21% Sintomi

DOMANDA 2 • Quale parametro è più utile monitorare durante chemioterapia per stratificare il rischio di cardiotossicità? 1) FEVS 2) dose totale di antracicline 3) Troponina I

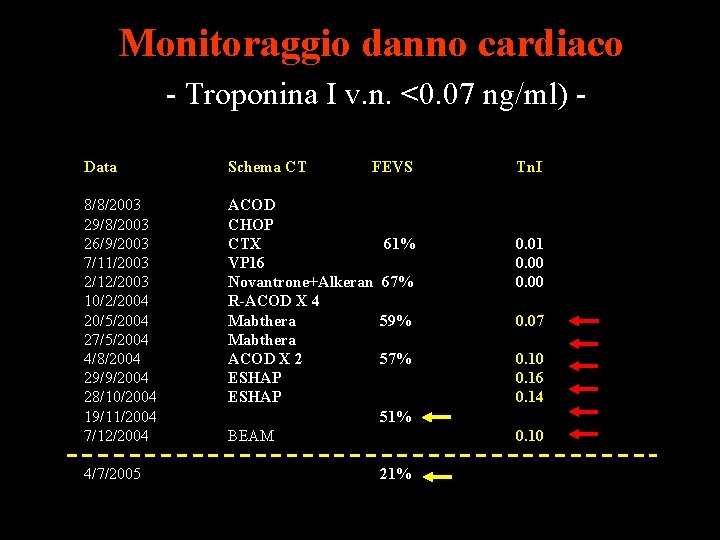
Monitoraggio danno cardiaco - Troponina I v. n. <0. 07 ng/ml) Data Schema CT 8/8/2003 29/8/2003 26/9/2003 7/11/2003 2/12/2003 10/2/2004 20/5/2004 27/5/2004 4/8/2004 29/9/2004 28/10/2004 19/11/2004 7/12/2004 ACOD CHOP CTX VP 16 Novantrone+Alkeran R-ACOD X 4 Mabthera ACOD X 2 ESHAP 4/7/2005 FEVS 61% Tn. I 67% 0. 01 0. 00 59% 0. 07 57% 0. 10 0. 16 0. 14 51% BEAM 0. 10 21%

DOMANDA 3 • Una volta evidenziata una CMP ipocinetica da chemioterapici, che terapia cardiologica utilizzereste per avere il massimo beneficio? 1) Digitale e diuretici 2) ACE-inibitori o Beta-bloccanti 3) ACE-inibitori + Beta-Bloccanti

Follow-up cardiologico • Da Febbraio 2006 ad oggi in terapia cardiologica con: ACE-inibitori + Beta-bloccanti + Diuretici • Stato attuale: non sintomi cardiologici Classe NYHA 2 FE 40%

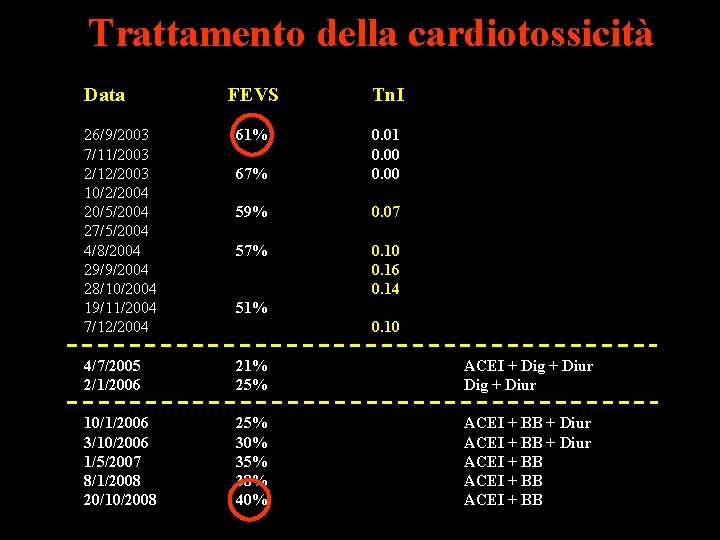
Trattamento della cardiotossicità Data FEVS Tn. I 26/9/2003 7/11/2003 2/12/2003 10/2/2004 20/5/2004 27/5/2004 4/8/2004 29/9/2004 28/10/2004 19/11/2004 7/12/2004 61% 67% 0. 01 0. 00 59% 0. 07 57% 0. 10 0. 16 0. 14 4/7/2005 2/1/2006 21% 25% ACEI + Dig + Diur 10/1/2006 3/10/2006 1/5/2007 8/1/2008 20/10/2008 25% 30% 35% 38% 40% ACEI + BB + Diur ACEI + BB 51% 0. 10

DOMANDA 4 • Quale strategia si è dimostrata maggiormente efficace nella prevenzione della CMP da chemioterapici? 1) Utilizzo di farmaci con minore potenziale cardiotossicità 2) Sospensione della chemioterapia dopo iniziale calo dell’ FEVS (>10%) 3) Trattamento con enalapril in pazienti ad alto rischio (Troponina I >0. 06 ng/ml)

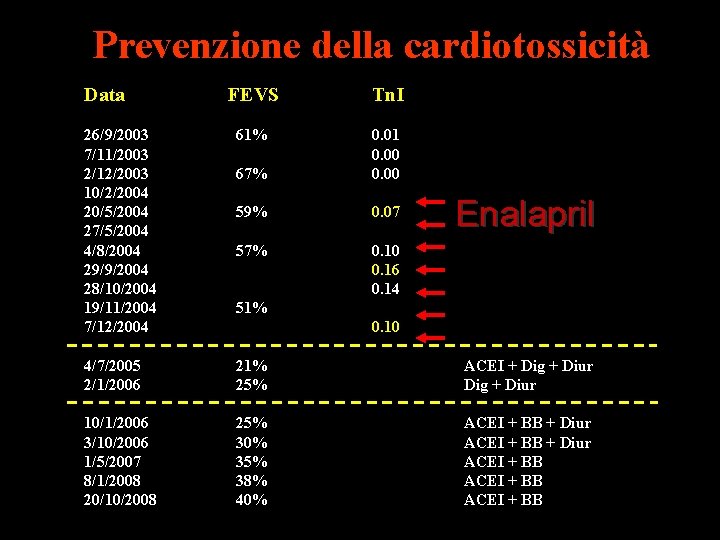
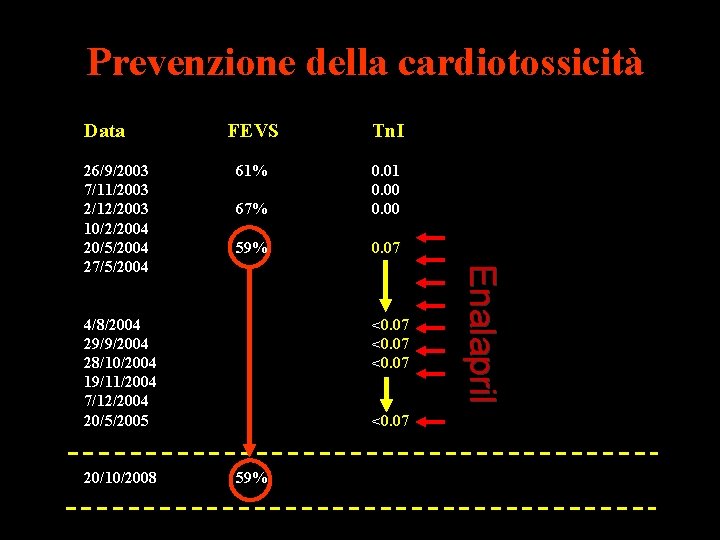
Prevenzione della cardiotossicità Data FEVS Tn. I 26/9/2003 7/11/2003 2/12/2003 10/2/2004 20/5/2004 27/5/2004 4/8/2004 29/9/2004 28/10/2004 19/11/2004 7/12/2004 61% 67% 0. 01 0. 00 59% 0. 07 57% 0. 10 0. 16 0. 14 4/7/2005 2/1/2006 21% 25% ACEI + Dig + Diur 10/1/2006 3/10/2006 1/5/2007 8/1/2008 20/10/2008 25% 30% 35% 38% 40% ACEI + BB + Diur ACEI + BB Enalapril 51% 0. 10

Prevenzione della cardiotossicità Data Tn. I 61% 67% 0. 01 0. 00 59% 0. 07 4/8/2004 29/9/2004 28/10/2004 19/11/2004 7/12/2004 20/5/2005 20/10/2008 <0. 07 59% Enalapril 26/9/2003 7/11/2003 2/12/2003 10/2/2004 20/5/2004 27/5/2004 FEVS

Grazie per l’attenzione! Guarigione oncologica Senza cardiotossicità ?
 Forum prevenzione incendi
Forum prevenzione incendi Prescrizioni normative sistemi controllo fumo
Prescrizioni normative sistemi controllo fumo Ufficio prevenzione infortuni
Ufficio prevenzione infortuni Inail civita castellana
Inail civita castellana Curarici
Curarici Caffè controindicazioni
Caffè controindicazioni Neuroplasticità
Neuroplasticità Vie enterali
Vie enterali Farmaci antibatterici
Farmaci antibatterici Escrezione dei farmaci
Escrezione dei farmaci Farmaci antimuscarinici
Farmaci antimuscarinici Farmaci ipolipidemizzanti cosa sono
Farmaci ipolipidemizzanti cosa sono Circolo enteroepatico farmaci
Circolo enteroepatico farmaci Ssri farmaci
Ssri farmaci Limbial
Limbial Halichondrin b
Halichondrin b Tabella equivalenza statine
Tabella equivalenza statine Farmaci venotropi
Farmaci venotropi Ssri farmaci
Ssri farmaci Glucosidi cardioattivi
Glucosidi cardioattivi Fv tv
Fv tv Diagnosi divisione in sillabe
Diagnosi divisione in sillabe Cisti del dotto tireoglosso diagnosi differenziale
Cisti del dotto tireoglosso diagnosi differenziale Diagnosi psicologica rimini riccione
Diagnosi psicologica rimini riccione Diagnosi dm 05/02/92
Diagnosi dm 05/02/92 Diagnosi cml
Diagnosi cml Esempi di diagnosi infermieristiche
Esempi di diagnosi infermieristiche Diagnosi infermieristiche ipertensione
Diagnosi infermieristiche ipertensione Cascata del complemento
Cascata del complemento Certificazione e diagnosi
Certificazione e diagnosi Biblioteca pianezza
Biblioteca pianezza Diagnosi funzionale redatta
Diagnosi funzionale redatta Diagnosi infermieristica obesità
Diagnosi infermieristica obesità Diagnosi funzionale esempio
Diagnosi funzionale esempio Terapia ocupacional sena
Terapia ocupacional sena Kognitywna terapia behawioralna
Kognitywna terapia behawioralna Unich fisioterapia
Unich fisioterapia Terapia neuroacustica
Terapia neuroacustica Terapia de pareja
Terapia de pareja Monica gomez terapeuta holistica
Monica gomez terapeuta holistica Terapia mrt
Terapia mrt La lectura como terapia
La lectura como terapia Terapia de pareja
Terapia de pareja Panipopitutarismo
Panipopitutarismo Centro siciliano di terapia della famiglia
Centro siciliano di terapia della famiglia Terapia manualna sierosław
Terapia manualna sierosław Terapia ocupacional uoc
Terapia ocupacional uoc Terapia dla nieśmiałych
Terapia dla nieśmiałych Salvador minuchin terapia familiar estructural
Salvador minuchin terapia familiar estructural Laso terapia
Laso terapia Anamnese terapia ocupacional idoso
Anamnese terapia ocupacional idoso Dietas oligoméricas
Dietas oligoméricas Acidi nucleici
Acidi nucleici Nutriia
Nutriia Terapia gestalt 9 zaleceń
Terapia gestalt 9 zaleceń Terapia w piaskownicy
Terapia w piaskownicy Meloterapie pentru copii
Meloterapie pentru copii Hivamat terápia
Hivamat terápia Modelos sistemicos terapia familiar
Modelos sistemicos terapia familiar Iridodialisi
Iridodialisi Terapia electroconvulsiva
Terapia electroconvulsiva Esostosi multipla
Esostosi multipla Arto fantasma terapia
Arto fantasma terapia Terapia gestalt ppt
Terapia gestalt ppt Funciones de la terapia dialéctico conductual
Funciones de la terapia dialéctico conductual Terapia antibiotica
Terapia antibiotica Modelo psicodinamico de constelaciones familiares
Modelo psicodinamico de constelaciones familiares Terapia educazionale del diabetico
Terapia educazionale del diabetico Terapia emozionale
Terapia emozionale Modelo de terapia de strong como influencia interpersonal
Modelo de terapia de strong como influencia interpersonal Ectima contagioso terapia
Ectima contagioso terapia